DOI: 10.11817/j.issn.1672-7207.2021.01.009
增压富氧气氛下NOx均相生成及SNCR反应机理研究
段元强,洪溥,仇兴雷,段伦博
(能源热转换及其过程测控教育部重点实验室,东南大学 能源与环境学院,江苏 南京,210096)
摘要:采用动力学计算与实验相结合的方式,对增压富氧气氛下的NOx均相生成和选择性非催化还原(selective non-catalytic reduction,SNCR)特性进行研究,分析温度、压力和组分体积分数等关键因素的影响。研究结果表明:对于NOx的均相生成反应,当反应压力为0.1~0.9 MPa时,最主要的NOx生成路径为NH3→NH2→H2NO→HNO→NO和HCN→CN→NCO→N2O;提高反应压力,上述NO和N2O主要生成路径基本保持不变;反应气氛中H2O分压提高使得部分H2NO中间体以及NO2产物进一步生成HONO,而HONO在H2O作用下快速分解为NO;而对于NOx的均相还原反应,提高反应压力可促进NO还原反应,但同时也在一定程度上可促进烟气中NO2的生成。
关键词:增压富氧燃烧;NOx;反应机理;均相生成;SNCR
中图分类号:TK16 文献标志码:A
文章编号:1672-7207(2021)01-0096-10
Study of homogeneous formation of NOx and SNCR reaction mechanism in pressurized oxy-fuel atmosphere
DUAN Yuanqiang, HONG Pu, QIU Xinglei, DUAN Lunbo
(Key Laboratory of Energy Thermal Conversion and Control, Ministry of Education, School of Energy and Environment, Southeast University, Nanjing 210096, China)
Abstract: The kinetic calculations and experiments were conducted to study the homogeneous formation and selective non-catalytic reduction(SNCR) characteristics of NOx in the pressurized oxy-fuel atmosphere, and the effects of key factors such as temperature, reaction pressure, and component volume fraction were analyzed. The results show that the two most important pathways of NOx homogeneous formation are NH3→NH2→H2NO→HNO→NO and HCN→CN→NCO→N2O when the reaction pressure ranges from 0.1 MPa to 0.9 MPa. With the increase of reaction pressure, these above-mentioned main formation pathways of NO and N2O remain unchanged. Part of the H2NO intermediates and NO2 will further generate HONO with the increase of H2O partial pressure,and then HONO will decompose rapidly into NO during the catalysis of H2O. For the homogeneous reduction and decomposition of NOx , increasing the reaction pressure promotes the NO reduction reactions, and it also promotes the generation of NO2 in the flue gas to a certain extent.
