真空碳热还原过程中二氧化硅的挥发行为
罗启1,2,3,刘大春1,2,3,曲涛1,3,田阳1,3,杨斌1,2,3,戴永年1,2,3
(1. 昆明理工大学 真空冶金国家工程实验室,云南 昆明,650093;
2. 昆明理工大学 云南省复杂有色金属资源清洁利用国家重点实验室,云南 昆明,650093;
3. 昆明理工大学 云南省有色金属真空冶金重点实验室,云南 昆明,650093)
摘要:为了解在真空碳热还原过程中SiO2的还原特性以及还原过程中的主要影响因素,对二氧化硅的还原过程进行热力学分析, 得出化学反应自由能和临界温度。在系统压力为2~200 Pa条件下,以分析纯SiO2和Fe2O3为原料,采用XRD,SEM,EDS和化学成分分析等手段,研究Fe/Si摩尔比、配碳量、反应时间、还原剂粒度和升温速率对硅的挥发率和还原反应速率的影响。实验结果表明:在100 Pa条件下,SiO2的临界反应温度为1 330~1 427 K 。SiO2发生气化反应生成的SiO气体挥发至石墨冷凝系统歧化生成Si和SiO2,造成硅的损失,且有部分SiO气体和石墨反应生成SiC;增大Fe/Si摩尔比和配碳量以及减小还原剂粒度均降低了硅的挥发率,提高了SiO2还原反应速率;延长反应时间和提高升温速率增加了硅的挥发率。
关键词:真空冶金;二氧化硅;三氧化二铁;挥发率
中图分类号:TF801;TF803.4 文献标志码:A 文章编号:1672-7207(2012)08-2900-09
Volatile behavior of silicon by carbothermic reduction in vacuum
LUO Qi1,2,3, LIU Da-chun1,2,3, QU Tao1,3, TIAN Yang1,3, YANG Bin1,2,3, DAI Yong-nian1,2,3
(1. National Engineering Laboratory of Vacuum Metallurgy,
Kunming University of Science and Technology, Kunming 650093, China;
2. State Key Laboratory Breeding Base of Complex Nonferrous Metal Resources Clear Utilization in Yunnan Province,
Kunming University of Science and Technology, Kunming 650093, China;
3. Key Laboratory of Non-Ferrous Metals Vacuum Metallurgy of Yunnan Province,
Kunming University of Science and Technology, Kunming 650093, China)
Abstract: In order to research the reduction characteristics of silica by carbothermic reduction in vacuum, the Gibbs free energy and critical temperature of the Si oxide deoxidized reaction by C were calculated and analyzed thermodynamically. The pure SiO2 powder was mixed to make pellets with Fe2O3 and coal powders, and the effects of Fe/Si molar ratio, carbon content, reaction time, reductant grain size and heating rate on the volatilization ratio and reaction rate of silica in carbothermic reduction were investigated by means of XRD, SEM, EDS and chemical composition analysis in vacuum (2-200 Pa). The results show that the critical temperature of SiO2 is 1 330-1 427 K when the system pressure is 100 Pa. After SiO2 converses to SiO, SiO disproportionats into Si and SiO2 in the graphite condensing system, and some of SiO with graphite transform into SiC. With the increase of Fe/Si molar ratio, carbon content and the decreases of reductant grain size, the volatilization ratio of silicon decreases, but the rate of reaction increases. With the rise of heating rate and reaction time, the volatilization ratio of silicon increases.
