文章编号:1004-0609(2011)12-3155-07
真空下碳热还原氧化铝的二次反应
冯月斌1, 2,杨 斌1,戴永年1
(1. 昆明理工大学 冶金与能源工程学院 真空冶金国家工程实验室,昆明 650093;
2. 昆明理工大学 理学院,昆明 650500)
摘 要:通过热力学分析和实验研究了真空条件下碳热还原氧化铝的二次反应。热力学分析表明:低温、高压有利于碳热还原氧化铝的产物Al2O、Al与CO的二次反应。分别绘制了Al2O和Al在一定分压下,与CO的二次反应平衡曲线图,给出了各二次反应的CO平衡分压与温度的关系,根据CO的分压和温度、利用二次反应平衡曲线图分析二次反应的产物。氧化铝与石墨的真空碳热还原实验研究证实:Al2O与CO降低温度首先生成Al4O4C和C,Al与CO降低温度首先生成Al4O4C和Al4C3,符合根据二次反应平衡曲线图分析得到的结论,说明二次反应平衡曲线图的合理性。
关键词:碳热还原;氧化铝;石墨;真空;Al2O;Al
中图分类号:TF 131; TF 8 文献标志码:A
Back-reactions of carbothermal reduction of alumina in vacuum
FENG Yue-bin1, 2, YANG Bin1, DAI Yong-nian1
(1. National Engineering Laboratory of Vacuum Metallurgy, Faculty of Metallurgy and Energy Engineering,
Kunming University of Science and Technology, Kunming 650093, China;
2. Faculty of Science, Kunming University of Science and Technology, Kunming 650500, China)
Abstract: The back-reactions of the carbothermal reduction of alumina in a vacuum were investigated. The thermodynamic analysis indicates that both low temperature and high pressure favor the back-reactions of Al2O3, Al and CO generated from the carbothermal reduction of alumina. The equilibrium curves for the back-reactions of Al2O and CO, and those of Al and CO are obtained, respectively, for certain partial pressures of Al2O and those of Al, showing the CO equilibrium partial pressure of the back-reactions as a function of the temperature. From the equilibrium curves, the species formed by the back-reactions are determined by the CO partial pressure and the temperature. The experiments on the alumina and graphite show that Al2O reacts back with CO to first form Al4O4C and C, and Al reacts back with CO to first form Al4O4Cand Al4C3, verifying the conclusions obtained from the equilibrium curves. The equilibrium curves of the back-reactions are reasonable.
Key words: carbothermal reduction; alumina; graphite; vacuum; Al2O; Al
碳热还原氧化铝是碳热法和歧化法提取铝的基 础[1-3],也是Serpek法生产AlN粉末的基础。但对碳热还原氧化铝反应机理的认识仍然存在着分歧,大体上可分为固-固相反应机理和气相反应机理[4-5]。