Key words: pressurized oxy-fuel combustion; NOx; reaction mechanism; homogeneous formation; SNCR
富氧燃烧是目前最具技术经济优势的燃煤电站CO2捕集技术之一,但大量的理论研究和示范装置的运行结果均表明,制氧系统和烟气净化压缩系统的高功耗成为制约该技术进一步工业化应用的根本性问题[1-2]。对于现有的空气燃烧电站,若将其改造为富氧燃烧,则其发电净效率至少降低10%[3-4],增压富氧燃烧就是在这一背景下被提出的。制氧系统和烟气净化压缩系统均是在压力下运行的,增压富氧燃烧将燃烧过程也置于压力下进行。相关研究表明[5-7],增压富氧燃烧技术具有系统功损小、杜绝漏风、烟气CO2纯度高和汽化潜热易回收等优点,近年来得到了广泛研究。NO和N2O是流化床煤燃烧过程中最主要的含氮污染物。在流化床增压富氧燃烧过程中,压力和气氛的改变会极大影响燃料N向气态NOx转化。流化床内燃料N的转化过程非常复杂,其中,关于压力和CO2对煤燃烧中NOx生成反应影响是研究热点。研究[8]表明,当压力从大气压力升压至0.5 MPa时,NO的排放量大幅度降低;继续升高压力,NO的排放将与压力不再相关。而压力对N2O排放的影响结果差异较大,SVOBODA等[9]的研究结果表明,在空气燃烧实验中,压力为1.0 MPa时N2O的排放比大气压力下的高,而工业装置的测试结果[8]表明,加压燃烧时N2O的排放比大气压力下的排放略低。
目前,关于增压富氧燃烧工况下NOx均相生成和还原特性的报道较少。本文作者利用反应动力学计算和研究高压、高CO2体积分数下NOx的均相生成路径,并在增压固定床反应器上开展NOx均相还原实验;此外,通过对气态产物进行实时监测,并结合反应动力学计算,探究运行压力对NH3均相还原NOx的影响途径。
1 研究方法
1.1 固定床反应器
NOx的均相还原实验在如图1所示的增压固定床上进行。该实验装置恒温段内径为24 mm,恒温段长为300 mm,热态设计压力为1.0 MPa,设计最高运行温度为1 000 ℃。在本实验中采用Thermo Fisher公司的Antaris IGS型傅里叶红外分气体分析仪对气体组分中NH3,NO和N2O的体积分数进行在线监测,这3种气体的体积分数测量精度均为1×10-6。
本实验中所使用的反应气体均由高压气瓶提供。实验前,使用与实验气氛相同的气体对系统进行充分吹扫。待吹扫完成后,打开预热器和主炉膛的电加热,进行升温。当系统达到设定温度后,通过同步调节入口气体流量和尾部阀门开度,使得炉内压力达到实验设定值。待系统压力和温度稳定后,进行气体组分测量。
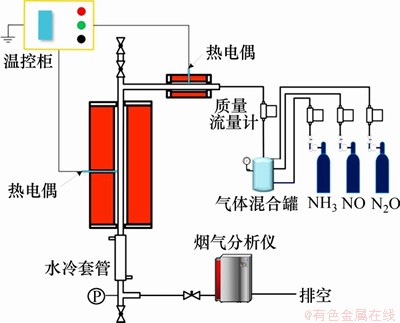
图1 增压固定床实验平台系统
Fig. 1 Schematics of pressurized fixed bed system
1.2 机理模型及验证
利用Chemkin Pro软件研究高压、高CO2分压下含氮前驱物NH3和HCN向NOx的气相生成路径。本研究所使用的模型为富氧气氛下的氮、硫综合反应模型,其包含88个组分和645步基元反应。该模型主要基于GRI-Mech 3.0[10-11],氮转化子模型主要来自于WARGADALAM等[12-13]的研究结果,同时也参考了HU等[14-15]的相关研究结果,而硫转化子模型主要来自于GLARBORG等[16]的研究,同时,在该模型中还考虑了H2,CO,N和S之间的交互反应[17]。反应器模型则采用柱塞流反应器PFR(plug flow reactor)。利用ALLEN等[18-19]的实验结果对上述模型进行验证,其结果如图2所示。从图2可以看出在实验工况范围内,NO及N2O体积分数的预测值和实验值的吻合度均较高,这证明上述反应模型可以用于预测压力下NOx生成/还原转化路径。
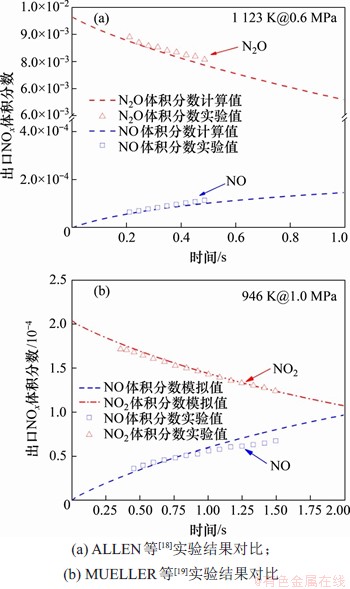
图2 本研究所使用的详细反应机理验证