Key words: vacuum metallurgy; silica; iron trioxide; volatilization ratio
国内外处理红土镍矿的方法主要包括[1-6]:回转窑干燥预还原—电炉熔炼法(RKEF)、烧结—鼓风炉硫化熔炼法、烧结—高炉还原熔炼法、还原焙烧—氨浸法和高压酸浸法。但这些方法都仅着眼于回收含量较低的镍、铁、钴,而含量极高的硅和镁都成为废渣,将其填埋弃置会对环境造成严重的二次污染[7-8],进行固化处理又导致金属资源流失[9]。云南元江镍矿镍储量43万t,平均含量(质量分数)为Ni 0.83%,Fe 11%,MgO 28%,SiO2 37%[10],极具综合利用价值。徐宝强等[10]利用真空碳热还原原理[11-16],提出真空碳热还原综合回收有价金属新工艺处理元江红土镍矿,在冷凝系统回收金属镁,镍、铁在渣中通过磁选得到富集。前期研究结果表明,镁被还原挥发的同时硅也发生类似反应,即在高温下SiO2气化生成低价SiO,继而SiO在冷凝区发生凝聚反应(歧化分解为SiO2和Si),不利于硅的回收,且污染炉体和冷凝系统,影响金属镁的纯度。雷斯等[17-18]在铁合金的冶炼过程中对Fe-Si-C系进行了研究,指出在硅铁的冶炼过程中不可避免会有SiO气体生成,从而造成硅的损失。何允平等[19]对工业硅生产的原理和工艺进行了阐述;李秋霞等[20]对低价氧化硅的歧化行为进行了热力学研究。但是在真空条件下,二氧化硅和三氧化二铁共热时硅的挥发行为未见报道。探索性实验研究表明,红土镍矿在真空碳热还原过程中镁的挥发率可以达到98%以上,但是氧化镁的存在对硅的挥发并没有影响。因此,有必要研究真空条件下二氧化硅和三氧化二铁共热时硅的挥发行为以及三氧化二铁对硅挥发的影响。基于此,本文作者根据红土镍矿的组成,以分析纯的SiO2和Fe2O3为原料,煤炭为还原剂,在热力学分析基础上对反应后渣相和冷凝物进行了XRD分析,并对渣相进行了SEM-EDS和化学成分分析,探讨二氧化硅真空碳热还原过程中硅的挥发行为,以便为红土镍矿真空碳热还原新工艺工业化应用中对硅的回收提供理论和技术依据。
1 实验
1.1 实验原料与设备
实验原料为粉状分析纯SiO2(质量分数为99.0%)和粉状分析纯Fe2O3(质量分数以Fe计,69.8%~ 70.1%)。还原剂煤炭分析结果(质量分数)见表1,其中灰分含SiO2 60.75%,Al2O3 26.3%,TiO2 2.47%。使用实验室自行设计的小型立式真空炉进行实验,其示意图见图1。
表1 煤炭分析结果(质量分数)
Table 1 Analysis results of coal %

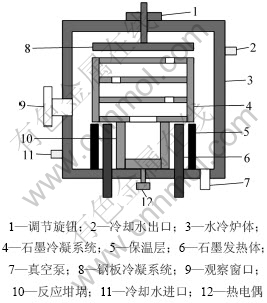
图1 真空炉示意图
Fig.1 Schematic diagram of vacuum furnace
1.2 实验研究方法
将煤炭球磨并筛分至小于0.106 mm,每次实验根据Fe/Si摩尔比和配碳量,称取一定量的Fe2O3,SiO2和煤炭,混合均匀,在6~8 MPa的压力下制成直径×高度为20 mm×25 mm试样后放入真空干燥箱,在500 ℃条件下干燥30~60 min,脱除吸附气体和水分。将样品置于坩埚中的密封真空炉。打开水冷装置,抽真空至极限(2 Pa),升温至一定温度后恒温,物料将在石墨坩埚内发生反应,挥发物在冷凝区冷凝。恒温一定时间以后停止加热,冷却至室温,开炉取样。实验过程中硅的挥发率计算式为:
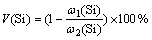
式中:V(Si)为硅的挥发率;ω1(Si)为渣中硅的质量分数;ω2(Si)为入炉原料中硅的质量分数。
采用日本理学公司Rigaku X线自动衍射仪(XRD)(TTRⅢ)对反应残料和冷凝物进行物相分析,使用Cu Kα,扫描区间为10°~95°;采用氟硅酸钾法测残料中硅元素的质量分数;采用Philips XL30ESEM- TMP型扫描电子显微镜观察反应残料的形貌,并用能谱仪(EDAX产PHOENIXTM)分析其表面元素含量。
2 理论分析
二氧化硅的还原过程极为复杂,对其机理的解释也较多[17-22],但是碳还原二氧化硅的总反应式是一致的[23]。查阅相关的热力学数据[24],运用“物质吉布斯自由能函数法”[25],计算真空碳热还原二氧化硅所涉及的化学反应的吉布斯自由能(ΔGT)与热力学温度(T)的关系,结果如表2所示。由表2可知:系统压力对体积增大的反应有显著的影响,而对反应(3),(4)和(9)并没有影响。
