二氧化硅在真空碳热-氯化法炼铝过程中的行为
袁海滨1,李秋霞2,朱富龙1,杨斌1,徐宝强1,戴永年1
(1. 昆明理工大学 真空冶金国家工程实验室,云南省有色金属真空冶金重点实验室,云南 昆明 650093;
2. 云南师范大学 化学化工学院,云南 昆明,650092)
摘要:采用XRD,EDS及热力学分析等方法,对二氧化硅在真空碳热-氯化法炼铝过程中的行为进行研究。研究结果表明:碳热还原过程SiO2在较低温度下发生碳热还原反应生成SiC,在更高的温度条件下碳热还原生成低价氧化硅SiO气体;另外还有一定量的SiC与Al4C3结合生成Al4SiC4。碳热还原过程生成的低价氧化硅SiO气体进入低温区歧解得到单质硅与二氧化硅;同时还有氧化铝碳热还原生成的低价氧化铝Al2O气体进入低温区与CO发生二次氧化反应生成氧化铝与碳,低价氧化铝Al2O与低价氧化硅SiO气体在低温区发生反应的可能性较小。碳热-氯化过程冷凝产物金属铝的EDS检测分析显示,SiO2碳热还原生成的低价氧化硅SiO歧解产物没有混入最终产物中,从而不会影响金属铝的纯度,该金属铝平均纯度达97.03%。
关键词:真空冶金;氯化铝;二氧化硅;铝
中图分类号:TF131;TF821 文献标志码:A 文章编号:1672-7207(2012)03-0803-06
Behavior of silica in vacuum metallurgy of aluminum production by carbothermic-chloride process
YUAN Hai-bin1, LI Qiu-xia2, ZHU Fu-long1, YANG Bin1, XU Bao-qiang1, DAI Yong-nian1
(1. National Engineering Laboratory for Vacuum Metallurgy, Key Laboratory for Nonferrous Metals Vacuum Metallurgy of Yunnan Province, Kunming University of Science and Technology, Kunming 650093, China;
2. School of Chemistry and Chemical Engineering, Yunnan Normal University, Kunming 650092, China)
Abstract: The behavior of silica in vacuum metallurgy of aluminum production by carbothermic-chloride process was investigated by XRD, EDS and thermodynamic analysis. The results show that SiC is formed at low temperature by SiO2 carbothermic reduction during alumina carbothermic reduction process, SiO(g) is generated at higher temperature which would disproportion into simple substance of silicon and silica at low temperatures, and Al4SiC4 is formed by a certain SiC reacting with Al4C3. Reoxidation reaction could occur between Al2O(g) and CO, which is generated by alumina carbothermic reduction. However, little possibility of reaction occurs between SiO(g) and Al2O(g) at low temperature area. SiO(g) disproportion product didn’t enter into aluminum metals by EDS analysis for the condensation products during carbothermic-chloride process, which means that final products can not be polluted by it, and the average purity of aluminum metal attains 97.03%.