Fig. 2 Verification of detailed reaction mechanism used in this study
2 增压富氧气氛NOx的均相生成机理
2.1 温度对NOx均相生成反应的影响
利用Chemkin Pro软件对增压富氧气氛下NO和N2O的均相生成机理进行分析。在反应压力为0.5 MPa,入口气体NH3,HCN,O2和H2O体积分数分别为0.1%,0.1%,5.0%和5.0%时,以CO2为平衡气体,反应温度对NOx气相生成的影响如图3所示。从图3可以看出:对于NO,各温度下NO峰值体积分数均低于6×10-5,这表明单独考虑均相生成反应时,含氮前驱物向NO的转化率较低;随着停留时间延长,NO出口体积分数呈现先增加后降低直至稳定的趋势;在700~950 ℃反应温度范围内,NO体积分数达到平衡的时间均小于1 s,且随着反应温度升高,NO的平衡体积分数也逐步升高;而对于N2O,在设定的反应时间12 s内,除温度700 ℃外,其余温度下N2O的体积分数均呈现快速上升后逐步下降趋势;当反应温度高于850 ℃时,N2O的分解和还原反应加剧,N2O体积分数快速降低直至完全分解还原为N2。
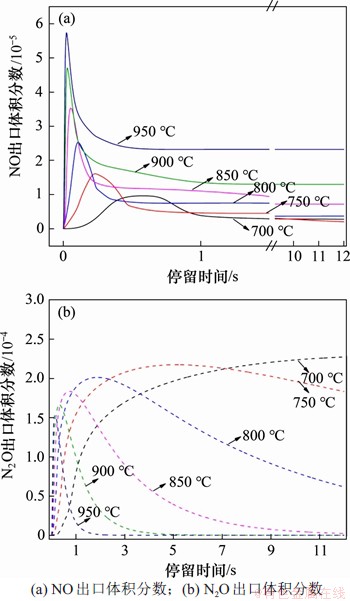
图3 反应温度对NOx均相生成反应的影响
Fig. 3 Effect of reaction temperature on homogeneous formation of NOx
2.2 压力对NOx均相生成反应的影响
在反应温度为850 ℃,入口气体NH3,HCN,O2和H2O体积分数分别为0.1%,0.1%,5.0%和5.0%时,以CO2为平衡气体,反应压力对NOx气相生成反应的影响如图4所示。从图4(a)可以看出:随着反应的进行,NO体积分数快速达到峰值后逐步下降,且NO达到峰值的时间随着反应压力的提高而缩短。反应压力提高会降低NO的最终体积分数,但与其前驱体(HCN和NH3)的入口体积分数相比,压力对NO气相生成反应的抑制作用较小。
在反应温度为850 ℃,入口气体NH3,HCN,O2和H2O体积分数分别为0.1%,0.1%,5.0%和5.0%时,以CO2为平衡气体,不同压力下各主要基元反应NO生成速率见图5。当反应压力为0.1 MPa时,NO的主要生成反应分别如下。
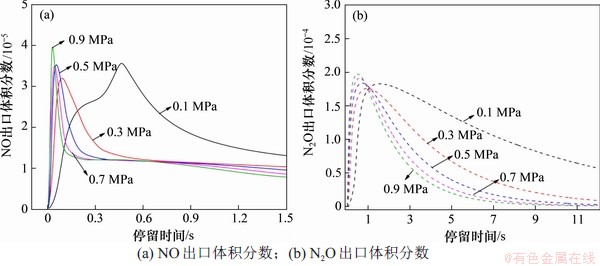
图4 反应压力对NOx均相生成反应的影响
Fig. 4 Effect of reaction pressure on homogeneous formation of NOx
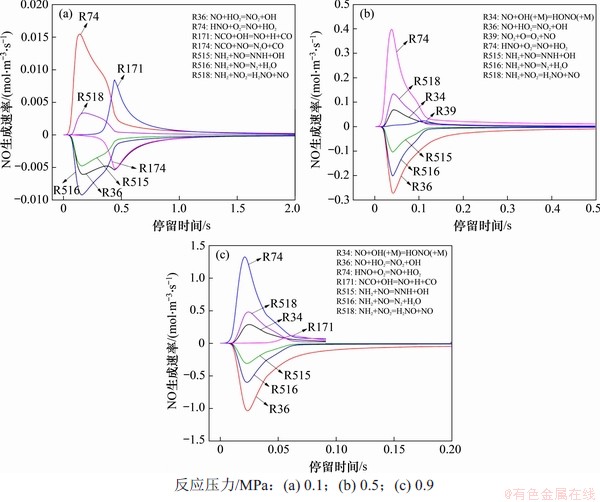
图5 NO生成率随反应时间变化曲线
Fig. 5 Production rate of NO with reaction time
R74:HNO+O2=NO+HO2;
R171:NCO+OH=NO+H+CO;
R518:NH2+NO2=H2NO+NO。
据推测,0.1 MPa下NO的主要有以下3条生成路径:
(a) NH3→NH2→H2NO→HNO→NO;
(b) HCN→CN→NCO→NO;
(c) HCN→NCO→NH2→HNO→NO。
其中,R74是NO生成的主导反应,这说明路径(a)是NO最主要生成路径,而NH3是NO的主要前驱体。
提高反应压力到0.5 MPa,可以发现NO的主要生成路径发生一定改变。加压促进了各基元反应的反应速率,同时,反应R34(即NO+OH(+M)=HONO(+M))也取代了R171成为NO的主要生成反应之一。进一步提高反应压力到0.9 MPa,可以发现NO的主要生成反应与0.5 MPa时基本保持不变,此时,NO的主要生成路径为
(d) NH3→NH2→H2NO→HNO→NO;
(e) NH3→NH2→H2NO→HONO→NO;
(f) HCN→NCO→NH2→HNO→NO。
