文章编号:1004-0609(2010)04-0777-07
氧化铝在碳热还原-氯化法炼铝过程中的行为
袁海滨1, 2, 3,冯月斌1, 2,杨 斌1, 2,郁青春1, 2,徐宝强1, 2,王鹏程1, 3,戴永年1, 2
(1. 昆明理工大学 真空冶金国家工程实验室,昆明 650093;
2. 昆明理工大学 云南省有色金属真空冶金重点实验室 冶金与能源工程学院,昆明 650093;
3. 昆明理工大学 材料科学与工程学院,昆明 650093)
摘 要:采用XRD、气相色谱仪、EDS及质量损失等手段与方法,在不同反应温度、系统压力、添加剂及反应时间对氧化铝在碳热及氯化过程进行研究。结果表明:碳热与氯化过程生成的气体主要是CO,含量达98.4%(质量分数)以上;碳热过程在50~100 Pa、高于1 693 K时,Al4O4C与Al4C3开始生成,且含量随着温度的升高与保温时间的延长而增加;在1 Pa及1 773 K时,Al4O4C碳热转化为Al4C3;分别添加10%Fe2O3与10%SiO2(质量分数),在40~100 Pa、1 803 K、保温120~150 min时,可使物料质量损失率达到26.70%与30.13%,促进碳热过程向生成Al4O4C与Al4C3方向进行;温度高于1 853 K不利于该反应的进行;碳热-氯化过程是Al2O3与Al4O4C、Al4C3及AlCl3共同反应生成低价氯化铝AlCl,气态AlCl进入低温区歧解得到金属铝。
关键词:氧化铝;碳热还原;氯化法;氯化铝;真空冶金
中图分类号:TF131;TF821 文献标志码:A
Thermal behavior of alumina in process of carbothermic reduction
and chloride to produce aluminum
YUAN Hai-bin1, 2, 3, FENG Yue-bin1, 2, YANG Bin1, 2, YU Qing-chun1, 2,
XU Bao-qiang1, 2, WANG Peng-cheng1, 3, DAI Yong-nian1, 2
(1.National Engineering Laboratory of Vacuum Metallurgy, Kunming University of Science and Technology,
Kunming 650093, China;
2. Key Laboratory of Nonferrous Metals Vacuum Metallurgy of Yunnan Province, Faculty of Metallurgy and
Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China;
3. Faculty of Material Science and Engineering, Kunming University of Science and Technology, Kunming 650093, China)
Abstract: Under conditions of different reaction temperatures, system pressures, additives and reaction time, the carbothermic behaviors and chloride behaviors of Al2O3 were investigated by XRD, gas chromatography, EDS and mass loss method. The results show that the gas forming in the process of carbothermic and chloride process is mainly CO, the content of CO reaches over 98.4%. Al4O4C and Al4C3 begin to generate under the conditions of 1 693 K and 50-100 Pa, and their contents increase with increasing temperature and prolonging reaction time. Under 1 Pa and 1 773 K, Al4O4C will transform into Al4C3. The mass loss rate of reactants reaches as high as 26.70% and 30.13% when added 10%Fe2O3 and 10%SiO2, respectively, under the conditions of 1 803 K, 120-150 min, and 40-100 Pa, which means that Fe2O3 and SiO2 can promote the carbothermic processes to generate Al4O4C and Al4C3, but the temperature over 1 853 K is not beneficial to progress the reaction. AlCl gas forms from AlCl3 reacting with Al2O3, Al4O4C and Al4C3 during alumina carbothermic reduction and chloride process, and AlCl gas disproportionates into Al and AlCl3 at low temperatures.