固-固相反应机理的基本观点为:氧化铝与碳直接发生固-固相反应,形成Al4O4C或Al2OC为中间产物,最终生成Al或Al4C3[6-9]。
气相反应机理的观点是氧化铝与碳之间的反应通过气相进行,生成含铝气体,该气体再次反应形成冷凝沉积物。早在1894年,STROUP[10]在高温下进行碳热还原氧化铝实验,发现氧化铝和石墨蒸发,随之又冷凝下来。ISH-SHALON[11]在氮气中进行碳热还原氧化铝实验,发现反应物表面有纤维状物质生成。HALMAN等[12]把氧化铝与活性炭在感应炉中氩气下加热,在石英管较冷部位发现了冷凝沉积物。这些实验现象说明氧化铝与碳反应生成气体产物,该气体产物再次反应形成冷凝沉积物。YU等[13]认为碳热还原氧化铝反应通过CO进行,生成Al、Al2O和AlO气体。LIHRMAN[14]认为1 806.65 ℃以下Al和Al2O是重要成分,而AlO、Al2O2和AlO2是次要成分。
本文作者研究真空下1 643 ~ 1 843 K碳热还原氧化铝,通过物相分析、扫描电镜分析和热力学分析,得出气相反应机理,认为氧化铝与碳通过气相反应生成Al2O、Al和CO气体,该气体产物发生二次反应生成冷凝沉积物[15-16]。
1 碳热还原氧化铝二次反应热力学分析
在1 200 ~ 1 900 K的温度范围内、系统压力小于100 Pa的条件下,碳热还原氧化铝生成气体产物Al2O、Al和CO,反应如下[15-16]:
Al2O3+2C=Al2O(g) +2CO(g) (1)
Al2O3+3C=2Al(g)+3CO(g) (2)
热力学分析表明[16]:反应(1)的初始反应温度低于反应(2)的,但反应(2)的吉布斯自由能随温度的升高而下降的趋势更大,使两个反应的吉布斯自由能与温度的关系曲线相交。在交点温度时,两个反应的吉布斯自由能相等。高于交点温度时,反应(2)的吉布斯自由能小于反应(1)的,应优先发生。该交点温度随系统压力的降低而降低。图1所示为反应(1)和(2)的吉布斯自由能相等时的温度与体系压力的关系曲线,在曲线左上区域,碳热还原氧化铝优先生成Al,在曲线右下区域,优先生成Al2O。
气体产物Al2O和Al在降低温度时,分别与CO发生二次反应,形成由Al4O4C、Al4C3、Al2O3和C组成的冷凝物[15-16]。
1.1 Al2O与CO的二次反应热力学分析
碳热还原氧化铝产物Al2O与CO可能发生的二次反应如下:
12Al2O(g)+8CO(g)=5Al4O4C(s)+Al4C3(s) (3)
9Al2O(g)+6CO(g)=5Al2O3(s)+2Al4C3(s) (4)
2Al2O(g)+2CO(g)=Al4O4C(s)+C(s) (5)
Al2O(g)+2CO(g)=Al2O3(s)+2C(s) (6)
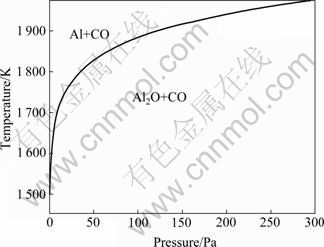
图1 反应(1)和(2)的吉布斯自由能相等时温度与体系压力的关系[16]
Fig.1 Relationship between temperature and system pressure at equal ?G of reaction (1) and reaction (2)
1.1.1 Al2O与CO的二次反应的吉布斯自由能
利用HSC软件的热力学数据,推导出在1 200 ~ 1 900 K的温度范围内反应(3)~(6)的吉布斯自由能与温度、压力的关系分别为式(7)~(10)所示。

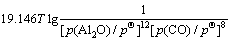 (7)
(7)

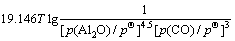 (8)
(8)
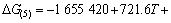
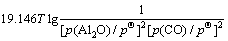 (9)
(9)
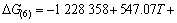
 (10)
(10)
假设氧化铝碳热还原反应在封闭体系中进行,则系统压力为碳热还原的气体产物的分压之和,气体产物的分压比符合反应方程式的计量关系。当氧化铝的碳热还原按照反应(1)进行时,系统压力等于Al2O与CO的分压之和,Al2O与CO的分压比为1:2。据此,可以由式(7)~(10)计算得到系统压力为105 Pa和150 Pa时二次反应(3)~(6)的吉布斯自由能与温度的关系,如图2所示。从图2可见,Al2O与CO的二次反应的吉布斯自由能随温度的升高和压力的降低而升高,低温、高压有利于二次反应的进行。体系压力为105 Pa时,在1 200~1 900 K的温度范围内,反应(3)~(6)的吉布斯自由能都为负值。体系压力为150 Pa时,反应(3)、(4)、(5)和(6)的初始反应温度分别为1 716、1 682、1 722和1 696 K,首先发生的反应为反应(5),其次为反应(3)。体系压力为150 Pa、温度低于1 600 K时,各反应的吉布斯自由能由低到高的顺序依次为反应(3)、(4)、(5)、(6)。
