文章编号:1004-0609(2008)08-1550-05
真空低价氟化铝歧化分解制备铝
李秋霞1, 2,陈为亮2,戴永年2
(1. 云南师范大学 化学化工学院,昆明 650092;
2. 昆明理工大学 真空冶金国家工程实验室,昆明 650093)
摘 要:利用“物质吉布斯自由焓函数法”,讨论不同压力下低价氟化铝生成及分解的热力学条件。结果表明:系统压力为100 kPa时,在1941.76 K以上才能生成低价氟化铝;而当系统压力降低到100~10 Pa时,在1 486.32~ 1 373.28 K 能生成低价氟化铝。同时,当系统压力小于300 Pa时在1 673 K下用氧化铝、氟化铝与还原剂碳在真空炉内实验,验证了理论研究结果,得到纯度为93.77%的金属铝;以焦碳作还原剂,铝土矿为原料在1 723 K进行了实验,得到纯度为95.13%的金属铝。
关键词:铝;真空冶金;歧化反应;低价氟化物;制备
中图分类号:TF 131; TF 8 文献标识码:A
Preparation of aluminum by disproportion of sub-fluoride in vacuum
LI Qiu-xia1, 2, CHEN Wei-liang2, DAI Yong-nian2
(1. School of Chemistry and Chemical Engineering ,Yunnan Normal University, Kunming 650092, China;
2. National Engineering Laboratory of Vacuum Metallurgy, Kunming University of Science and Technology, Kunming 650093, China)
Abstract: Thermodynamics process and experiment of aluminum extracting from bauxite and Al2O3, were studied. The temperature, at which alumina, aluminum fluoride and carbon react to form aluminum sub-fluoride at different pressures, was evaluated with “Substance Gibbs’ Free Energy Functional Determinant” method. The results show that the reaction temperature can be considerable declined in vacuum, such as the temperature dropped to 1 486.32?1 373.28 K in the pressure range from 100 Pa to 10 Pa, but the temperature of the reaction needed is more than 1 941.76 K under 100 kPa. The metal aluminum with the purity of 93.77% is obtained by the experiment alumina, aluminum fluoride and carbon in a vacuum furnace when the system pressure is less than 300 Pa at 1 673 K; the aluminum metal with the purity of 95.13% is also gotten at 1 723 K by using coke for reducing bauxite.
Key words: aluminum; vacuum metallurgy; disproportion; sub-fluoride; preparation
金属铝是性能优异、用途广泛、关联度大的基础有色金属材料,在国民经济发展中具有不可替代的重要作用[1]。目前铝冶炼工艺虽然有了迅猛发展,但电解铝生产的电能耗平均每吨还在1.4~1.5 kW?h时,每吨电解铝电力成本约7 000元,占生产成本的6%左右;生产1 t电解铝将直接排放二氧化碳约12 t、二氧化硫92.5 kg,同时还存在含氟污染物的排放等问题[2?5];又由于铝原子有3个外层电子,它们的离子化势分别 为[6]:一级5.98 eV、二级18.82 eV、三级28.44 eV。铝的离子化势都很小,所以既可形成Al+,也可形成Al2+和Al3+,常见的化合物中为Al3+。近些年来,人们对利用低价化合物法提取铝进行了较多的研究[7?12],低价铝化物是铝的一种高温稳定、低温分解的气态物质,即在常温下是不存在的,在一定温度下分解为金属铝和三氧化铝,即通过低价铝化物的生成及分解可从含氧化铝的原料中制备金属铝。低价化合物法简化了目前铝冶金的两段生产工艺和设备,原料要求低,采用真空冶金进行冶炼,“三废”将减少。该方法包括低价硫化物法、低价氯化物法和低价氟化物法,其中低价氟化铝(AlF)歧化分解法对设备没有腐蚀性,有一定的优势。为此,本文作者对低价氟化物AlF的生成及利用歧化分解反应提取铝进行了初步的研究。
1 生成低价氟化铝的热力学研究
碳与氧化铝、三氟化铝反应,在高温下生成低价氟化铝(AlF),反应方程式可表示为式(1)和(2),低价氟化铝歧化分解反应方程式可表示为式(3):


利用“物质吉布斯自由能函数法”[13]对上述3式进行热力学研究,其依据如式(4)~(8):