Key words: vacuum metallurgy; AlCl; SiO2; aluminum
金属铝因其具有优异的物理化学性质而被广泛应用于国民经济生产中[1]。目前世界上铝的生产主要采用冰晶石-氧化铝融盐电解法炼铝工艺[2],然而,在过去的100多年里,人们从未放弃研究新的方法来代替电解法。其中炼铝新方法主要有常压碳热还原法[3-4]、真空碳热还原法[5]以及真空碳热-卤化(含硫化)法[6-8]等,但常压碳热还原法存在还原温度高(通常需 2 000 ℃以上)且金属铝与渣相难以分离的缺点[9-10],而真空碳热还原法与碳热-卤化法由于在真空条件下进行,能降低金属铝的还原温度,且金属铝易于与渣相分离,从而能保证金属铝的纯度[11]。真空碳热还原法与碳热-卤化法炼铝过程,在原料的选择上,没有冰晶石-氧化铝融盐电解法要求严格,含铝的原料可以是氧化铝,也可以是含铝的矿物(如铝土矿等),通常铝的矿物原料中普遍含有二氧化硅等氧化物,因此,二氧化硅在炼铝过程的行为引起研究者的高度重视。目前,国内外学者对二氧化硅在SiO2-C二元系中的碳热过程研究较多[12-15],李秋霞等[16]对二氧化硅在真空碳热-氟化法炼铝过程的行为有过研究,但二氧化硅在真空碳热-氯化法炼铝过程中的行为研究鲜见报道。本文作者使用分析纯氧化铝、石墨还原剂、无水氯化铝、二氧化硅为原料,对二氧化硅在该法炼铝过程的行为进行试验研究与热力学验证。
1 实验
实验用氧化铝、石墨、无水氯化铝、二氧化硅均为分析纯。具体步骤如下:称取摩尔比为3:1的石墨与氧化铝共计20.0 g,再分别添加质量分数为0~10.0% 的SiO2,混合均匀,在2~4 MPa的压力下制成(直 径×高)20 mm×5 mm块后放入干燥箱内,在150 ℃下干燥180 min后,取出并置于真空炉内坩埚中,密封真空炉。打开水冷装置系统,抽真空至极限始升温,至1 803~1 853 K始恒温60~90 min后,加热炉底无水氯化铝升华装置至373~403 K(50~200 Pa),无水氯化铝升华并沿着导气管进入高温反应坩埚内进行碳热-氯化反应,此过程恒温40~90 min。待炉内系统压力降低并稳定后,关闭所有加热系统,继续抽真空至室温。关闭水冷系统,开炉取样并称量质量。
采用Rigaku D/max-3B型X线衍射仪分析反应残渣与冷凝产物的物相,Cu Ka辐射源,扫描区间为10°~100°,管电压为50 kV,管电流为100 mA。采用Philips XL30ESEM-TMP型扫描电子显微镜观察冷凝产物的形貌,用能谱仪(EDAX产PHOENIXTM)分析冷凝产物表面元素含量。
2 结果与分析
2.1 碳热还原过程的XRD分析
在氧化铝与石墨中添加一定量的SiO2后,在1 803~ 1 853 K及50~100 Pa的条件下,碳热还原保温60~ 90 min,残渣的XRD分析结果如图1所示。

图1 SiO2添加量不同时氧化铝碳热还原过程残渣的XRD谱
Fig.1 XRD patterns of residues of Al2O3 carbothermic reduction under different SiO2 additions
由图1可知:添加一定量的SiO2后,渣相中的Al4O4C的衍射峰强度出现一定程度上的减弱,Al4C3的衍射峰完全消失,而渣相中产生了2种新的物相,即Al4SiC4和SiC,其中Al4SiC4的衍射峰较为明显。另外,渣相中还有未完全碳热还原的氧化铝和碳(石墨)。图2所示为含SiO2物料经碳热还原后,在冷凝器入口处收集(靠近高温碳热还原区域)得到的冷凝产物的XRD谱。由图2可以看出:冷凝物中含有C和Si(单质硅),Al2OC和Al2O3。据此,推断碳热还原过程可能发生如下反应:
SiO2+3C=SiC+2CO (g) (1)
2Al2O3+3C=Al4O4C+2CO (g) (2)
Al2O3+2C=Al2O(g)+2CO(g) (3)
Al2O3+Al4C3=3Al2OC (4)
Al2O3+3C=Al2OC+2CO(g) (5)
SiO2+2C=Si+2CO(g) (6)
SiO2+2SiC=3Si+2CO(g) (7)