Key words: alumina; carbothermic reduction; chlorination; AlCl; vacuum metallurgy
自从冰晶石-氧化铝融盐电解法在工业上使用以来,就一直有人研究其他的炼铝方法[1]。近些年来,人们对常压下碳热还原氧化铝炼铝进行较多的研究。多数学者认为[2-5],该过程分两步反应:2 173~2 473 K时,2Al2O3+9C=Al4C3+6CO;高于2 473 K时,Al2O3+Al4C3=6Al+3CO。GRUNERT等[6]提出,低温区(2 173~2 273 K)碳与氧化铝反应除了生成碳化铝和一氧化碳,该过程还有金属铝蒸气和低价氧化铝(Al2O)气体产生,且反应速率随温度的升高而增大。而LINDSTAD等[7]使用CnHm(1≤n≤12;2≤m≤26)天然气作为高温热源(2 228~2 273 K),认为该过程先生成金属铝、低价氧化铝(Al2O)和一氧化碳混合蒸气,该混合蒸气又引入另一密闭容器内与预先预热好的氧化铝与碳反应生成碳化铝,将生成的碳化铝引入另一容器内与氧化铝反应,生成金属铝和一氧化碳。FRUEHAN等[8-10]使用活性炭与氧化铝反应(2 233~ 2 293 K),得到与LINDSTAD[7]一致的研究结果。HALMANN等[11]的研究认为过程分3步进行:1) 高温下,2Al2O3+3C=Al4O4C+2CO;2) 在稍高的温度下,Al4O4C+6C=Al4C3+4CO;3) 在更高的温度下,Al4O4C+Al4C3=8Al+4CO。另外,BRUNO等[12-13]、STEVENSON[14]和TROUP[15]在铝硅合金生产并进一步提取纯铝的过程中,也有与HALMANN等[11]研究的类似反应:
1) 反应温度为1 773~1 873 K时,
3SiO2+9C= 3SiC+6CO;
2) 反应温度为1 873~2 173 K时,
2Al2O3+3C=Al4O4C+ 2CO;
3) 反应温度为2 223~2 473 K时,
Al4O4C +3SiC=4Al+3Si+ 4CO。
常压碳热还原氧化铝的研究较多,而氧化铝在该过程中的行为也存在两步法与三步法两种学说。然而,对于真空下碳热氯化,氧化铝在低价氯化铝法炼铝过程中的行为,目前尚未见到报道。为此,本文作者对氧化铝在真空碳热过程与氯化过程中的行为进行热力学研究及实验验证。
1 实验
1.1 实验原料与设备
主要实验原料:氧化铝(分析纯)、石墨(大于99.85%)、无水氯化铝(分析纯)。实验设备:使用实验室自行设计的真空炉进行实验[16]。
1.2 实验过程
称取一定摩尔比的石墨与氧化铝,混合均匀,在1~8 MPa的压力下制成d 20 mm×5 mm块后放入干燥箱内,在150 ℃下干燥180 min后,取出后置于真空炉内坩埚中,密封真空炉。抽真空至极限,开始打开水冷装置,升温至一定温度始加热炉底氯化铝升华装置(不充入氯化铝气体时,研究氧化铝在碳热过程中的行为;充入氯化铝气体时,研究氧化铝在碳热氯化过程中的行为)。恒温至炉内真空压力稳定后,停止加热,继续抽真空至室温。开炉取样并称取质量。
1.3 分析测试
采用台湾Agilent Technologies公司生产的6890N(G1530N)气相色谱仪(GS)对反应气体进行成分分析;用日本理学公司Rigaku X射线自动衍射仪(D/max-3B)对样品进行物相分析;用美国伊达克斯(EDAX)有限公司PHOENIXTM能谱仪(EDS)进行元素种类与含量的表征。
2 结果与讨论
2.1 气相色谱分析
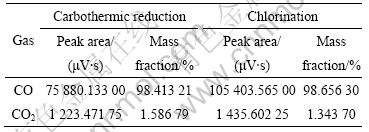
表1所列为1 703~1 753 K碳热过程与1 753 K氯化过程(均为60 min)的气体气相色谱分析。由色谱分析原理[17]可知,峰面积与该气体含量成正比,因此采用峰面积乘以相应的质量校正因子归一化法进行定量分析(峰面积近似等于峰高乘半宽)。从表1可看出,碳热过程与氯化过程的主要气体为一氧化碳(CO),含量均在98.4%以上,而CO2在1.3~1.6%。据此,本文作者对碳热及氯化过程的热力学分析,均假定生成气体近似为100%CO。
表1 碳热与氯化过程气体的气相色谱分析