根据文献[14]得到:式(1)、(2) 和(3)的 分别为2 058.277、1 755.657和?714.626 kJ/mol。设反应在真空系统中进行,若体系残压p(residual)分别为1×105、1×104、1×103、100和10 Pa。根据文献[13]查找式(1)和(2)中各物质的
分别为2 058.277、1 755.657和?714.626 kJ/mol。设反应在真空系统中进行,若体系残压p(residual)分别为1×105、1×104、1×103、100和10 Pa。根据文献[13]查找式(1)和(2)中各物质的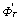 数据和式(5)~(8)计算得到不同温度和压力下式(1)和(2)的?
数据和式(5)~(8)计算得到不同温度和压力下式(1)和(2)的?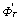 和?GT,其中不同温度范围下Qp的依据分别为式(9)和(10):
和?GT,其中不同温度范围下Qp的依据分别为式(9)和(10):

不同压力下式(1)和(2)的最低反应温度(即?GT = 0)列于表1。
表1 式(1)和(2)在不同压力下反应的最低温度
Table 1 Initial temperature of reactions (1) and (2) at different pressures

由图1和表1可以看出:体系压力p减小时,?GT也减小,反应需要的温度也降低,即降低体系的压力可以使反应温度降低。当体系在100 kPa时生成低价氟化铝的最低温度为1 941.76 K,体系压力降低到100~10 Pa间时,反应的最低温度可以降低到1 486.32~ 1 373.28 K。由此可以看出:降低反应压力可大大降低反应温度。

图1 式(1)和(2)在不同压力和温度下?GT—T关系
Fig.1 Relationships between ?GT and T of reactions (1) and (2) at different pressures and temperatures
2 低价氟化铝歧化分解的热力学研究
类似的,根据式(5)~(8)以及Qp = 计算得到式(3)在不同压力和温度下的?GT,?GT与T的关系见图2,不同压力下反应(3)最低温度(即?GT =0)列于表2。
计算得到式(3)在不同压力和温度下的?GT,?GT与T的关系见图2,不同压力下反应(3)最低温度(即?GT =0)列于表2。
表2 式(3)在各体系压力下反应的温度
Table 2 Reaction of temperature reaction (3) at different pressures

由图2和表2可以看出:体系残余压力p减小时,?GT升高,反应需要的最低温度也降低。即降低体系压力将使歧化分解反应温度降低。体系压力降低到100~10 Pa间,反应所需的温度就可以降低到1 075.71~ 985.63 K。即当体系压力控制在100~10 Pa时,温度控制在973~1 073 K可以使低价氟化铝发生歧化分解。

图2 式(3)在不同压力和温度下的?GT—T关系
Fig.2 Relationships between ?GT and T of reaction (3) at different pressures and temperatures
3 实验
3.1 实验原料
铝土矿和工业氧化铝(中州铝厂提供,主要成分见表3和表4)、煤(见表5)、工业氟化铝(云南铝厂提供)
表3 铝土矿主要化学成分
Table 3 Main chemical composition of bauxite (mass fraction, %)

表4 工业氧化铝的化学成分
Table 4 Chemical composition of alumina (mass fraction,%)
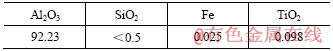
表5 煤种及主要性能指标
Table 5 Coal grades selected and their performance indices