SiO2+C=SiO(g)+CO(g) (8)
碳热还原过程产生的气态物质进入低温冷凝区域内,可能发生如下反应:
Al2O(g)+2SiO(g)=2Si+Al2O3 (9)
Al2O(g)+2CO(g)=Al2O3+2C (10)
2SiO(g)=SiO2+Si (11)
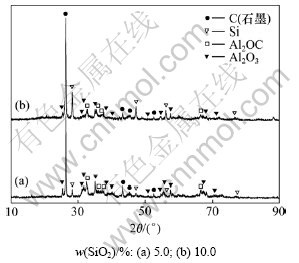
图2 含SiO2物料经碳热还原后冷凝物的XRD谱
Fig.2 XRD patterns of condensates after carbothermic reduction with different SiO2 additions
2.2 碳热还原过程的热力学分析
在系统压力为100 Pa的条件下,对上述反应方程式(1)~(11)进行热力学分析[17],结果见图3和4所示。
对图3和图4中各式的?GT 与T关系曲线进行线性拟合,根据拟合的线性方程计算各反应式在系统压力为100 Pa下的初始反应温度,分别如表1和表2所示。

图3 100 Pa时式(1)~(8)碳热还原过程?GT与T的关系
Fig.3 Relationships between ?GT and T of Eqs. (1)-(8) during carbothermal reduction
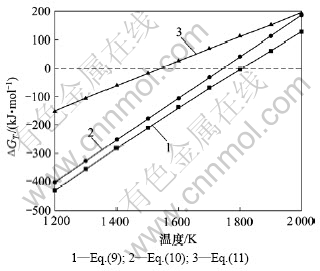
图4 100 Pa时式(9)~(11)冷凝过程?GT与T的关系
Fig.4 Relationships between ?GT and T of Eqs. (9)-(11) during condensation process
表1 100 Pa时式(1)~(8)碳热还原过程的初始反应温度
Table 1 Initial temperatures for Eqs.(1)-(8) by carbothermic reduction process at 100 Pa

表2 100 Pa时式(9)~(11)碳热还原过程的初始反应温度
Table 2 Initial temperatures for Eqs.(9)-(11) by carbothermic reduction process at 100 Pa

据图1可知:碳热还原过程在不加入SiO2时,渣相中含有一定量的Al4C3,而添加SiO2后Al4C3衍射峰消失,Kamal[18]指出:由于SiO2碳热反应生成SiC,而SiC的存在将抑制Al4C3的生成。然而,结合Si-Al-C-O相图[19]分析,结果见图5所示。本文作者认为:Al4C3是被SiC所消耗转化成结构稳定的Al4SiC4,1 mol Si消耗4 mol Al,从而不利于碳热-氯化法炼铝过程金属铝直收率的提高[20],该过程发生如下反应:
Al4C3+SiC=Al4SiC4 (12)
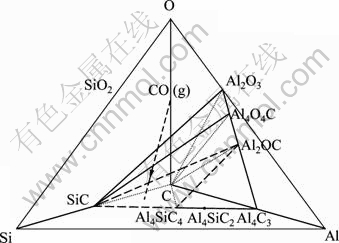
图5 Si-Al-C-O相图
Fig.5 Phase diagram of Si-Al-C-O
Forster等采用显微镜照相、X线和化学分析法对于不同质量配比下的Al2O3-C二元系反应生成的产物进行物相鉴定分析,认为该过程存在中间化合物Al4O4C和Al2OC,而这些化合物的存在现已被其他研究者证实[21]。结合Si-Al-C-O相图[19]分析可知:Al2OC可能由式(4)或式(5)生成,而据图3的热力学分析结果可知:在100 Pa时,式(4)与式(5)反应进行的初始温度分别为1 827.0 K和1 708.9 K。因此,可推断Al2OC的生成来自于反应式(5):Al2O3+3C=Al2OC+2CO(g),Al2OC随Al2O3-C系统碳热还原反应生成的CO气体沿着系统抽真空方向而被带入冷凝器底部,从冷凝器底部收集的物料的XRD分析显示,物料中含有一定量的Al2OC(见图2)。