Table 1 Gas phase chromatography analysis of carbothermic reduction and chlorination
2.2 氧化铝在碳热过程中的行为
2.2.1 温度对氧化铝在碳热过程中行为的影响
X射线衍射仪不仅能测定晶体的结构,而且可对样品的某一物相的相对含量进行初步判断。根据X射线衍射强度公式可知,某一物相的相对含量的增加,其衍射线的强度亦随之增加[18]。氧化铝与碳(石墨)摩尔比为1?3,系统压力50~100 Pa、反应时间为90 min,考察反应温度对氧化铝在碳热过程中行为的影响,其残渣的XRD谱如图1所示。
由图1可以看出:在1 693 K时,氧化铝与碳没发生反应,残渣仍为Al2O3和C;当温度升高至1 703 K时,开始生成Al4O4C及少量的Al4C3,并随着温度的升高,Al4O4C的衍射强度亦增强,Al4C3的衍射强度也有所增强;氧化铝与碳的衍射强度随之而减弱。这说明当温度高于1 693 K时,Al2O3和C发生碳热反应生成Al4O4C与Al4C3,Al4O4C及Al4C3随着温度的升高其相对含量也增加。下面就系统压力为100 Pa的条件,对碳热过程生成Al4O4C与Al4C3的可能反应方程式进行热力学分析(见图2)[19-21]。
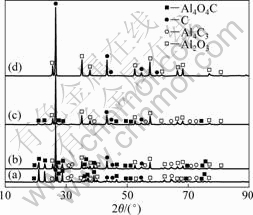
图1 不同温度下氧化铝碳热行为残渣的XRD谱
Fig.1 XRD patterns of residues of Al2O3 carbothermic reaction at different temperatures: (a) 1 853 K; (b) 1 753 K; (c) 1 703 K; (d) 1 693 K
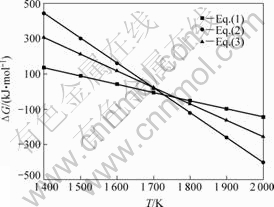
图2 100 Pa下氧化铝碳热过程的ΔGT和T关系[19-21]
Fig.2 Relationship between ΔGT and T of reaction Al2O3 with C at 100 Pa[19-21]
2Al2O3(s)+3C(s)=Al4O4C(s)+2CO(g) (1)
2Al2O3(s)+9C(s)=Al4C3(s)+6CO(g) (2)
Al4O4C(s)+6C(s)=Al4C3(s)+4CO(g) (3)
分析表明,式(1)、(2)和(3)在系统压力为100 Pa时,初始反应温度分别为1 700、1 720和1 730 K。说明在相同系统压力下,1 700 K左右时,Al2O3与C发生碳热反应优先生成Al4O4C;当温度高于1 700 K时,才有Al4C3生成。据此也能说明氧化铝与碳的热反应产物中,Al4O4C的衍射强度高于Al4C3的。根据GITLESEN等[22]对Al-C-O三元系的研究指出,当Al2O3被C还原时,一次反应产物是Al4O4C,这是一种热力学上稳定的化合物;当进一步同碳反应时,另一种产物是碳化铝。此热力学分析与实验结果同GITLESEN等[22]的研究较为吻合。另外,上述热力学分析表明,Al4C3也有可能来自于Al4O4C与C的反应。
2.2.2 系统压力对氧化铝在碳热过程中行为的影响
Al2O3与C在高温下碳热过程属于固态反应物生成的产物中有气态物质。因此,从热力学角度[21], ,系统压力对反应有显著影响。在氧化铝与碳(石墨)摩尔比为1?3,反应温度为1 773 K、反应时间为90 min的条件下,考察系统压力为1 Pa与100 Pa时,系统压力对氧化铝在碳热过程中行为的影响,其残渣的XRD谱如图3所示。
,系统压力对反应有显著影响。在氧化铝与碳(石墨)摩尔比为1?3,反应温度为1 773 K、反应时间为90 min的条件下,考察系统压力为1 Pa与100 Pa时,系统压力对氧化铝在碳热过程中行为的影响,其残渣的XRD谱如图3所示。
由图3可知,当系统压力为1Pa时,残渣中不含Al4O4C,而含较多的Al4C3;而在相同温度下,系统压力为100 Pa时,残渣中Al4O4C的衍射强度明显强于Al4C3的。根据碳热反应方程式(1)、(2)和(3),由于该过程是增容反应,则在相同温度下,系统压力越小, 越有利于气体CO的扩散,促进反应向生成Al4C3方向进行。即在热力学条件(高温低压)更优的情况下,氧化铝在碳热过程中主要发生式(2)与(3)的反应。