与0.1 MPa时相比,高压下的中间产物HONO部分改变了NO的生成路径。HONO的主要生成路径有2条,分别是R43(即NO2+HO2=HONO+O2)和R87(即H2NO+NO2=HONO+HNO)。总压提高后,O2和H2O的分压随之提高,这促进了反应R18(即HO2+OH=H2O+O2)向着逆反应方向进行,提高了反应气氛中HO2的体积分数,而HO2体积分数提高会促进反应R36(即NO+HO2=NO2+OH)进行,将生成的NO氧化为NO2。但生成的NO2仍会进一步反应生成HONO并发生反应R34(即NO+OH(+M)=HONO(+M)),而在反应R34中H2O的第三体强化系数为5.0,H2O分压的提高会极大提高反应R34的逆反应速率,使其取代R171成为NO的主要生成反应之一。从以上分析可以看出:在反应初期,总压提高和高H2O分压的强化作用会使得NO生成反应加剧,NO快速达到体积分数峰值;在反应后期,随着反应持续进行,生成的NO会逐步与NCO和NH2发生还原反应生成N2,或者先与NCO反应生成N2O,再进一步分解为N2。
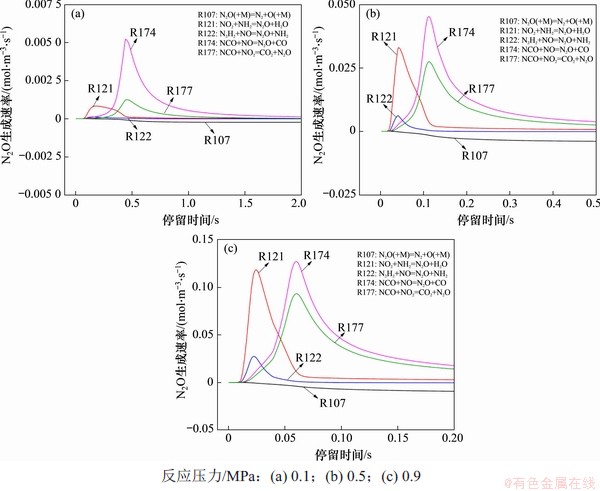
图6 N2O生成率随反应时间的变化
Fig. 6 Production rate of N2O with reaction time
从图4(b)可以看出:N2O体积分数的变化趋势与NO体积分数的变化趋势类似,均先快速升高并达到峰值,然后,随着反应时间延长,N2O体积分数逐步降低,直至完全分解为N2为止。在反应温度为850 ℃,入口气体NH3,HCN,O2和H2O体积分数分别为0.1%,0.1%,5.0%和5.0%时,以CO2为平衡气体,不同压力下各主要基元反应N2O生成速率见图6。从图6可以看出:随着压力升高,N2O的主要生成和消耗反应并未发生改变;高压同步促进了N2O主要生成和消耗反应的速率,但对于反应R121(即NO2+NH2=N2O+H2O)的促进作用最明显。O2和H2O的分压提高促进了R18逆反应的进行,提高了反应气氛中HO2的体积分数,促进了反应R36向着正方向进行,并提高了NO2的体积分数。而反应气氛中NO2体积分数的提高会极大提高R121的反应速率,这使得在0.9 MPa下R121成为最主要的N2O生成反应,此时,N2O的主要生成反应路径为:
(g) HCN→CN→NCO→N2O;
(h) NH3→NH2→N2O。
随着反应的持续进行,NCO和NH2等中间产物被逐步消耗,N2O生成反应速率降低,而N2O分解反应R107(即N2O(+M)=N2+O(+M))成为主导反应,将前期生成的N2O分解为N2。
在反应温度为850 ℃,入口气体NH3,HCN,O2和H2O体积分数分别为0.1%,0.1%,5.0%和5.0%时,以CO2为平衡气体,0.1 MPa和0.9 MPa下NOx的主要气相生成反应路径见图7。从图7可以看出反应压力的提高对NOx气相生成反应的影响主要体现为:1) 高H2O和O2分压改变了气氛中NCO和HO2等基团的体积分数,从而改变了各主要基元反应的份额;2) 高压改变了NO反应路径。高H2O分压使得一部分H2NO中间体以及NO2产物生成HONO,而HONO会在H2O的作用下快速分解为NO。但整体来说,通过NH3→NH2→H2NO→HONO→NO这一路径生成的NO占比较少,增压并没有改变NO最主要的生成路径。
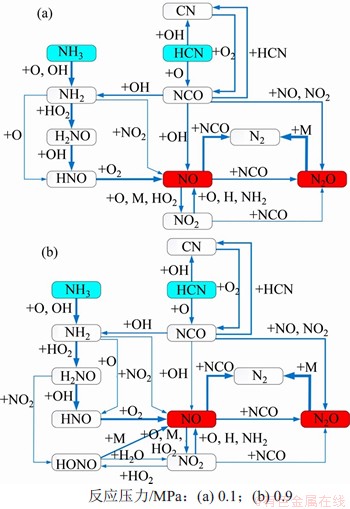
图7 不同反应压力下的NO,N2O气相生成路径
Fig.7 Gas-phase formation pathways of NO and N2O under different reaction pressures
2.3 反应气氛对NOx均相生成反应的影响
在反应温度为850 ℃,停留时间为4 s,入口气体NH3,HCN,O2和H2O体积分数分别为0.1%,0.1%,5.0%和5.0%时,平衡气体为CO2或N2,不同平衡气氛下NOx的气相生成体积分数随压力的变化如图8所示。从图8可以看出:在反应工况下,NO的出口体积分数要远低于N2O的体积分数;当反应压力为0.1 MPa时,N2气氛下的NO体积分数要略高于CO2气氛下的NO体积分数;随着反应压力逐步升高,NO体积分数呈现下降趋势;当反应压力大于0.3 MPa时,CO2气氛下的NO出口体积分数略高于N2气氛下的NO出口体积分数,但两者的差距非常小;而对于N2O,在本反应的压力范围内,N2气氛下N2O出口体积分数要始终低于CO2气氛下N2O出口体积分数。
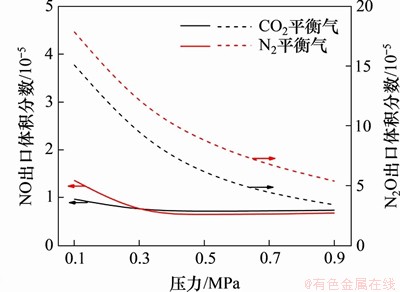