考虑到真空还原实验一般在2~200 Pa下进行,因此对反应(1)~(14)在系统压力为100 Pa下的吉布斯自由能(ΔGT)与热力学温度(T)的关系进行计算,结果如图2所示。从图2可以看出:在100 Pa条件下:反应(7)的起始温度高于反应(1)的起始温度,与反应(2)的起始温度接近,即在碳对二氧化硅还原的同时,不可避免会有SiC和SiO生成,其起始反应温度分别为1 330 K和1 427 K;SiO气体在高温条件下与C接触容易生成SiC或者Si,但是C捕获SiO的能力有限,因此SiO气体容易挥发,在冷凝区歧化分解为Si和SiO2;SiO2破坏SiC生成SiO或者Si的起始反应温度分别为1 562 K和1 723 K,因此SiC具有很高的化学稳定性,难于分解,是工业硅生产中导致炉底上涨的主要原因;Fe2O3与C在465 K时首先生成Fe3O4,继而Fe3O4在676 K时生成FeO,当温度高于706 K时,有金属Fe生成;游离Fe的存在有助于破坏碳热还原过程中容易生成的SiC,其起始反应温度为1 083 K。
表2 二氧化硅真空碳热还原过程中可能发生的主要化学反应[24,25]
Table 2 Primary reactions of silicon by vacuum carbothermic reduction[24,25]
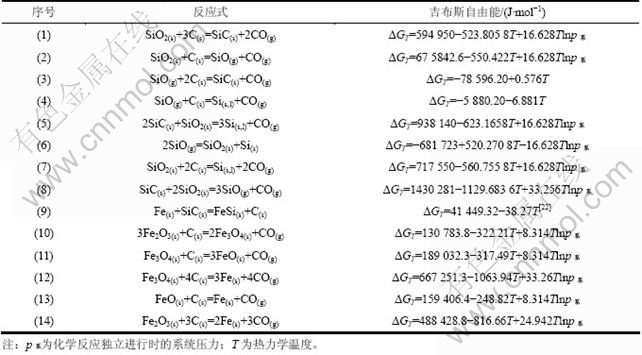
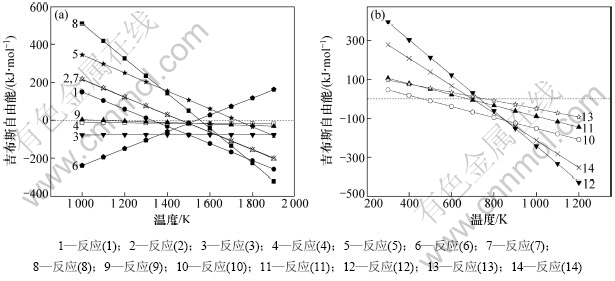
图2 100 Pa下吉布斯自由能和温度的关系
Fig.2 Relationships between Gibbs free energy and temperature at 100 Pa
3 结果与讨论
3.1 二氧化硅的真空碳热还原行为
根据化学方程式(7)和(14)计算配碳模数M,当M=1.0时,配碳量为化学计量。对SiO2与Fe2O3真空碳热还原得到的渣相进行XRD分析,结果如图3所示,实验条件如下:系统压力为2~200 Pa,配碳量M=1.1,反应温度为1 450 ℃,反应时间为180 min。从图3可知:当Fe/Si摩尔比为1:4.67时,渣中主要物相除SiC,FeSi和SiO2外,还掺杂有FeSi2,没有发现铁的氧化物,说明Fe2O3反应完全,SiO2有剩余;随着Fe/Si摩尔比增大,SiO2和FeSi2的特征衍射峰有所减弱,FeSi的特征衍射峰逐渐增强;当Fe/Si摩尔比为1:2时,渣中物相种类发生改变,SiO2和FeSi2的特征衍射峰均消失,只有SiC和FeSi的特征衍射峰。说明铁的增加,加快了SiO2的还原反应速率,才会使SiO2的特征衍射峰消失,并且Fe-Si合金中Si的含量升高,从而使FeSi的特征衍射峰加强。这是因为Fe可以溶解Si,使Si离开反应区并且放出热量,从而改善了SiO2还原的热力学条件;另一方面,Fe的增加,游离的Fe与Si在高温下形成合金,二组元活度均呈负偏差[26],其中以Fe组元的负偏差为大,因此,Fe的存在将使SiO2的还原反应起始温度大大降低。
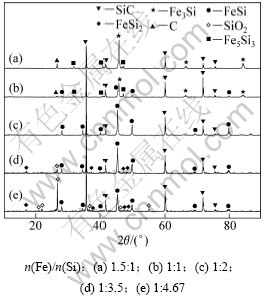
图3 不同Fe/Si摩尔比时渣相XRD图谱
Fig.3 XRD patterns of slags with different mole ratios of Fe to Si
继续增大Fe/Si摩尔比到1:1时,出现了Fe3Si,Fe5Si3和C的特征衍射峰,且SiC和FeSi的特征衍射峰有所减弱;当Fe/Si摩尔为1.5:1时,FeSi的特征衍射峰消失,SiC的特征衍射峰明显减弱,Fe5Si3和C的特征衍射峰明显加强。其原因是:铁的增加,促进了铁与碳化硅作用生成硅铁合金和石墨的反应,增强了铁破坏碳化硅的能力。雷斯[17]认为:在1 500 K时反应开始进行,在1 500~1 600 K之间反应激烈进行;热力学计算表明,在1 083~1 900 K内,有铁存在时,碳化硅不稳定,因为铁破坏碳化硅反应自由能的变化值为负值。