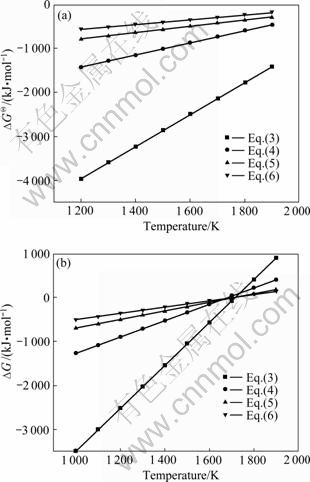
图2 系统压力不同时Al2O与CO二次反应的吉布斯自由能与温度的关系
Fig.2 Relationship between ?G and temperature of back- reactions between Al2O and CO at different system pressures: (a) 105 Pa; (b) 150 Pa
1.1.2 Al2O与CO的二次反应的平衡图
假设Al2O与CO发生二次反应时,只按照反应(3)~(6)中的任一个反应进行。则反应的自由度为f=(4-1)-3+2=2,影响反应平衡的因素有温度、压力和气相组成。当Al2O的分压一定时,CO的分压p(CO)为温度T的函数,p(CO)=φ(T)。
由各反应的吉布斯自由能二项式 ,
, ,可得到反应(3)~(6)的平衡关系分别为式(11)~(14)。
,可得到反应(3)~(6)的平衡关系分别为式(11)~(14)。
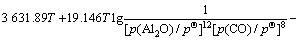
 (11)
(11)
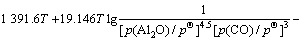
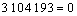 (12)
(12)
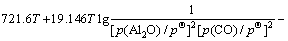
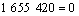 (13)
(13)
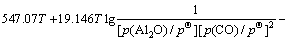
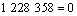 (14)
(14)
据此,绘制出Al2O的分压为0.5 Pa和50 Pa时的二次反应的平衡曲线,如图3所示。
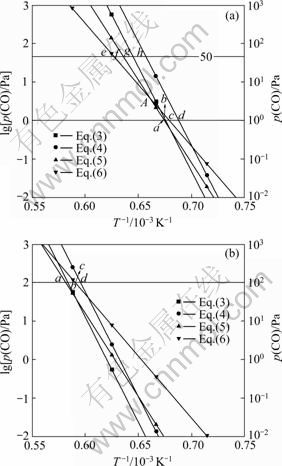
图3 不同Al2O分压时Al2O与CO二次反应的平衡曲线图
Fig.3 Equilibrium curves for back-reactions between Al2O and CO at different partial pressures of Al2O: (a) p(Al2O)=0.5 Pa; (b) p(Al2O)=50 Pa
由图3可知:碳热还原氧化铝以反应(1)进行生成Al2O和CO,Al2O的分压为0.5 Pa和50 Pa时,CO的分压分别为1 Pa和100 Pa,二次反应(5)、(3)、(6)和(4)的平衡点分别为a、b、c和d。碳热还原氧化铝生成的气体产物从反应区上升,温度逐渐降低,首先到达a点发生反应(5)生成Al4O4C和C,反应(3)的平衡点b点与a点极为接近,也容易发生反应(3)生成Al4O4C和Al4C3。由于氧化铝碳热还原反应生成的Al2O和CO的物质的量的比为1:2,二次反应按式(5)和(3)进行时,二者的反应比分别为1:1和3:2,反应后的气体总压降低,CO与Al2O的分压比增大,达到反应(5)和(6)的平衡曲线的交点A以上时,优先发生反应(6)。假设碳热还原氧化铝生成的Al2O和CO的分压分别为50 Pa和100 Pa,降低温度按照反应(5)和(3)反应后,Al2O的分压为0.5 Pa时,CO的分压应大于50 Pa。Al2O和CO的分压分别为0.5 Pa和50 Pa时,反应(6)、(5)、(3)和(4)的平衡点分别为e、f、g和h。因此,继续降低温度,首先到达反应(6)的平衡点,发生反应(6)生成Al2O3和C。根据二次反应平衡曲线图可以判断不同条件下发生的二次反应。
1.2 Al与CO的二次反应热力学分析
碳热还原氧化铝产物Al与CO可能发生的二次反应如下:
8Al(g)+4CO(g)=Al4O4C(s)+Al4C3(s) (15)
6Al(g)+3CO(g)=Al2O3(s)+Al4C3(s) (16)
4Al(g)+4CO(g)=Al4O4C(s)+3C(s) (17)
2Al(g)+3CO(g)=Al2O3(s)+3C(s) (18)
1.2.1 Al与CO的二次反应的吉布斯自由能