根据图3与表1的热力学分析,比较SiO2参与的碳热还原反应方程式(1),(6),(7)和(8)可知:该过程发生反应的先后顺序依次为:式(1),式(6),式(8),式(7),即SiO2碳热过程先生成SiC,在较高的温度下生成单质金属硅及低价氧化硅SiO气体,在更高的温度下才发生SiO2与SiC生成单质硅的反应。然而,在本实验条件下,碳热还原过程渣相的XRD检测显示,该过程没有单质硅的生成,而在冷凝物中检测到一定量的单质硅。因此,可确定SiO2在碳热过程主要发生式(1),(8)以及式(12)反应。
SiO2在碳热还原过程反应生成的低价氧化硅SiO气体具有高温稳定、低温歧解的性质,SiO气体进入低温冷凝区而歧解得到单质硅及氧化硅,见图2所示,歧解生成的氧化硅呈非晶状态,因此,在图2的XRD检测显示为较多的非尖锐衍射峰。
另外,图4和表2热力学分析显示:式(9),(10)和(11)在100 Pa的系统压力下的初始温度分别为 1 806.4,1 745.5和1 544.6 K。由此可说明,该过程发生反应的先后顺序依次为:式(11),式(10),式(9),即最先发生低价氧化硅SiO气体的歧解反应,其次是式(10)低价氧化铝与一氧化碳的反应,而式(9)低价氧化铝与低价氧化硅气体的初始反应温度较高,然而低价氧化硅已在较低温度条件下发生歧解反应,因此,发生式(9)反应的可能性较小。
2.3 碳热-氯化过程冷凝产物的EDS分析
物料经碳热还原后保温60~90 min,加热升华装置至373~403 K,无水氯化铝升华进入高温反应坩埚内与碳热还原后的渣相进行碳热-氯化反应,相关反应如下[11]:
Al4O4C(s)+3C(s)+2AlCl3(g)→6AlCl(g)+4CO(g) (13)
Al4C3(s)+Al2O 3(s)+3AlCl3(g)→9AlCl(g)+3CO(g) (14)
Al4O4C(s)+Al4C3(s)+Al2O3(s)+3C(s)+5AlCl3(g)→15AlCl(g)+7CO(g) (15)
碳热-氯化反应生成的低价氯化铝AlCl气体进入低温区发生歧解反应生成金属铝与三氯化铝,由于金属铝与三氯化铝的冷凝温度不同而分别在不同温度区域冷凝。对碳热-氯化过程的冷凝产物金属铝进行SEM与EDS分析,结果分别见图6和表3所示。
表3 碳热-氯化过程冷凝产物金属铝的EDS分析结果(质量分数)
Table 3 EDS analysis results for condensation products by carbothermic-chloride process %

文献[22]研究指出:在系统压力为10~100 Pa时,低价氯化铝AlCl歧解得到金属的初始温度为950~ 1 050 K,而100 Pa下式(11)低价氧化硅SiO气体歧解得到单质硅与二氧化硅的初始温度为1 544.6 K。由此可知,低价氧化硅SiO与低价氯化铝AlCl气体歧解的初始温度不同而分别将在不同的温度区域发生反应。实验结果显示,在较低的冷凝区得到金属铝,而其他冷凝物在温度较高的区域得到。表3中冷凝产物金属铝的EDS检测分析也说明,SiO2碳热还原生成的低价氧化硅SiO歧解产物没有混入最终产物金属铝中,从而不会影响金属铝的纯度。从表3可知:金属铝纯度较高,其平均纯度达97.03%。
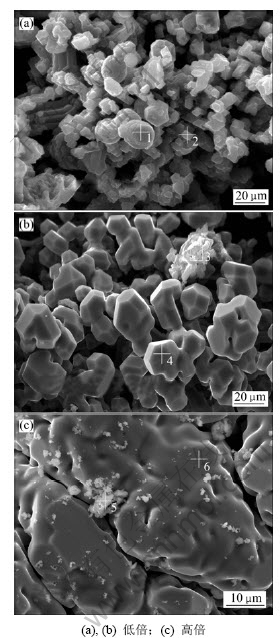
图6 冷凝产物的SEM像
Fig.6 SEM images of condensation products
3 结论
(1) 碳热还原过程的XRD与热力学分析表明,SiO2在较低温度下发生碳热还原反应生成SiC,在更高的温度条件下碳热还原生成低价氧化硅SiO气体;由于1 mol Si消耗4 mol Al,生成结构稳定的Al4SiC4,从而不利于碳热-氯化法炼铝过程金属铝直收率的 提高。
(2) 碳热还原过程生成的低价氧化硅SiO气体进入低温区歧解得到单质硅与二氧化硅,歧解生成的二氧化硅呈非晶状态;同时还有氧化铝碳热还原生成的低价氧化铝Al2O气体进入低温区与CO发生二次氧化反应生成氧化铝与碳。而热力学研究指出,低价氧化铝Al2O与低价氧化硅SiO气体在低温区发生反应的可能性较小。
(3) 碳热-氯化过程冷凝产物金属铝的EDS检测分析显示,SiO2碳热还原生成的低价氧化硅SiO歧解产物没有混入最终产物中,从而不会影响金属铝的纯度,该金属铝的平均纯度达97.03%。