对于用氧化铝为原料的烧结实验研究得出:按10煤3 g 、5煤7 g、16煤10 g和氧化铝5 g的比例混合制团,在520 ℃下进行烧结[15],可以预防喷料发生,使之具有良好的焦结效果并含有足量的还原用碳。
3.2 实验操作
1) 将实验所需氟化铝称量后置于异型坩埚底部。
2) 按实验方案将氧化铝混合煤混合均匀或铝土矿与焦炭,压团,置于坩埚中部,安装好蒸馏盘底盘再放蒸馏盘,密封真空炉。
3) 抽真空直到极限真空时升温,到520 ℃左右保温约30 min,再快速升温,直到主体反应需要的温度,恒温,等炉内残余压力保持恒定时,反应完毕。
4) 切断电源,继续抽真空和保持水冷直到室温;停泵和水;取样。
4 实验结果
4.1 工业氧化铝实验
残余压力为300 Pa以下,反应温度在1 300~1 550 ℃范围内都可以得到金属铝,当温度为 1 400~1 450 ℃时得到的金属铝状态最佳。不同温度得到纯度不同的金属铝,如表6所列。从表6可以看出:温度控制在1 400 ℃以上,金属铝的纯度可以达到92.54%以上。图3所示为1 400 ℃和150 Pa时金属铝的EDS谱及化学成分,可见其杂质含量很少;其中所含的氧的第一来源为实验后自身氧化导致的,第二来源为在分析过程中激光照射金属铝表面引起的氧化所导致。图4所示为金属铝的SEM像。可以看出:金属铝颗粒的切面呈金属状,纯度较高,同时金属铝颗粒周围附有氟化物,这是因为反应(3)歧化分解为金属铝和氟化物是同时进行,两者分离不及时引起。
表6 各温度下金属铝的纯度
Table 6 Purity of aluminum at different temperatures (mass fraction,%)


图3 金属铝EDS谱
Fig.3 Energy-dispersive spectra of Al metal (1 400 ℃, 150 Pa)

图4 金属铝的SEM像
Fig.4 SEM micrograph of Al metal (1 400 ℃, 150 Pa)
4.2 铝土矿实验
残余压力为300 Pa以下,用铝土矿为原料,用焦炭作为还原用炭,反应温度在1 300~1 550 ℃范围内都可以得到金属铝。图5所示为1 450 ℃和150 Pa时金属铝的EDS谱,图6所示为金属铝的SEM像。可以看出:金属铝颗粒纯度较高,还含有少量的硅,由于铝土矿中的二氧化硅也发生了类似的反应,先生成气态低价氧化硅再歧化分解为金属硅;金属铝颗粒周围的粉状物为氟化物,同样是因为歧化分解反应分解出的金属铝和氟化物是同时进行,两者分离不及时引起。

图5 金属铝EDS谱
Fig.5 Energy-dispersive spectra of Al (1 450 ℃, 150 Pa)