利用HSC软件的热力学数据,推导出在1 200~ 1 900 K的温度范围内反应(15)~(18)的吉布斯自由能与温度、压力的关系分别为式(19)~(22):

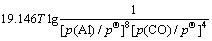 (19)
(19)
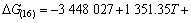
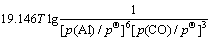 (20)
(20)
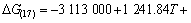
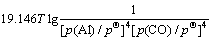 (21)
(21)
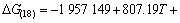
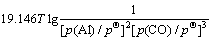 (22)
(22)
氧化铝的碳热还原按照反应(2)进行时,系统压力等于Al与CO的分压之和,Al与CO的分压比等于2:3。据此,可以由式(19)~(22)计算得到系统压力为105 Pa和125 Pa时二次反应(15)~(18)的吉布斯自由能与温度的关系,如图4所示。从图4可见,Al与CO的二次反应的吉布斯自由能随温度的升高和压力的降低而升高,低温、高压有利于二次反应的进行。体系压力为105 Pa时,在1 200~1 900 K的温度范围内,反应(15)~(18)的吉布斯自由能都为负值。体系压力为125 Pa时,反应(15)、(16)、(17)和(18)的初始反应温度分别为1 821、1 807、1 788和1 760 K,首先发生的反应为反应(15),各反应的吉布斯自由能由低到高的顺序依次为反应(15)、(16)、(17)、(18)。
1.2.2 Al与CO的二次反应的平衡图
同Al2O与CO的二次反应类似,Al的分压一定时,p(CO)=φ(T),由各反应的吉布斯自由能二项式,可得到反应(15)~(18)的平衡关系式分别为式(23)~ (26):
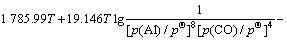
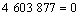 (23)
(23)
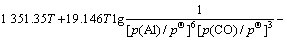
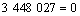 (24)
(24)

 (25)
(25)
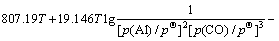
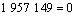 (26)
(26)
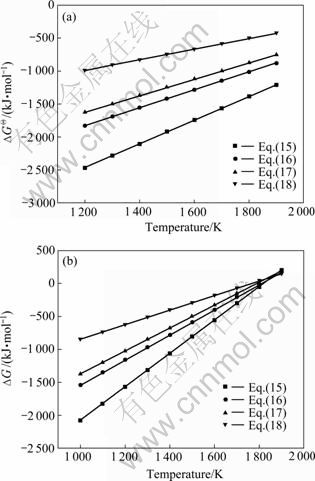
图4 不同系统压力时Al与CO二次反应的吉布斯自由能与温度的关系
Fig.4 Relationship between ?G and temperature of back- reactions between Al and CO at different system pressures: (a) 105 Pa; (b) 125 Pa
据此,绘制出Al的分压为0.5 Pa和50 Pa时二次反应的平衡曲线,如图5所示。
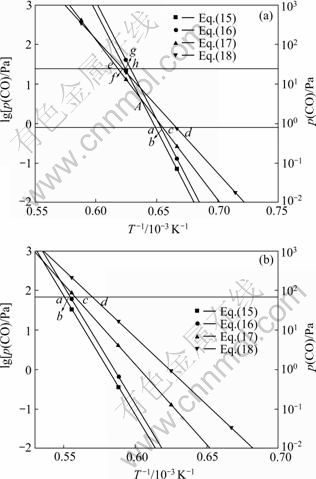
图5 不同Al分压时Al与CO的二次反应的平衡曲线图
Fig.5 Equilibrium curves for back-reactions between Al and CO at different partial pressures of Al: (a) p(Al)=0.5 Pa; (b) p(Al)=50 Pa
碳热还原氧化铝以反应(2)进行生成Al和CO,Al的分压为0.5 Pa和50 Pa时,CO的分压分别为0.75 Pa、和75 Pa,二次反应(15)~(18)的平衡点分别为a、b、c和d。碳热还原氧化铝生成的气体产物从反应区上升,温度逐渐降低,首先到达a点发生反应(15)生成Al4O4C和Al4C3。由于氧化铝碳热还原反应生成的Al和CO的物质的量的比为2:3,二次反应按式(15)进行时,二者的反应比为2:1,反应后的气体总压降低,CO与Al的分压比增大,达到反应(15)和(17)的平衡曲线的交点A以上时,发生反应(17)。假设碳热还原氧化铝生成的Al和CO的分压分别为50 Pa和75 Pa,降低温度按照反应(15)反应后,Al的分压为0.5 Pa时,CO的分压应为25.5 Pa,二次反应(17)、(18)、(15)和(16)的平衡点分别为e、f、g和h,继续降低温度首先到达反应(17)的平衡点,发生反应(17)生成Al4O4C和C,如图5(a)所示。根据二次反应平衡曲线图可以判断不同条件下发生的二次反应。