图1所示的冷凝区只有在石墨冷凝系统收集到了淡黄色的块状冷凝物,而在钢板冷凝系统并没有收集到冷凝物。说明SiO气体挥发至石墨冷凝系统就已经发生了凝集反应(歧化分解为Si和SiO2)。对冷凝物进行XRD分析,结果如图4所示。从图4可知:冷凝物主要由Si,SiO2和SiC组成,说明SiO2气化生成了SiO气体,继而SiO气体在石墨冷凝系统发生了凝聚反应,并且有部分SiO气体和石墨发生反应(3)生成SiC。
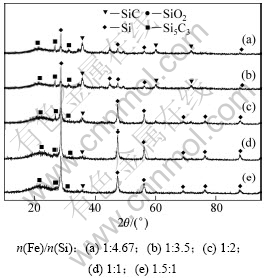
图4 不同Fe/Si摩尔比时冷凝物XRD图谱
Fig.4 XRD patterns of condensate with different mole ratios of Fe to Si
根据气体状态方程PV=nRT[25]可知,气体温度T和体积V一定时,体系压力P与气体分子数n成正比,因此在真空炉内氧化物的还原反应速率可以用系统压力来表征。当还原反应由缓慢变得激烈时,产生的CO气体增多,系统压力会增大;当还原反应由激烈变得缓慢时,产生的CO气体减少,系统压力会变小;当还原反应结束时,就不再有CO气体产生,系统压力将稳定在真空炉的极限值。因此,在实验过程中,以15 ℃/min的速率升温,当温度达到1 450 ℃时开始保温,在保温阶段每5 min记录一次系统压力。图5所示为配碳模数M=1.1,反应温度1 450 ℃,不同Fe/Si摩尔比条件下,保温阶段反应时间和系统压力的关系。
从图5可以看出:在反应开始阶段,虽然Fe/Si摩尔比不同,但是系统压力基本一样为200 Pa,而且随着时间的延长迅速下降;反应时间在40 min内,Fe/Si摩尔比越大,系统压力越大,还原反应速率越大;在反应60 min时,Fe/Si摩尔比为1.5:1的物料最先趋于稳定值,其次是Fe/Si摩尔比为1:1的物料在105 min时趋于稳定值,其他3个物料在180 min内未达到稳定值。说明SiO2的还原反应速率在反应前期较大,在反应后期,逐渐减小;铁的增加,有利于提高SiO2与C的还原反应速率。其原因是:铁可以无限溶解硅,使硅离开反应区,从而改善了硅还原的热力学条件,减少了硅的损失,并且硅溶解在铁中放出的热量,占热平衡进项中总热量的2.5%~3%[17,22],若炉料中没有铁,则这部分热量也没有了;另一方面,从化学反应动力学因素考虑,还原过程一般认为有以下几步:固-固界面处氧化物与碳反应;产物CO气体通过产物层扩散;氧化物和碳通过产物层扩散;接触界面的反应。还原反应开始阶段,球团中氧化物与碳接触条件良好,能使反应顺利进行,反应速率较大;此后,还原反应的进行需要氧化物和碳通过产物层的扩散才能完成,使得反应速率逐渐减小。
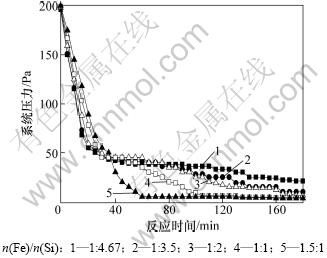
图5 反应时间与系统压力的关系
Fig.5 Relationships between time of reaction and system pressure
3.2 Fe/Si摩尔比对硅挥发率的影响
当系统压力为2~200 Pa,配碳量M=1.0,反应温度为1 450 ℃,反应时间为90 min和180 min时,Fe/Si摩尔比与硅挥发率的关系如图6所示。从图6可以看出:反应时间90 min,Fe/Si摩尔比从1:4.67升高到1.5:1时,硅的挥发率从12.18%降低至0.456%;反应时间180 min,Fe/Si摩尔比从1:4.67升高到1.5:1时,硅的挥发率从18.4%降低至0.8%,硅的挥发率随Fe/Si摩尔比的增大,呈明显的下降趋势。热力学分析表明:在810 ℃时,Fe与SiC开始反应,产物是硅铁和石墨,并且随着温度的升高,吉布斯自由能越来越负,反应的驱动力就越大。因此在1 450 ℃时,铁的增加,促进了反应(1)抑制了反应(2)的进行;另一方面,球团中铁的氧化物逐渐被还原为金属铁,形成较致密的外壳,球团孔隙率降低,导致球团内外气体扩散阻力增大,从而阻碍了残余SiO气体的挥发。
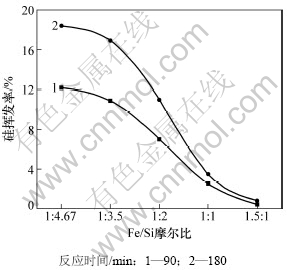
图6 Fe/Si摩尔比对硅挥发率的影响
Fig.6 Effect of Fe/Si mole ratio on silicon volatilization