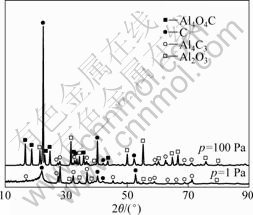
图3 不同系统压力时氧化铝碳热行为残渣的XRD谱
Fig.3 XRD patterns of Al2O3 carbothermic reduction residues at different system pressures
2.2.3 添加剂与反应时间对氧化铝在碳热过程中行为的影响
在氧化铝与碳(石墨)摩尔比为1?3,反应温度为 1 753~1 853 K、系统压力60~109 Pa、.反应时间为60~150 min的条件下,添加Fe2O3和SiO2,考察添加剂对氧化铝在碳热过程中行为的影响,其中分别添加Fe2O3和SiO2时,残渣质量损失分别如图4和5所示。
热力学分析[19-21]表明,Fe2O3+3C=2Fe+3CO在系统压力为100 Pa、反应温度为1 000 K时,ΔGT=-212.386 kJ,即吉布斯自由能具有较大的负值,则金属铁较容易被还原。实验结果显示:添加5%~15%的Fe2O3均能得到少许几颗金属,对金属做EDS分析,发现该金属为Al-Fe-C-O合金。从而说明金属铁在高温下被还原后呈熔融状,而熔融状的铁又将Al2O3与C包裹。从图4(a)可以看出,在1 803 K、保温90 min时,添加Fe2O3后残渣的质量损失明显高于未添加者的,且残渣的质量损失随着Fe2O3添加量(0%~15%)的增加而增大,质量损失为22.93%~23.90%。

图4 添加Fe2O3时氧化铝碳热行为残渣的质量损失
Fig.4 Mass loss of Al2O3 carbothermic reaction residues by adding Fe2O3
从图4(b)可以看出,添加10% Fe2O3,在1 803 K、保温120~150 min时,残渣的质量损失随着反应时间的延长而增加,质量损失为26.65%~26.70%。虽然Fe2O3被碳热还原而消耗一部分C,且还原的铁高温下又将Al2O3与C包裹,但添加Fe2O3后残渣的质量损失率增大,促进氧化铝碳热过程反应的进行,其原因是少量均匀分散在氧化铝与碳中的Fe2O3被还原后生成少量CO气体,而该CO气体的排出又使得反应物料变得疏松多孔,从而使得Al2O3与C反应生成的CO也能顺利地从物料中排出,促使碳热过程向生成Al4O4C与Al4C3方向进行。
添加5%~10%的SiO2在1 753~1 803 K时反应冷凝物经XRD检测,均有一定量的单质硅Si及SiO2。据文献[23],当系统压力为10~100 Pa,1 352~1 463 K时二氧化硅与还原剂碳可发生反应生成低价氧化硅SiO气体,而低价SiO气体在低于1 535 K(10~100 Pa)时即可歧化分解得到单质硅,与二氧化硅其反应方程如下:
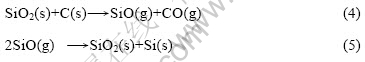
由图5(a)可知,在1 753 K、添加5% SiO2时,随着反应时间的延长,残渣的质量损失率也随之增加;120~150 min时,质量损失增幅较小,为23.95%~24.25%。由图5(b)可知,在1 703~1 853 K内添加10% SiO2,恒温120min时,残渣质量损失先增(1 803 K时达到最大质量损失为30.13%)后减小趋势,其原因在于:SiO2与还原剂碳在一定温度内的反应生成SiO与CO气体,而SiO与CO气体的排出在一定程度上也能很好地分散物料,使氧化铝碳热过程生成的CO也能顺利地从疏松的孔中排出而促使反应进行;但当温度升高至1 853 K时,物料表面发生熔融并平铺,物料被致密地覆盖,使CO气体无法排出,从而阻碍氧化铝碳热过程向生成Al4O4C与Al4C3方向进行。另外,高温下SiO2也易与石墨质坩埚反应生成SiC,使得物料与坩埚紧密粘在一起而导致坩埚在一定程度上受损。因此,实验中应尽可能控制物料在适宜温度范围内反应。
2.3 氧化铝在碳热-氯化过程中的行为
为了研究氧化铝在碳热-氯化过程中的行为,氯化过程不添加Fe2O3与SiO2。当氧化铝与碳(石墨)摩尔比为1?3,氯化铝过量2~4倍、系统压力在70~150 Pa、待炉内温度为1 753~1 853 K时,开始加热氯化铝升华装置至403 K,恒温60 min后,其残渣的检测结果如图6所示。