图8 N2和CO2平衡气对NOx均相生成反应的影响
Fig. 8 Effect of N2 and CO2 balance gas on homogeneous formation of NOx
在温度为850 ℃,压力为0.5 MPa,入口气体NH3,HCN,O2和H2O体积分数分别为0.1%,0.1%,5.0%和5.0%,平衡气体为CO2或N2时,2种气氛下NO和N2O敏感性分析结果如图9所示。从图9(a)可以看到:平衡气由N2变为CO2对NO生成和还原反应敏感性的影响较小。而对于N2O,平衡气体由N2变为CO2对其生成和分解的影响主要有两点:1) CO2气氛下NO体积分数略高于N2气氛下的NO体积分数,这有利于R174的进行,在一定程度上促进了N2O的生成反应;2) 对于第三体反应R107,CO2的第三体强化系数要大于N2的第三体强化系数,这有利于N2O分解反应的发生。
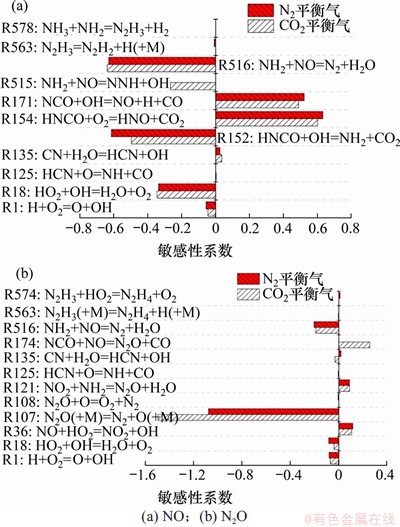
图9 NO和N2O气相生成反应敏感性系数
Fig.9 Sensitivity coefficient of gas-phase formation reactions of NO and N2O
3 增压富氧气氛下的SNCR反应机理
在循环流化床煤燃烧过程中,对NOx的还原主要有2条途径[20]:一是通过配风控制,在炉内特定区域形成还原性气氛,利用CO、焦炭还原NOx;二是采用选择性非催化还原法(SNCR)在炉顶或旋风分离器的特定温度区间内喷入适量的NH3或尿素溶液,对NOx进行还原。UR RAHMAN等[21]采用动力学模型计算的方法分析了在较高压力下,温度、氧体积分数、水分、NH3体积分数、SO2体积分数等参数对SNCR性能的影响。目前,对于增压富氧气氛下的SNCR反应相关研究较少。
为了研究增压富氧气氛对均相SNCR反应的影响,利用增压固定床实验台,通过配气在反应器入口加入一定量的NH3和NOx,研究温度、压力以及还原剂喷入量等运行参数对NOx还原反应的影响。在本实验中,反应压力设定为0.1(即常压),0.3,0.5,0.7和0.9 MPa,而反应温度区间为700~950 ℃。在整个实验过程中,通过调节气体流量和尾部针型阀开度使炉内气体的流速维持在0.075 m/s,在恒温段的停留时间约为4 s。具体实验工况如表1所示。
表1 增压富氧气氛SNCR实验工况表
Table 1 Matrix of the SNCR experiment in the pressurized oxy-fuel atmosphere

在本文中选用氧体积分数为5%,这是因为根据煤种和实际燃烧状况不同,循环流化床锅炉SNCR反应段的氧气体积分数在1%~6%之间[22]。同时根据UR RAHMAN等[21]的研究结果,对于增压富氧气氛下的SNCR反应,随着O2体积分数提高,最佳反应温度及温度窗口均向低温移动,但NOx出口体积分数的变化趋势并未改变。同时,本实验也考虑了二次反应的影响,并在固定床出口段设置了水冷夹套,将气体快速冷却至150 ℃以下,降低二次反应的发生以及实验误差。
3.1 温度、压力对NH3还原NO的影响
当入口气体NH3,NO和O2体积分数分别为0.05%,0.05%和5.00%,CO2为平衡气体时,3种压力下运行温度对NH3还原NO反应的影响如图10(a)所示。从图10(a)可以看出:随着温度逐步升高,NO出口体积分数呈现先降低后增加的趋势,这意味着气相SNCR反应存在一个最佳温度区间,为800~900 ℃;当温度低于800 ℃时,NO的还原反应速率较慢,出口烟气中的NO仍然维持一个较高水平;而当温度高于900 ℃时,NH3被氧化并生成更多的NO,造成脱硝效率降低。
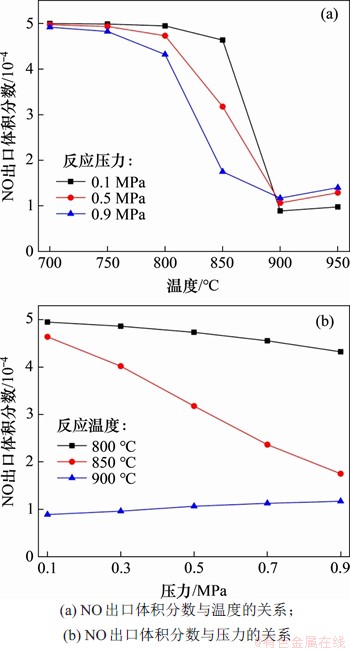
图10 反应温度和压力对NH3还原NO反应的影响
Fig. 10 Effect of reaction temperature and pressure on reduction of NO by NH3