参考文献:
[1] 顾松青. 我国的铝土矿资源和高效低耗的氧化铝生产技术[J]. 中国有色金属学报, 2004, 14(S1): 91-97.
GU Song-qing. Alumina production technology with high efficiency and low consumption from Chinese bauxite resource[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(S1): 91-97.
[2] 杨重愚. 轻金属冶金学[M]. 北京: 冶金工业出版社, 1996: 1-3.
YANG Chong-yu. Light metals metallurgy[M]. Beijing: Metallurgical Industry Press, 1996: 1-3.
[3] Johansen K, Aune J A. Method and reactor for production of aluminum by carbothermic reduction of alumina: US, 6440193B1[P]. 2002-08-27.
[4] Aune J A, Enebakk Y, Johansen K. Method and reactor for production of aluminum by carbothermic reduction of alumina: US, 6805723B2[P]. 2004-10-19.
[5] 王鹏程, 戴永年. 氧化铝真空碳热还原实验研究[D]. 昆明: 昆明理工大学冶金与能源工程学院, 2009: 26-71.
WANG Peng-cheng, DAI Yong-nian. Experimental study on alumina carbothermic reduction in vacuum[D]. Kunming: Kunming University of Science and Technology. Faculty of Metallurgy and Energy Engineering, 2009: 26-71.
[6] 吴国元, 刘大春, 戴永年. 低价硫化物法从氧化铝直接炭还原制铝的动力学研究[J]. 真空科学与技术学报, 2004, 24(4): 263-266, 270.
WU Guo-yuan, LIU Da-chun, DAI Yong-nian. Dynamic study of Al extraction from Al2O3 by Al sub-sulphidization[J]. Chinese Journal of Vacuum Science and Technology, 2004, 24(4): 263-266, 270.
[7] 王平艳, 刘谋盛, 戴永年. 真空碳热还原氯化法从铝土矿炼铝[J]. 真空科学与技术学报, 2006, 26(5): 377-380.
WANG Ping-yan, LIU Mou-sheng, DAI Yong-nian. Vacuum metallurgy of Al from bauxite by carbothermic reaction- chlorination[J]. Chinese Journal of Vacuum Science and Technology, 2006, 26(5): 377-380.
[8] 李秋霞, 陈为亮, 戴永年. 真空低价氟化铝歧化分解制备铝[J]. 中国有色金属学报, 2008, 18(8): 1550-1554.
LIU Qiu-xia, CHEN Wei-liang, DAI Yong-nian. Preparation of aluminum by disproportion of sub-fluoride in vacuum[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(8): 1550-1554.