当Fe/Si摩尔比为1:3.5,反应时间为90 min时,反应残料的背散射电子图像和EDS分析结果如图7所示。从图7可知:主要物相为白色颗粒状的Fe-Si合金,分布广泛,平均粒径在10 um左右,灰色的SiC呈聚集态,灰黑色的SiO2粒径为40~100 um,与反应前相比粒径明显减小。此外还掺杂了微量的Al和Mg的氧化物,这是煤炭灰分中带入的杂质,但是在XRD分析(图8)中未发现这些氧化物,这是由于这些氧化物杂质含量不够高,达不到XRD的检测限所致。二氧化硅与三氧化二铁含碳球团在还原过程中的固相反应,导致铁晶粒很难形成和长大,一方面,铁晶粒的形成需要克服成核壁垒,另一方面,生成的铁晶粒扩散至含Si和SiC的表面发生固相反应而消失,并生成新相(Fe-Si合金)。形成的新相以及碳化硅就在二氧化硅的边界生长,随着反应的进行,新相以及碳化硅层把还原剂和二氧化硅隔开,使得二氧化硅的还原变的困难。
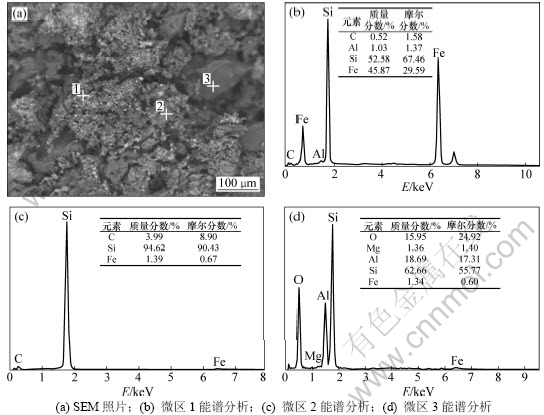
图7 n(Fe)/n(Si)=1:3.5反应90 min时残料的SEM分析
Fig.7 SEM image of slag with n(Fe)/n(Si)=1:3.5 after reacting for 90 min
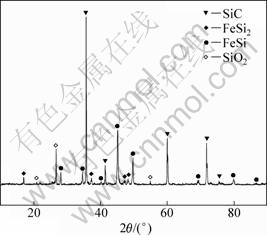
图8 当n(Fe)/n(Si)=1:3.5反应90 min时残料的XRD图谱
Fig.8 XRD patterns of slag at n(Fe)/n(Si)=1:3.5 after reacting for 90 min
3.3 配碳量对硅挥发率的影响
当系统压力2~200 Pa,配碳模数M=1.0,1.1,1.2,反应温度1 450 ℃,反应时间90 min时,Fe/Si摩尔比与硅挥发率的关系如图9所示。从图9可以看出:配碳模数M=1.0时,硅的挥发率最大,配碳模数M=1.2时,硅的挥发率最小。当Fe/Si摩尔比为1:3.5,配碳量从M=1.0增加到1.2时,硅的挥发率从12.77%降低至8.83%。说明配碳量的增加可以降低硅的挥发率。其原因是:在煤基直接碳热还原过程中,以间接还原为主直接还原为辅,铁、硅的氧化物与碳直接接触中断后将会导致还原反应停滞,此时碳通过产物层的扩散才能使反应继续进行,配碳量的增加,增加了C与SiO的接触面积,增强了C捕获SiO气体的能力,促进了反应(3)和(4)的进行,使SiO转变为SiC或者Si。
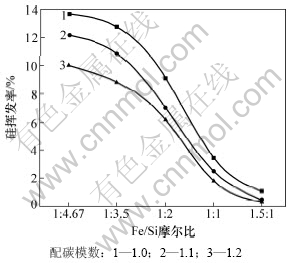
图9 配碳量对硅挥发率的影响
Fig.9 Effect of reductant content on silicon volatilization
3.4 反应时间对硅挥发率的影响
在系统压力2~200 Pa,Fe/Si摩尔比为1:3.5,配碳量为M=1.0,1.1,1.2条件下,反应时间对SiO2真空碳热还原挥发率的影响如图10所示。由图10可见:当M=1.2,反应时间从90 min延长到180 min时,硅的挥发率有所增大,从8.83%增大到12.23%,再从180 min延长到225 min时,硅的挥发率变化不大为12.35%。说明随着反应时间增加,硅的挥发率呈明显增大趋势,最后达到一定的稳定值,225 min时二氧化硅的还原过程基本完成。
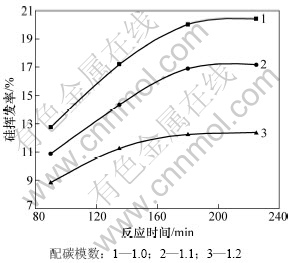
图10 反应时间对硅挥发率的影响
Fig.10 Effect of reaction time on silicon volatilization
对配碳量M=1.0条件下的渣相进行XRD分析,结果如图11所示。从图11可以看出:当反应时间为90 min时,除有明显的SiO2,SiC和FeSi特征衍射峰存在外,还有Fe5Si3的特征衍射峰存在;当反应时间为135 min时,物相种类没有发生变化,但是SiO2的特征衍射峰明显减弱,SiC和FeSi特征衍射峰明显增强;当反应时间为180 min时SiO2的特征衍射峰继续减弱,Fe5Si3转变为FeSi2;当反应时间为225 min时,SiO2的特征衍射峰消失,物相组成是SiC和FeSi,没有检测到其他物相。说明延长反应时间有利于SiO2还原过程的进行,硅的挥发率也就随着增大,当SiO2的还原过程结束时,硅的挥发率不再增加。
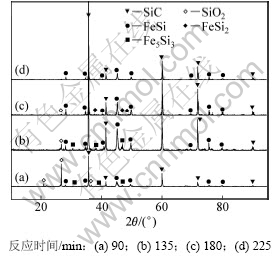
图11 配碳量M=1.0时渣相XRD图谱