在800,850和900 ℃下NO出口体积分数随压力变化情况如图10(b)所示。从图10(b)可见:在800 ℃和850 ℃下,NO出口体积分数随着压力升高呈现下降趋势;而在900 ℃时,随着系统总压升高,NO体积分数略有上升。这是因为在900 ℃时NH3的氧化反应较强烈,总压升高,O2分压和NH3初始分压也相应升高,NH3氧化反应加剧,并导致出口NO体积分数略上升。
利用Chemkin Pro对850 ℃时的NO生成路径进行模拟,发现当运行压力从0.1 MPa升高到0.9 MPa后,NO的主要还原路径发生了一定改变。当运行压力为0.1 MPa时,最主要的NO还原反应按反应速率从大至小依次为反应R516,R515和R36。压力增加会促进上述3个反应进行,且反应R516始终占据主导地位。而压力对反应R36的促进作用要大于对R515的作用,这使得在0.9 MPa时,NO还原反应按反应速率从大至小依次为R516,R36和R515,这在一定程度上会导致出口气体中NO2体积分数升高。
R36:NO+HO2=NO2+OH
R515:NH2+NO=NNH+OH
R516:NH2+NO=N2+H2O
3.2 NH3体积分数对NO还原反应的影响
在反应温度为850 ℃,入口气体NH3体积分数分别为0.04%,0.05%和0.10%,NO体积分数为0.05%,O2体积分数为5%,CO2为平衡气体时,不同喷氨体积分数对NO还原效率的影响如图11所示。从图11可以看出:在0.5 MPa下,当喷氨体积分数为5×10-4(即氨氮的物质的量比为1:1)时的脱硝效率仅为36.5%,要远低于实际燃烧过程中的脱硝效率[20-23],这说明气相SNCR反应在总脱硝反应中的占比较小,床料及飞灰对NO还原反应的催化作用占比较大。而进一步提高喷氨体积分数,当氨氮的物质的量比为2:1时,单纯的气相脱硝效率可达96.1%。
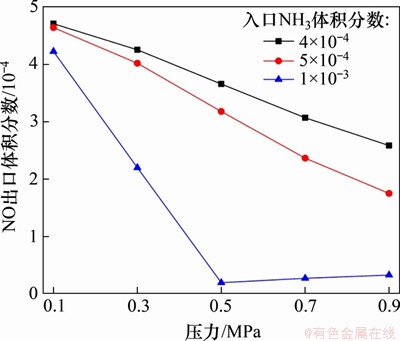
图11 NH3体积分数对NO气相还原反应的影响
Fig. 11 Effect of NH3 mass fraction on gas-phase reduction of NO
3.3 NH3体积分数对N2O还原反应的影响
NH3还原N2O的总包反应为3N2O+2NH3=4N2+3H2O,N2O与NH3的理论化学当量比为3:2。在反应温度为850 ℃,入口气体NH3体积分数分别为0,0.035%,0.040%和0.100%,N2O体积分数为0.05%,O2体积分数为5.00%时,以CO2为平衡气体,不同喷氨体积分数对N2O还原效率的影响如图12所示。从图12可以看到:当NH3体积分数从0升高到3.5×10-4时,N2O的出口体积分数显著降低,此时,反应器入口的N2O与NH3的物质的量比接近于理论化学当量比;当NH3体积分数提高到1.0×10-3时,在相同反应压力下,N2O出口体积分数进一步下降。通过分析可以发现,添加适量NH3后,N2O还原的主导反应仍然为反应R107(即N2O(+M)=N2+O(+M)),这说明在850 ℃和SNCR法脱除N2O的过程中,主要是N2O自身的热分解反应。在反应前期,由于NH3体积分数较高,反应R122(即N2H2+NO=N2O+NH2)的速率较快,但随着NH3持续被消耗,该反应速率降低。从图12还可以看出,当系统总压小于0.5 MPa时,总压升高对N2O气相分解的促进作用较明显。
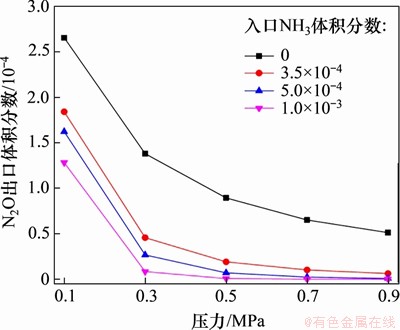
图12 NH3体积分数对N2O还原反应的影响
Fig. 12 Effect of NH3 mass fraction on gas-phase reduction of N2O
4 结论
1) 在增压富氧气氛下,NOx的主要气相生成路径为NH3→NH2→H2NO→HNO→NO和HCN→CN→NCO→N2O。总压提高并未改变NOx的主要生成路径,但总压升高增加了H2O和O2初始分压,改变了气氛中NCO和HO2等基团体积分数,促进了基元反应的速率,并改变了各主要基元反应发生的程度。
2) 对于NOx的次要生成路径,增压后高H2O分压使得少部分的H2NO中间体以及NO2产物生成HONO,而HONO则会在H2O作用下快速分解为NO。
3) 提高系统压力会促进气相SNCR反应的进行。不同的是,对于N2O,提高压力并不会改变其主要还原路径;但对于NO,提高反应压力在促进NO还原反应的同时,也会在一定程度上促进烟气中NO2的生成。