2 实验
2.1 实验原料及实验过程
将氧化铝(分析纯)和石墨(纯度99.8%)以摩尔比1:3进行配比,混匀后,以压力2 MPa时的粉末压成直径为20 mm圆柱状料块,每块料的质量约为5 g。
反应物料置于真空炉内的石墨坩埚中,真空度稳定后,升温到一定的温度,保持该温度30 min。
2.2 实验结果与讨论
根据碳热还原氧化铝优势反应分析(见图1),设计了两个实验。
实验Ⅰ:反应温度为1 843 K,炉内压力最高达150 Pa。该条件位于反应(1)和(2)的吉布斯自由能相等时的温度与压力的关系曲线的右下区域,碳热还原氧化铝产物主要为Al2O和CO。
实验Ⅱ:反应温度为1 843 K,炉内压力最高为38 Pa,该条件位于曲线的左上区域,碳热还原氧化铝产物主要为Al和CO。
图6所示为两个实验的冷凝物的XRD谱。实验Ⅰ得到的冷凝物的XRD谱表明其中Al4O4C和C的含量最高,证实二次反应热力学分析得出的Al2O与CO首先发生反应(5)生成Al4O4C和C的结论。实验Ⅱ得到的冷凝物的XRD谱表明其中Al4C3和Al的含量最高,Al4O4C的次之。冷凝物中较高的铝含量直接说明该实验条件下产生了铝气体。根据热力学分析结果,Al气体与CO的二次反应优先发生反应(15)生成Al4O4C和Al4C3,接着发生反应(17)生成Al4O4C和C,因此Al4O4C的含量应高于Al4C3,但实验得到了相反的结果,这是因为冷凝下来的固体铝也与CO发生二次反应形成Al4C3和Al2O3,导致Al4C3的含量超过Al4O4C的,该反应早已被证实[17],XRD谱中不可忽视的Al2O3的衍射峰也说明了该反应的发生。
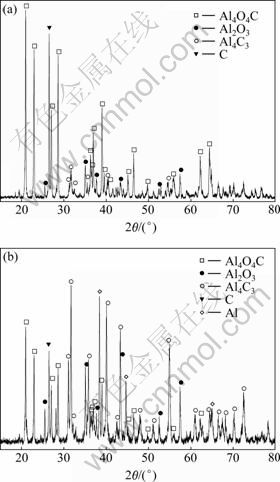
图6 冷凝物的XRD谱
Fig.6 XRD patterns of condensates: (a) ExperimentⅠ; (b) ExperimentⅡ
实验Ⅰ采用塔式冷凝器,在温度更低的冷凝部位还得到少量主要由Al2O3和C组成的冷凝物,其XRD谱如图7所示。证实了Al2O与CO二次反应热力学分析得出的结论:二次反应(5)及(3)进行后,CO的含量增大,达到反应(5)和(6)的平衡曲线的交点A以上时,优先发生反应(6)生成Al2O3和C。
实验Ⅱ采用管式冷凝器,冷凝区温度没有明确的分区,冷凝物中除了含有较多的Al4C3、Al4O4C和Al外,C的含量也较高,说明也该也发生了反应(17)。
随着温度和气体组成的改变,二次反应按不同的反应式进行。因此,反应得到的冷凝物中,首先发生的反应的产物含量较高,同时还含有其他各反应的产物。
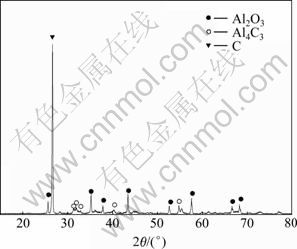
图7 实验Ⅰ温度更低部位的冷凝物的XRD谱
Fig.7 XRD pattern of condensates at much lower temperature zone in experimentⅠ
3 结论
1) 真空碳热还原氧化铝的气体产物Al2O、Al与CO发生二次反应形成由Al4O4C、Al4C3、Al2O3和C组成的冷凝物,低温、高压有利于二次反应的进行。
2) 碳热还原氧化铝生成Al2O和CO时,二次反应首先生成Al4O4C和C;碳热还原氧化铝生成Al和CO时,二次反应首先生成Al4O4C和Al4C3。随着各气体分压和温度的改变,发生不同的二次反应,可以通过二次反应平衡图分析不同条件下的二次反应产物。
REFERENCES
[1] Bruno M J. Aluminum carbothermic techonology[R]. Washington: US Department of Energy Golden Field Office, 2004.
[2] 冯月斌, 戴永年, 王平艳. 歧化法生产和精炼铝的研究概况[J]. 轻金属, 2009(3): 12-15.
FENG Yue-bin, DAI Yong-nian, WANG Ping-yan. Research of the production and refining of aluminum by disproportion[J]. Light Metals, 2009(3): 12-15.
[3] 李秋霞, 陈为亮, 戴永年. 真空低价氟化铝歧化分解制备铝[J]. 中国有色金属学报, 2008, 18(8): 1550-1554.