Fig.11 XRD patterns of slags with M=1.0 after reacting for different time
3.5 还原剂粒度对硅挥发率的影响
在系统压力2~200 Pa,配碳量M=1.0,Fe/Si摩尔比为1:3.5,反应温度1 450 ℃,反应时间180 min条件下,考察还原剂煤炭的粒度对硅挥发率的影响。当煤炭的粒度为0.25,0.15,0.106,0.075和0.058 mm,二氧化硅真空碳热还原过程中硅的挥发行为如图12所示。从图12可知:当还原剂粒度从0.25 mm减小到0.058 mm时,硅的挥发率有所下降,从22.13%下降到18.43%。其原因是:煤粉和二氧化硅在6~8 MPa压力下制备的含碳球团在真空条件下发生还原反应,可理解为碳和二氧化硅紧密接触而发生反应(1)和(2),随着反应的进行,碳和二氧化硅被新相阻隔,反应(1)和(2)只有通过扩散才能继续进行,否则停止。反应(1)和(2)分别涉及固体氧化物和碳,因此这2种固体反应物的粒度便会对含碳球团的反应产生一定的影响。还原剂粒度的减小,增大了碳的比表面积,促进了反应(1)和(2)的进行,但更是增大了碳捕获SiO的能力,促进了反应(3)和(4)的进行。因此,还原剂粒度的减小,降低了硅的挥发率。
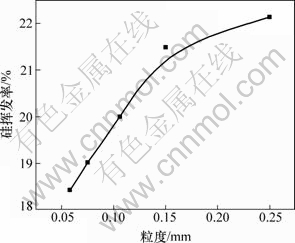
图12 还原剂粒度对硅挥发率的影响
Fig.12 Effect of reductant grain size on silicon volatilization
3.6 升温速率对硅挥发率的影响
当系统压力2~200 Pa,配碳量M=1.0,Fe/Si摩尔比为1:3.5,反应温度1 450 ℃,反应时间180 min条件下,升温速率与硅挥发率的关系如图13所示。从图13可知:升温速率对硅的挥发率有一定的影响。升温速率从15 ℃/min增大到55 ℃/min 时,硅的挥发率增加了4.94%,为24.94%;继续增大升温速率,硅挥发率的增幅减小,当升温速率为135 ℃/min时,硅的挥发率达到26.02%。文献[27]研究表明:煤基直接还原过程中铁氧化物的反应逐级进行。由热力学计算可知,在100 Pa条件下,铁的一系列氧化物与碳最终并完全反应生成金属铁的温度为706 K以上,并且随着温度的升高,吉布斯自由能越来越负,相应反应就越容易发生。因此,当升温速率提高,缩短了球团传热的时间以及三氧化二铁的还原时间,从而减少了升温阶段铁的生成量,根据Fe-C相图[28]可知,铁的减少将不利于形成包裹SiO2与C的熔体,此熔体的形成有利于SiO2与C的还原反应,且可以抑制SiO气体的挥发。因此升温速率的提高不利于反应(1)的进行,促进了反应(2)的进行,从而增大了硅的挥发率。
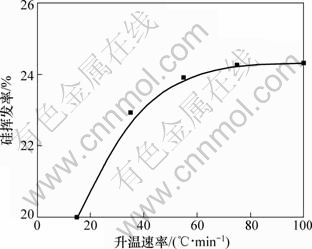
图13 升温速率对硅挥发率的影响
Fig.13 Effect of heating rate on silicon volatilization
4 结论
(1) 在100 Pa条件下,SiO2与C在1 330 K时开始反应生成SiC, 在1 427 K时开始有SiO气体和Si生成,SiO气体容易挥发且温度低于1 538 K时极不稳定,容易歧化分解为Si和SiO2,Fe2O3经过一系列还原反应生成金属Fe的起始反应温度为706 K。
(2) 铁对二氧化硅真空碳热行为有显著影响,Fe-Si-C体系随着铁的增加,SiC逐渐转变为FeSi2,FeSi,Fe5Si3和Fe3Si,并且加快了SiO2的还原反应速率,减少SiO气体的挥发。
(3) SiO2发生气化反应生成了SiO气体,在石墨冷凝系统歧化生成Si和SiO2,且有部分SiO气体和石墨反应生成了SiC。
(4) 增加配碳量和减小还原剂的粒度可有效降低硅的挥发率,提高升温速率和延长反应时间硅的挥发率将增大。
参考文献:
[1] GUO Xue-yi, LI Dong, Park K H, et al. Leaching behavior of metals from a limonitic nickel laterite using a sulfation-roasting-leaching process[J]. Hydrometallurgy, 2009, 99: 144-150.