[9] Choate W, Green J. Technoeconomic assessment of the carbothermic reduction process for aluminum production[J]. Light Metal, 2006, 135(10): 445-450.
[10] Lindstad T. Method for recovering aluminum vapor and aluminum suboxide from off-gases during production of aluminum by carbothermic reduction of alumina: US, 6530970B2[P]. 2003-03-11.
[11] 袁海滨, 冯月斌, 杨斌, 等. 氧化铝在低价氯化铝法炼铝过程中的行为[J]. 中国有色金属学报, 2010, 20(4): 777-783.
YUAN Hai-bin, FENG Yue-bin, YANG Bin, et al. Disproportion reaction of alumina in vacuum metallurgy of aluminum[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(4): 777-783.
[12] LI Ke-zhi, WEI Jian, LI He-jun, et al. Silicon assistant carbothermal reduction for SiC powders[J]. Journal of University of Science and Technology Beijing, 2008, 15(4): 484-488.
[13] Pivac B, Dubcek P, Capan I, et al. Structural analysis of annealed amorphous SiO/SiO2 superlattice[J]. Thin solid films, 2008, 516(8): 6796-6799.
[14] Silva P C, Figueiredo J L. Production of SiC and Si3N4 whiskers in C+SiO2 solid mixtures[J]. Materials Chemistry and Physics, 2001, 72(10): 326-331.
[15] Kovacevic I, Dubcek P, Duguay S, et al. Silicon nanoparticles formation in annealed SiO/SiO2 multilayers[J]. Physica E, 2007, 38(12): 50-53.
[16] 李秋霞, 朱冬梅, 刘永成, 等. 二氧化硅在真空低价法制备铝过程中的歧化行为研究[J]. 真空科学与技术学报, 2009, 29(1): 35-37.
LI Qiu-xia, ZHU Dong-mei, LIU Yong-cheng, et al. Disproportion reaction of silica in vacuum metallurgy of aluminum[J]. Chinese Journal of Vacuum Science and Technology, 2009, 29(1): 35-37.
[17] Barin I. 纯物质热化学数据手册[M]. 程乃良, 牛四通, 徐桂英, 译. 北京: 科学出版社, 2003: 17-1505.
Barin I. Thermochemical date of pure substances[M]. CHENG Nai-liang, NIU Si-tong, XU Gui-ying, trans. Beijing: Science Press, 2003: 17-1505.
[18] Kamal J. Processing of ceramic matrix SiC-Al composites[J]. Journal of Materials Processing Technology, 1999, 38(2): 361-368.
[19] Baud S, Thevenot F, Pisch A, et al. High temperature sintering of SiC with oxide additives (Ⅰ): Analysis in the SiC-Al2O3 and SiC-Al2O3-Y2O3 systems[J]. Journal of the European Ceramic Society, 2003, 23(1): 1-8.
[20] 袁海滨, 朱富龙, 杨斌, 等. 氧化铝真空碳热还原-氯化法炼铝的工艺研究[J]. 真空科学与技术学报, 2010, 30(6): 582-587.
YUAN Hai-bin, ZHU Fu-long, YANG Bin, et al. Process of aluminum production by alumina carbothermic reduction- chlorination in vacuum[J]. Chinese Journal of Vacuum Science and Technology, 2010, 30(6): 582-587.
[21] 邱竹贤. 铝冶金物理化学[M]. 上海: 上海科学技术出版社, 1985: 350-351.
QIU Zhu-xian. Physical chemistry for aluminum metallurgy[M]. Shanghai: Shanghai Science and Technology Press, 1985: 350-351.
(编辑 陈爱华)
收稿日期:2011-04-20;修回日期:2011-06-28
基金项目:国家自然科学基金—云南联合基金重点资助项目(u0837604);高等学校博士学科点专项科研基金资助项目(20095314110003)
通信作者:袁海滨(1984-),男,江西吉安人,硕士,从事有色金属真空冶金研究;电话:0871-5161583;E-mail: yuanhaibin101@163.com