王平艳等[24]在碳热-氯化实验的研究过程中,使用氧化铝(或铝土矿)、煤作还原剂、无水氯化铝作氯化剂,认为碳热-氯化过程发生的主要反应如下:
图5 添加SiO2时氧化铝碳热行为残渣的质量损失
Fig.5 Mass loss of alumina carbothermic reaction residues by adding SiO2
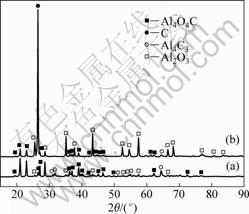
图6 碳热-氯化前后残渣的XRD谱
Fig.6 XRD patterns of residues before (a) and after (b) carbothemic-chloride process
Al2O3(s)+AlCl3(g)+3C(s)→3CO(g)+3AlCl(g) (6)
对式(6)进行分析[19-21]发现,在101 Pa、1 500 K时,其ΔGT=-20.041 kJ。而本文作者在50~100 Pa、 1 693 K,氧化铝与碳(石墨)摩尔比为1?3,反应时间为90 min时,从图1的衍射分析可知,该条件下不发生碳热反应,没有Al4O4C与Al4C3生成,该残渣经无水氯化铝氯化60 min后,冷凝器内无金属铝生成,则该过程并没有发生式(6)反应。而在1 753~1 853 K,由图6可知,残渣在氯化后,渣相中Al4O4C与Al4C3衍射强度,特别是低角度区Al4O4C与Al4C3衍射强度均明显比氯化前(即碳热过程)的衍射强度弱;氯化后,C与Al2O3衍射强度凸显。由此可推断,碳热过程生成的Al4O4C与Al4C3参与了氯化反应而非C与Al2O3直接参与氯化反应,即如下反应方程式:

对碳热-氯化过程可能发生的方程式(7)、(8)和(9)在100 Pa的系统压力条件下,进行热力学分析[19-21](见图7)。
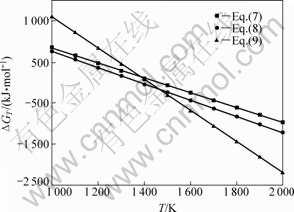
图7 100 Pa下氧化铝碳热-氯化过程的ΔGT和T关系
Fig.7 Relationship between ΔGT and T of alumina carbothermic process at 100 Pa
由图7可知,式(7)、(8)和(9)在100 Pa的系统压力下初始反应温度分别为1 450、1 380和1 415 K。当实验条件为70~150 Pa,1 753~1 853 K时,上述各反应式均可发生。
碳热-氯化过程生成的低价氯化铝AlCl气体进入低温区,歧解得到金属铝及氯化铝,其反应方程式为3AlCl(g)=AlCl3(g)+2Al(l,s)。对冷凝器内收集的冷凝产物进行XRD分析,其结果如图8(a)所示。
图8(b)从上至下为冷凝器顶、1#、2#、3#与4#冷凝盘。由于冷凝器内由上至下存在温度梯度,即温度逐渐升高,因此,在较低温度的冷凝器顶部收集到的金属铝衍射峰较强,纯度较高;而冷凝盘中Al4C3由下至上衍射强度逐渐减弱,即其含量逐渐减少,这是因为发生如下反应:4Al+3C=Al4C3。当T=1 000 K时,ΔGT=-156.678 kJ,由上至下随着温度的升高,AlCl歧解得到的Al极易与石墨质冷凝器的C结合而生成Al4C3。
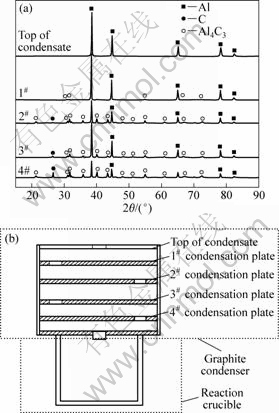
图8 冷凝产物的XRD谱与反应装置简图
Fig.8 XRD patterns (a) of condensates and schematic diagram of reaction (b)
3 结论
1) 气相色谱分析显示, 氧化铝在碳热与氯化过程产生气体主要为CO,含量均达98.4%以上。
2) 碳热过程在50~100 Pa、高于1 693 K时,Al4O4C与Al4C3开始生成且含量随着温度的升高与保温时间的延长而增加;随着温度的继续升高或者系统压力的减小,Al2O3及Al4O4C碳热转化为Al4C3。
3) 在40~100 Pa、1 803 K和保温120~150 min时,分别添加10%Fe2O3与10%SiO2即可使物料质量损失达到26.70%与30.13%,促进碳热过程向生成Al4O4C与Al4C3方向进行,温度过高反而不利于反应的进行。