参考文献:
[1] 郑楚光, 赵永椿, 郭欣. 中国富氧燃烧技术研发进展[J]. 中国电机工程学报, 2014, 34(23): 3856-3864.
ZHENG Chuguang, ZHAO Yongchun, GUO Xin. Research and development of oxy-fuel combustion in China[J]. Proceedings of the CSEE, 2014, 34(23): 3856-3864.
[2] HOSSAINPOUR S. Thermo-economic evaluation of 300 MW coal based oxy-fuel power plant integrated with organic Rankine cycle[J]. International Journal of Greenhouse Gas Control, 2019, 88: 383-392.
MADDAHI L, HOSSAINPOUR S. Thermo- economic evaluation of 300MW coal based oxy-fuel power plant integrated with organic Rankine cycle[J]. International Journal of Greenhouse Gas Control, 2019, 88: 383-392.
[3] ESCUDERO A I, ESPATOLERO S, ROMEO L M, et al. Minimization of CO2 capture energy penalty in second generation oxy-fuel power plants[J]. Applied Thermal Engineering, 2016, 103: 274-281.
[4] DARDE A, PRABHAKAR R, TRANIER J P, et al. Air separation and flue gas compression and purification units for oxy-coal combustion systems[J]. Energy Procedia, 2009, 1(1): 527-534.
[5] XIA Fei, YANG Zhiwei, ADEOSUN A, et al. Pressurized oxy-combustion with low flue gas recycle: computational fluid dynamic simulations of radiant boilers[J]. Fuel, 2016, 181: 1170-1178.
[6] ZEBIAN H, MITSOS A. Pressurized OCC (oxy-coal combustion) process ideally flexible to the thermal load[J].Energy, 2014, 73: 416-429.
[7] ZEBIAN H, MITSOS A. Pressurized oxy-coal combustion: ideally flexible to uncertainties[J]. Energy, 2013, 57: 513-526.
[8] CUENCA M A, ANTHONY E J. Pressurized fluidized bed combustion[M]. Springer Science & Business Media, 1995: 90-100.
[9] SVOBODA K, POHORELY M. Influence of operating conditions and coal properties on NOx and N2O emissions in pressurized fluidized bed combustion of subbituminous coals[J]. Fuel, 2004, 83(7/8): 1095-1103.