LI Qiu-xia, CHEN Wei-1iang, DAI Yong-nian. Preparation of aluminum by disproportion of sub-fluoride in vacuum[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(8): 1550-1554.
[4] 刘新宽, 马明亮, 周敬恩. 碳热还原法制备氮化铝反应机制的研究进展[J]. 硅酸盐通报, 1999(1): 35-39.
Liu Xin-kuan, Ma Ming-liang, Zhou Jing-en. Review of mechanisms of carbothermal reduction nitridation reactions[J]. Bulletin of the Chinese Ceramic Society, 1999(1): 35-39.
[5] 李亚伟, 李 楠, 袁润章. Al2O3-C-N2系统反应热力学初步探讨[J]. 硅酸盐通报, 2001(4): 3-8.
Li Ya-wei, Li Nan, YUAN Run-zhang. Thermodynamical discussion of the reaction evolution in Al2O3-C-N2 system[J]. Bulletin of the Chinese Ceramic Society, 2001(4): 3-8.
[6] Cox J H, Pidgeon L M. The aluminum-oxygen-carbon system[J]. Canadian Journal of Chemistry, 1963, 41: 671-683.
[7] Hyeong Uk Joo, Woo-Sik Jung. Coversion of basic dicarboxylate Al(Ⅲ) complexes to aluminum carbide under a flow of argon[J]. Journal of Alloys and Compounds, 2008, 465(1/2): 265-268
[8] Yuan Hai-bin, Yang Bin, Yu Qing-chun, Xu Bao-qiang, Zhu Yu-yan, Feng Yue-bin, Dai Yong-nian. Reaction mechanism of AlCl generated by carbothermic chloride to produce aluminum in vacuum[C]//Proceeding of the 9th Vacuum Metallurgy and Surface Engineering Conference. Beijing: Electronics Industry Press, 2009: 39-45.
[9] Yuan Hai-bin, YANG Bin, XU Bao-qiang, YU Qing-chun, FENG Yue-bin, DAI Yong-nian. Aluminum production by carothermo-chlorination reduction of alumina in vacuum[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(8): 1505-1510.
[10] Stroup P T. Carbothermic smelting of aluminum[J]. Transaction of Metallurgical Society of Aime, 1964, 230(3): 355-372.
[11] Ish-Ahalom M. Formation of aluminum oxynitride by carbothermal reduction of aluminum oxide in nitrogen[J]. Journal of Materials Science Letters, 1982, 1(4): 147-149.
[12] Halmann M, Frei A, Ateinfeld A. Carbothermal reduction of alumina: Thermochemical equilibrium calculation and experimental investigation[J]. Energy, 2007, 32(12): 2420-2427.
[13] Yu J K, Ueno S, Li H X, Hiragushi K. Improvement of graphitization of isotropic carbon by Al2O3 formed from aluminum chelate compound[J]. Journal of the European Ceramic Society, 1999, 19(16): 2843-2848.
[14] Lihrmann J M. Thermodynamics of the Al2O3-Al4C3 system Ⅲ. Equilibrium vapour pressures and activation energies for volatilization[J]. Journal of European Ceramic Society, 2008, 28(3): 649-656.
[15] FENG Yue-bin, YANG Bin, DAI Yong-nian. Carbothermal reduction of alumina in a vacuum[C]//Advanced Material Research. Switzland: Trans Tech Publications, 2011, 156/157: 1688-1691.
[16] 冯月斌,杨 斌,戴永年. 真空下碳热还原氧化铝热力学[J]. 中国有色金属学报, 2011, 21(7): 1748-1755.
FENG Yue-bin, YANG Bin, DAI Yong-nian. Thermodynamics of the carbothermal reduction of alumina in a vacuum[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(7): 1748-1755.
[17] 东北工学院有色系轻金属冶炼教研室. 专业轻金属冶金学: 电冶炼部分[M]. 北京: 冶金工业出版社, 1960: 417.
Teaching and Research Group of Light Metal Smelting, Department of Non-Ferrous Metallurgy, North-East Technical College. Special Light Metal Metallurgy: Electric Smelting Part [M]. Beijing: Metallurgical Industry Press, 1960: 417.
(编辑 李艳红)
基金项目:国家自然科学基金-云南省联合基金(u0837604)
收稿日期:2010-11-26;修订日期:2011-04-30
通信作者:杨 斌,教授,博士;电话/传真:0871-5161583;E-mail: kgyb2005@126.com