4) 在70~150 Pa、1 753~1 853 K、氯化过程恒温60 min时,碳热-氯化过程是Al2O3联合Al4O4C、Al4C3及AlCl3反应,而非Al2O3联合C与AlCl3反应生成低价氯化铝AlCl,气态AlCl进入低温区歧解得到Al且金属铝在温度较高的冷凝区极易与石墨质冷凝器的C结合而生成Al4C3。
REFERENCES
[1] 邱竹贤. 有色金属冶金学[M]. 北京: 冶金工业出版社, 2007, 3: 86.
QIU Zhu-xian. Non-ferrous metal metallurgy[M]. Beijing: Metallurgical Industry Press, 2007, 3: 86.
[2] JOHANSEN K, AUNE J A. Method and reactor for production of aluminum by carbothermic reduction of alumina: US6440193B1[P]. 2002-08-27.
[3] AUNE J A, JOHANSEN K. Method and reactor for production of aluminum by carbothermic reduction of alumina: US6805723B2[P]. 2004-10-19.
[4] WILLIAM D E, ROBERT H J, RAMAN R S, WILLIAM S F. Process for the production of aluminum: US4099959[P]. 1978-11-07.
[5] DEWING E W, SOOD R S, SOUTHAM F W. Carbothermic production of aluminum: US4261736[P]. 1981-04-14.
[6] GRUNERT E, TRONCHE L, MERCIER GRENOBLE J. Reduction of alumina: US2974032[P]. 1961-03-07.
[7] LINDSTAD T. Method for recovering aluminum vapor and aluminum suboxide from off-gases during production of aluminum by carbothermic reduction of alumina: US6530970B2[P]. 2003-03-11.
[8] FRUEHAN R J, CARKIN G. The pressure of Al2O and Al in Equilibrium with a Al4C3- Al2O3 (Saturated) Slag at 1 950 ℃ to 2 020 ℃[J]. Metallurgical and Materials Transactions, 2004, 10: 35B, 5.
[9] FRUEHAN, LI Y, CARKIN G. Method using selected carbons to react with Al2O and Al Vapors in the carbothermic production of aluminum: US6849101[P]. 2005-02-01.
[10] FRUEHAN R J, LI Y, CARKIN G. Mechanism and rate of reaction of Al2O, Al, and CO vapors with carbon[J]. Metallurgy and Materials Transactions, 2004, 35(4): 617-623.
[11] HALMANN M, FREI A, STEINFELD A. Carbothermal reduction of alumina: thermochemical equilibrium calculations and experimental investigation[J]. Energy, 2007, 32: 2420-2427.