[10] SMITH G P, GOLDEN DM, FRENKLACH M, et al. GRI-MECH 3.0[EB/OL]. [2020-10-01]. http: //combustion. berkeley.edu/gri-mech/.
[11] 王学斌, 刘梓晗, 韩旭, 等. 加压富氧燃烧下SO3生成特性的动力学机理研究[J]. 工程热物理学报, 2017, 38(6): 1357-1361.
WANG Xuebin, LIU Zihan, HAN Xu, et al. Dynamics mechanism investigation on the formation of SO3 during pressurized oxy-fuel combustion[J]. Journal of Engineering Thermophysics, 2017, 38(6): 1357-1361.
[12] WARGADALAM V J, LOFFLER G, WINTER F, et al. Homogeneous formation of NO and N2O from the oxidation of HCN and NH3 at 600-1 000 ℃[J]. Combustion and Flame, 2000, 120(4): 465-478.
[13] HASHEMI H, HANSEN S, TOFTEGAARD M B, et al. A model for nitrogen chemistry in oxy-fuel combustion of pulverized coal[J]. Energy & Fuels, 2011, 25(10): 4280-4289.
[14] HU Fan, LI Pengfei, GUO Junjun, et al. Evaluation, development, and validation of a new reduced mechanism for methane oxy-fuel combustion[J]. International Journal of Greenhouse Gas Control, 2018, 78: 327-340.
[15] HU Fan, LI Pengfei, WANG Kai, et al. Evaluation, development, and application of a new skeletal mechanism for fuel-NO formation under air and oxy-fuel combustion[J]. Fuel Processing Technology, 2020, 199: 106256.
[16] GLARBORG P, KUBEL D, DAM-JOHANSEN K, et al. Impact of SO2 and NO on CO oxidation under post-flame conditions[J]. International Journal of Chemical Kinetics, 1996, 28(10): 773-790.
[17] ALLEN M T, YETTER R A, DRYER F L. High pressure studies of moist carbon monoxide/nitrous oxide kinetics[J]. Combustion and Flame, 1997, 109(3): 449-470.
[18] ALLEN M T, YETTER R A, DRYER F L. The decomposition of nitrous oxide at 1.5 P 10.5 atm and 1103 T 1173 K[J]. International Journal of Chemical Kinetics, 1995, 27(9): 883-909.
[19] MUELLER M A, YETTER R A, DRYER F L. Kinetic modeling of the CO/H2O/O2/NO/SO2 system: implications for high-pressure fall-off in the SO2+O(+M)=SO3(+M) reaction[J]. International Journal of Chemical Kinetics, 2000, 32(6): 317-339.
[20] 黄中, 杨娟, 车得福. 大容量循环流化床锅炉技术发展应用现状[J]. 热力发电, 2019, 48(6): 1-8.
HUANG Zhong, YANG Juan, CHE Defu. Application and development of large-scale circulating fluidized bed boiler[J]. Thermal Power Generation, 2019, 48(6): 1-8.
[21] UR RAHMAN Z, WANG X, ZHANG J, et al. Kinetic study and optimization on SNCR process in pressurized oxy-combustion[J]. Journal of the Energy Institute, 2021, 94: 263-271.
[22] 薛现恒, 邓雨生, 段伦博, 等. 基于410 t/h Compact型流化床锅炉的SNCR影响因素探究[J]. 锅炉技术, 2019, 50(3): 30-35.
XUE Xianheng, DENG Yusheng, DUAN Lunbo, et al. The research of the influences of the SNCR on a 410 t/h circulating fluidized bed boiler[J]. Boiler Technology, 2019, 50(3): 30-35.
[23] ZHAO J, WEI X, LI T, et al. Effect of HCl and CO on nitrogen oxide formation mechanisms within the temperature window of SNCR[J]. Fuel, 2020, 267: 117231.
(编辑 陈灿华)
收稿日期: 2020 -10 -15; 修回日期: 2020 -11 -12
基金项目(Foundation item):国家重点研发计划项目(2018YFB0605301) (Project(2018YFB0605301) supported by the National Key Research and Development Program of China)
通信作者:段伦博,博士,教授,从事洁净煤技术、二氧化碳减排、固体废弃物处理、先进能源材料研究;E-mail: duanlunbo@seu.edu.cn
引用格式: 段元强, 洪溥, 仇兴雷, 等. 增压富氧气氛下NOx均相生成及SNCR反应机理研究[J]. 中南大学学报(自然科学版), 2021, 52(1): 96-105.
Citation: DUAN Yuanqiang, HONG Pu, QIU Xinglei, et al. Study of homogeneous formation of NOx and SNCR reaction mechanism in pressurized oxy-fuel atmosphere[J]. Journal of Central South University(Science and Technology), 2021, 52(1): 96-105.