[2] Coto O, Galizia F, Hernadez I, et al. Cobalt and nickel recoveries from laterite tailings by organic and inorganic bio-acids[J]. Hydrometallurgy, 2008, 94(1/4): 18-22.
[3] XU Yan-bin, XIE Yan-ting, YAN Lan, et al. A new method for recovering valuable metals from low-grade nickeliferous oxide ores[J]. Hydrometallurgy, 2005, 80(4): 280-285.
[4] Agatzini S L, Zafiratos I G, Spathis D. Beneficiation of a greek serpentine nickeliferous ore. Part I: Mineral processing[J]. Hydrometallurgy, 2004, 74(3/4): 259-265.
[5] Soler J M, Cama J, Gal?S, et al. Composition and dissolution kinetics of garnierite from the Loma de Hierro Ni-laterite deposit, Venezuela[J]. Chemical Geology, 2008, 249(1/2): 191-202.
[6] CHEN Sheng-li, GUO Xue-yi, SHI Wen-tang, et al. Extraction of valuable metals from low-grade nickeliferous laterite ore by reduction roasting-ammonia leaching method[J]. J Cent South Univ Technol, 2010, 17(4): 765-769.
[7] 李建华, 程威, 肖志海. 红土镍矿处理工艺综述[J]. 湿法冶金, 2004(12): 4-8.
LI Jian-hua, CHENG Wbi, XIAO Zhi-hai. Overview of the exaction processing on laterite[J]. Hydrometallurgy, 2004(12): 4-8.
[8] ZHAI Yu-chun, MU Wen-ning, LIU Yan, et al. A green process for recovering nickel from nickeliferous laterite ores[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S1): s65-s70.
[9] Pelino M, Kararnanov A, Pisciella P, et al. Vitrification of electric arc furnace dusts[J]. Waste Manage Ment, 2002, 22(8): 945-949.
[10] 徐宝强, 裴红彬, 杨斌, 等. 真空碳热还原脱除红土镍矿中镁的研究[J]. 真空科学与技术学报, 2011, 31(3): 341-347
XU Bao-qiang, PEI Hong-bin, YANG Bin, et al. The carbothermic reduction process for magnesium removal from nickel laterite in vacuum[J]. Chinese Journal of Vacuum Science and Technology, 2011, 31(3): 341-347.
[11] 戴永年, 杨斌. 有色金属材料的真空冶金[M]. 北京: 冶金工业出版社, 2003: 7-343.
DAI Yong-nian, YANG Bin. Non-ferrous metal materials vacuum metallurgy[J]. Beijing: Metallurgical Industry Press, 2003: 7-343.
[12] 郁青春, 杨斌, 马文会, 等. 氧化镁真空碳热还原行为研究[J]. 真空科学与技术学报, 2009, 29(S1): 68-71.
YU Qing-chun, YANG Bin, MA Wen-hui, et al. Study of carbothermic reduction of magnesia in vacuum[J]. Chinese Journal of Vacuum Science and Technology, 2009, 29(S1): 68-71.
[13] 王介超, 刘晓荣, 许斌, 等. 转炉污泥对氧化球团还原性的影响[J]. 中南大学学报: 自然科学版, 2011, 42(2): 287-293.
WANG Jie-chao, LIU Xiao-rong, XU Bin, et al. Effect of converter sludge on reduction degree of pellets[J]. Journal of Central South University: Science and Technology, 2011, 42(2): 287-293.
[14] 朱德庆, 翟勇, 潘建, 等. 煤基直接还原—磁选超微细贫赤铁矿新工艺[J]. 中南大学学报: 自然科学版, 2008, 39(6): 1132-1138.
ZHU De-qing, ZHAI Yong, PAN Jian, et al. Beneficiation of super microfine low-grade hematite ore by coal-based direct reduction-magnetic concentration process[J]. Journal of Central South University: Science and Technology, 2008, 39(6): 1132-1138.