[12] BRUNO M J. Production of aluminum-silicon alloy and ferrosilicon and commercial purity aluminum by the direct reduction process[R]. Pittsburgh: Aluminum Company of America, 1978.
[13] BRUNO M J. Overview of ALCOA direct reduction process technology[C]//McGeer J P. Light Metals 1984. Warrendale: TMS, 1984: 1571-1590.
[14] STEVENSON D T. Feasibility of an aluminum-silicon blast-arc process[C]//McGeer J P. Light Metals 1984. Warrendale: TMS. 1984: 1613-1631.
[15] TROUP R L. Feasibility of an aluminum-silicon blast furnace[C]//McGeer J P. Light Metals 1984. Warrendale: TMS. 1984: 1591-1612.
[16] 戴永年, 王平艳, 杨 斌, 等. 一种直接从铝矿中提炼铝的真空炉: CN200520099859[P]. 2005-10-18.
DAI Yong-nian, WANG Ping-yan, YANG Bin, et al. A direct metallurgy of Al from bauxite by vacuum furnace: CN200520099859[P]. 2005-10-18.
[17] 孙传经. 气相色谱分析原理与技术[M]. 北京: 化学工业出版社, 1979, 12: 7, 277.
SUN Chuan-jing. Principle of gas chromatography and technology[M]. Beijing: Chemical industry Press, 1979, 12: 7, 277.
[18] 黄新民, 解 挺. 材料分析测试方法[M]. 北京: 国防工业出版社, 2008: 54.
HUANG Xin-min, XIE Ting. Method of material analysis and test[M]. Beijing: National Defence Industry Press, 2008: 54.
[19] LIHRMANN J M. Thermodynamic of the Al2O3-Al4C3 system (Ⅰ)—Thermochemical functions of Al oxide, carbide and oxycarbides between 298 and 2 100 K[J]. Journal of the European Ceramic Society, 2008, 28: 633-642.
[20] 伊赫桑·巴伦著. 纯物质热化学数据手册[M].第3版. 程乃良, 牛四通, 徐桂英, 译. 北京: 科学出版社, 2003: 209, 403.
BARIN I. Thermochemical date of pure substances [M]. 3rd ed. CHENG Nai-liang, NIU Si-tong, XU Gui-ying, transl. Beijing: Science Press, 2003: 209, 403.
[21] 天津大学物理化学教研室编. 物理化学[M]. 北京: 高等教育出版社, 2005: 33-131.
Physical Chemistry Staff Room of Tianjing University. Physical chemistry[M]. Beijing: Higher Education Press, 2005: 33-131.
[22] GITLESEN G, HERSTAD O, MOTZFELDT K. Selected topics in high temperature chemistry[M]. Oslo: Universitetsforlaget, 1966: 143-144.
[23] 李秋霞, 朱冬梅, 刘永成, 周小奎, 戴永年. 二氧化硅在真空低价法制备铝过程中的歧化行为研究[J]. 真空科学与技术学报, 2009, 29(1): 35-37.
LI Qiu-xia, ZHU Dong-mei, LIU Yong-cheng, ZHOU Xiao-kui, DAI Yong-nian. Disproportion reaction of silica in vacuum metallurgy of aluminum[J]. Chinese Journal of Vacuum Science and Technology, 2009, 29(1): 35-37.
[24] 王平艳, 刘谋胜, 戴永年. 真空碳热还原氯化法从铝土矿炼铝[J]. 真空科学与技术学报, 2006, 26(5): 377-380.
WANG Ping-yan, LIU Mou-sheng, DAI Yong-nian. Vacuum metallurgy of Al from bauxite by carbothermic reaction- chlorination[J]. Chinese Journal of Vacuum Science and Technology, 2006, 26(5): 377-380.
(编辑 李艳红)
基金项目:国家自然科学基金-云南省联合基金重点资助项目(u0837604)
收稿日期:2009-06-05;修订日期:2009-11-15
通信作者:杨 斌,教授,博士;电话:0871-5161583;E-mial:kgyb2005@126.com