图6 金属铝的SEM像
Fig.6 SEM micrographs of Al (1450 ℃, 150 Pa)
5 结论
1) 在100 kPa时,氧化铝、氟化铝与还原剂碳反应在1 941.76 K以上才能生成低价氟化铝;而当系统残余压力在100~10 Pa时,在1 486.32~1 373.28 K 以上即可以生成低价氟化铝;形成低价氟化铝后进行歧化分解反应。体系压力在100~10 Pa范围内,即歧化分解温度在 1 075.71~ 985.63 K间。
2) 采用真空碳热还原进行实验,以氧化铝、氟化铝为原料,在系统压力为150 Pa,反应温度在1 400 ℃时,在冷凝区得到能谱分析纯度为93.77%的金属铝,如果考虑分析时氧元素的误差,其纯度远大于93.77%,而且原料中的硅元素在此条件下没有被还原出来,说明反应温度和冷凝温度都比较好,便于硅与铝的分离;当以铝土矿、氟化铝为原料,在系统压力为150 Pa,反应温度在1 450 ℃得到纯度为95.13%的金属铝,硅含量为4.87%,说明矿石中的二氧化硅被还原出来,而且以气态硅化合物的形式在冷凝区与铝同时冷凝下来,控制冷凝区的温度使硅与铝分离可以提高金属铝纯度,为下一步实验研究提供了依据。
REFERENCES
[1]顾松青. 我国的铝土矿资源和高效低耗的氧化铝生产技术[J]. 中国有色金属学报, 2004, 14(S1): 91?97.
GU Song-qing. Alumina production technology with high efficiency and low consumption from Chinese bauxite resource[J]. The Chinese Journal of nonferrous Metals, 2004, 14(S1): 91?97.
[2]刘新星, 陈 帆, 海热提. 世界铝工业的可持续发展[J]. 轻金属, 2005(7): 3?6.
LIU Xin-xing, CHEN Fan, HAI Re-ti. The sustainable development of aluminum industry in the world[J]. Light Metals, 2005(7): 3?6.
[3]邱竹贤. 铝冶金物理化学[M]. 上海: 上海科学技术出版社, 1985: 346?347.
QIU Zhu-xian. Physical chemistry of aluminum metallurgy[M]. Shanghai: Shanghai Science & Technology Press, 1985: 346?347.
[4]邱竹贤. 21世纪伊始铝电解工业的新进展[J]. 中国工程科学, 2003, 5(4): 41?46.
QIU Zhu-xian. Development of aluminium electrolytic industry at the beginning of the 21st century[J]. Engineering Science, 2003, 5(4): 41?46.
[5]戚大光, 任锁堂. 炭热还原铝土矿的研究[J]. 化工冶金, 1989, 10(4): 1?9.
QI Da-guang, REN Suo-tang. Carbothermic reduction of aluminum-bearing ores[J]. Engineering Chemistry & Metallurgy, 1989, 10(4): 1?9.
[6]黄佩丽, 田荷珍. 基础元素化学[M]. 北京: 北京师范大学出版社, 1994: 282?283.
HUANG Pei-li, TIAN He-zhen. Basic elements chemistry[M]. Beijing: Beijing Normal University Press, 1994: 282?283.
[7]吴国元, 刘大春, 戴永年. 低价硫化物法从氧化铝直接炭还原制铝的动力学研究[J]. 真空科学与技术学报, 2004, 24(4): 263?266, 270.
WU Guo-yuan, LIU Da-chun, DAI Yong-nian. Dynamics study of extraction from Al2O3 by Al sub-sulphidization[J]. Chinese Journal of Vacuum Science and Technology, 2004, 24(4): 263?266, 270.
[8]吴国元, 戴永年. 铝膜的低价硫化铝歧化反应制备法[J]. 中国有色金属学报, 2003, 13(5): 1302?1305.
WU Guo-yuan, DAI Yong-nian. Filming on steel using sub-sulphide of Al[J]. The Chinese Journal of Nonferrous Metals, 2003, 13(5): 1302?1305.
[9]李秋霞, 陈为亮, 郑东帮, 戴永年. 真空下低价硫化物法直接提取铝的热力学研究[J]. 真空科学与技术学报, 2006, 26(2): 150?154.
LI Qiu-xia, CHEN Wei-liang, ZHENG Dong-bang, DAI Yong-nian. Thermodynamic study of direct aluminium extraction from aluminium sub-sulphide[J]. Chinese Journal of Vacuum Science and Technology, 2006, 26(2): 150?154.
[10]王平艳, 刘谋盛, 戴永年. 真空碳热还原氯化法从铝土矿炼铝[J]. 真空科学与技术学报, 2006, 26(5): 377?380.
WANG Ping-yan, LIU Mou-sheng, DAI Yong-nian. Vacuum metallurgy of AI from bauxite by carbothermic reduction- chlorination[J]. Chinese Journal of Vacuum Science and Technology, 2006, 26(5): 377?380.
[11]OTHMER D F. Method for producing aluminum metal directly form ore. U S 3793003[P]. 1974?10?10.
[12]ADAMS C M Jr. Aluminum production. U S 4188207[P]. 1980?2?12.
[13]叶大伦, 胡健华. 实用无机物热力学数据手册[M]. 第二版. 北京: 冶金工业出版社, 2002: 75?369.
YE Da-lun, HU Jian-hua. The handbook of inorganic thermodynamic data[M]. Beijing: Metallurgical Industry Press, 2002: 75?369.
[14]傅献彩. 物理化学[M]. 北京: 高等教育出版社, 1989: 250?386.
FU Xian-cai. Physical chemistry[M]. Beijing: Higher Education Press, 1989: 250?386.
[15]李秋霞, 罗志敏, 熊今春, 戴永年. 真空下低价化合物法提取铝的新工艺?原料烧结实验研究[J]. 真空, 2006, 43(2): 54?58.
LI Qiu-xia, LUO Zhi-min, XIONG Jin-chun, DAI Yong-nian. Experimental study on sintering to extract Al by low-valency compounds in vacuum[J]. Vacuum, 2006, 43(1): 54?58.
基金项目:国家重点基础研究发展计划资助项目(2007CB616908)
收稿日期:2007-11-13;修订日期:2008-03-25
通讯作者:李秋霞,副教授;电话:0871-5161583;E-mail: qiuxiali03@163.com
(编辑 何学锋)