[15] QI Tian-gui, LIU Nan, LI Xiao-bin, et al. Thermodynamics of chromite ore oxidative roasting process[J]. J Cent South Univ Technol, 2011, 18(1): 83-88.
[16] LI Rong-ti, PAN Wei, Masamichi Sano. Kinetics and mechanism of carbothermic reduction of magnesia[J]. Metallurgical and Materials Transactions B, 2003, 34(4): 433-437.
[17] 雷斯. 铁合金冶炼[M]. 周进华, 于忠, 译. 北京: 冶金工业出版社, 1981: 32-44.
PbICC M A. Ferroalloy smelting[M]. ZHOU Jin-hua, YU Zhong, trans. Beijing: Metallurgical Industry Press, 1981: 32-44.
[18] 刘卫. 铁合金生产[M]. 北京: 冶金工业出版社, 2005: 59-61.
LIU Wei. Ferroalloy production[M]. Beijing: Metallurgical Industry Press, 2005: 59-61.
[19] 何允平, 王恩慧. 工业硅生产[M]. 北京: 冶金工业出版社, 1989: 39-49.
HE Yun-ping, WANG En-hui. Industrial silicon production[M]. Beijing: Metallurgical Industry Press, 1989: 39-49.
[20] 李秋霞, 朱冬梅, 刘永成, 等. 二氧化硅在真空低价法制备铝过程中的歧化行为研究[J]. 真空科学与技术学报, 2009, 29(1): 35-37.
LI Qiu-xia, ZHU Dong-mei, LIU Yong-cheng, et al. Disproportion reaction of silica in vacuum metallurgy of aluminum[J]. Chinese Journal of Vacuum Science and Technology, 2009, 29(1): 35-37.
[21] 杨栋, 冯乃祥, 王耀武, 等. 碳热还原法制取铝硅合金的反应机理及其动力学[J]. 中国有色金属学报, 2011, 21(1): 227-235.
YANG Dong, FENG Nai-xiang, WANG Yao-wu, et al. Reaction mechanism and kinetics of preparation of aluminium-silicon alloys by carbothermal reduction method [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(1): 227-235.
[22] 李春德. 铁合金冶金学[M]. 北京: 冶金工业出版社, 1991: 15-45.
LI Chun-de. Metallurgy of ferrous alloys[M]. Beijing: Metallurgical Industry Press, 1991: 15-45.
[23] 何允平, 王金铎. 工业硅科技新进展[M]. 北京: 冶金工业出版社, 2003: 37-39.
HE Yun-ping, WANG Jin-zhe. New progress of science and technology of industrial silicon[M]. Beijing: Metallurgical Industry Press, 2003: 37-39.
[24] 叶大伦, 胡建华. 实用无机物热力学数据手册[M]. 2版. 北京: 冶金工业出版社, 2002: 175-925.
YE Da-lun, HU Jian-hua. Thermochemical date of inorganic substance[M]. 2nd ed. Beijing: Metallurgical Industry Press, 2002: 175-925.
[25] 傅献彩, 沈文霞, 姚天扬, 等. 物理化学[M]. 5版. 北京: 高等教育出版社, 2005: 204-386.
FU Xian-cai, SHEN Wen-xia, YAO Tian-yang, et al. Physical chemistry[M]. 5th ed. Beijing: Higher Education Press, 2005: 204-386.
[26] 吴贤熙. Al-Si-Fe三元合金组元活度的计算[J]. 中国有色金属学报, 1999, 9(3): 627-663.
WU Xian-xi. Activity calculation of each component in Al-Si-Fe ternary system[J]. The Chinese Journal of Nonferrous Metals, 1999, 9(3): 627-663.
[27] 黄柱成, 蔡凌波, 张元波, 等. Na2CO3和CaF2强化赤泥铁氧化物还原研究[J]. 中南大学学报: 自然科学版, 2010, 41(3): 838-844.
HUANG Zhu-cheng, CAI Ling-bo, ZHANG Yuan-bo, et al. Reduction of iron oxides of red mud reinforced by Na2CO3 and CaF2[J]. Journal of Central South University: Science and Technology, 2010, 41(3): 838-844.
[28] 黄希祜. 钢铁冶金原理[M]. 3版. 北京: 冶金工业出版社, 2005: 313.
HUANG Xi-gu. Iron and steel metallurgy principles[M]. 3rd ed. Beijing: Metallurgical Industry Press, 2005: 313.
(编辑 赵俊)
收稿日期:2011-07-11;修回日期:2011-09-19
基金项目:国家自然科学基金资助项目(51004058);云南省应用基础研究项目(2010CI009,2011FB040)
通信作者:刘大春(1966-),男,陕西西安人,博士,教授级高工,从事有色金属的真空冶金及新材料制备研究;电话:13608858239;E-mail:lcd_2002@sina.com;2008luoqi@163.com