


Trans. Nonferrous Met. Soc. China 22(2012) 1224-1231
Characterization of adsorptive capacity and mechanisms on adsorption of copper, lead and zinc by modified orange peel
FENG Ning-chuan1,2, GUO Xue-yi1
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Basic Medical Science, Ningxia Medical University, Yinchuan 750004, China
Received 9 March 2011; accepted 28 February 2012
Abstract: A novel adsorbent was prepared by modifying orange peel with sodium hydroxide and calcium chloride. The morphological and characteristics of the adsorbent were evaluated by infrared spectroscopy (IR), scanning electron microscopy (SEM) and N2-adsorption techniques. The adsorption behavior of Cu2+, Pb2+ and Zn2+ on modified orange peel (SCOP) was studied by varying parameters like pH, initial concentration of metal ions. Equilibrium was well described by Langmuir equation with the maximum adsorption capacities for Cu2+, Pb2+ and Zn2+ of 70.73, 209.8 and 56.18 mg/g, respectively. Based on the results obtained in batch experiments, breakthrough profiles were examined using a column packed with SCOP for the separation of small concentration of Pb2+ from an excess of Zn2+ followed by elution tests. Ion exchange with Ca2+ neutralizing the carboxyl groups of the pectin was found to be the predominant mechanism.
Key words: orange peel; chemical modification; adsorption capacity; adsorption mechanism
1 Introduction
The release of heavy metals into our environment is still large. In certain areas of the world it is even increasing. The pollution of water resources due to the disposal of heavy metals has been an increasing worldwide concern for the last few decades. It is well known that some metals have poisonous or otherwise harmful effects on many forms of life. The numerous metals, which are significantly toxic to human beings and ecological environments, include copper, lead, cadmium, zinc and nickel, etc. Currently used water treatment technologies involving chemical precipitation, evaporation, electrochemical treatment, and the use of ion exchange resins are expensive and sometimes ineffective, especially when metals are present in solution at very low concentrations [1,2]. Therefore, numerous approaches have been studied for the development of cheap and effective metal sorbents, such as fly ash [3], peat [4,5], microbial biomass [6], and agricultural byproducts [7-9]. Another cheap and unconventional adsorbent particularly suited to adsorption is fruit residue, such as apple, banana, and orange peel [10-12]. The use of orange peel as an adsorbent material presents strong potential due to its high content of cellulose, pectin (galacturonic acid), hemicellulose and lignin. These components bear various polar functional groups including carboxylic and phenolic acid groups to be involved in metal binding [13,14] and are biopolymers admittedly associated to the removal of heavy metals [15]. As a low-cost adsorbent, orange peel is an attractive option for the adsorption removal of dissolved metals.
Some of the advantages of using plant wastes for wastewater treatment include simple technique, requiring little processing, good adsorption capacity, selective adsorption of heavy metal ions, low cost, free availability and easy regeneration. However, the application of untreated plant wastes as adsorbents can also bring several problems, such as low adsorption capacity, high chemical oxygen demand (COD) and biological chemical demand (BOD) as well as total organic carbon (TOC), due to release of soluble organic compounds contained in the plant materials [16-18]. The increase of the COD, BOD and TOC can cause depletion of oxygen content in water and can threaten the aquatic life. Therefore, plant wastes need to be modified or treated before being applied to decontamination of heavy metals [19,20]. In the present study, a simple and economic preparation of the adsorbent from orange peel was performed and adsorption experiments were conducted to determine the metal adsorption mechanisms. The effects of pH and initial concentration of metal ions on the adsorption of Cu2+, Pb2+, and Zn2+ ions were also investigated.
2 Experimental
2.1 Chemicals
Aqueous solutions of Cu2+, Pb2+, and Zn2+ ions for the batch experiments were prepared by dissolving corresponding individual metal chlorides (CuCl2·2H2O, ZnCl2·7H2O) of analytical grade or nitrate (Pb(NO3)2) salts in 0.1 mol/L hydrochloric acid solution and in a solution of 0.1 mol/L N-2-hydroxyethylpiperazine-N-2- ethanesulphonic acid (HEPES), a buffer reagent, followed by mixing these two solutions at an ordinary volume ratio to adjust pH, while dilute sodium hydroxide solution was used to prepare solutions of higher pH. In addition, nitrate salts of Pb2+ and Zn2+ of analytical grade were used to prepare sample solution of chromatographic separation test.
2.2 Preparation of adsorbent from orange peel
Orange peel (OP) was used as the raw material for the preparation of adsorbent. The OP, which was collected from a local plantation, was cut into small pieces, washed several times with double distilled water and dried at 60 °C. The product was crushed and sieved to obtain an average particle size lower than 0.45 mm and then treated with sodium hydroxide and calcium chloride solutions to improve the capacity of metal adsorption. For this purpose, 100 g of dried OP was soaked in 500 mL ethanol, 250 mL NaOH (0.8 mol/L) and 250 mL CaCl2 (0.8 mol/L) solution for 20 h. After repeated decantation and filtration, the modified biomass (SCOP) was washed with double distilled water until the solutions reached a pH value of 7.0, and then dried and final sample was obtained for the test use.
2.3 Instruments used for biosorbent characterizations
The specific surface area values of OP and SCOP were measured by BET N2 adsorptions on a Quantasorb model surface area analyzer (ASAP 2010). Surface morphology of the adsorbent was determined by using a JSM-5600LV scanning electron microscope equipped with energy dispersive X-ray (EDX) analysis. The potassium dichromate method was used to measure the chemical oxygen demand (COD). FTIR spectroscopy was used to identify the chemical groups present in the adsorbent. Spectra of the adsorbent before and after modifying were recorded in the wavenumber range 400-4000 cm-1 using a JASCO-410 model FT-IR spectrometer with the samples prepared as KBr discs. The calcium and copper concentrations in SCOP before and after the adsorption were quantified by using a Philips Magix 2424 X-ray fluorescence spectrometer.
2.4 Batchwise adsorption tests
Batch adsorption experiments were conducted at 298 K by agitating 0.10 g of adsorbent with 25 mL of metal ions solution of desired concentration in 100 mL stoppered conical flask using a shaking thermostat machine at a speed of 120 r/min. The effect of solution pH on the equilibrium adsorption of Cu2+, Pb2+ and Zn2+ was investigated under similar experimental conditions between pH 2.0 and 6.0. The effect of contact time on batch experiments was examined by varying the contact time of suspensions from 0 to 120 min. In the isotherm experiments, 0.10 g of adsorbent was added in 25 mL of metal ions solution at various concentrations. Once the pre-set contact time (12 h) reached, the samples were withdrawn and centrifuged at 4000 r/min for 5 min and the supernatant solutions were analyzed for the residual metal ion concentration and for the Ca2+ ions released by the SCOP by using Shimadzu model AA-6650 atomic absorption spectrophotometer. The amount of adsorption at equilibrium (qe) was calculated by the following equation (1) and the adsorption efficiency, A, of the metal ion was calculated from equation (2):
 (1)
(1)
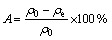 (2)
(2)
where ρ0 and ρe are the initial and equilibrium concentrations of Cu2+, Pb2+ and Zn2+ ions, respectively; V is the volume of the solutions; m is the amount of adsorbent used (g). All the adsorption experiments were conducted in duplicate, and the mean values were calculated.
2.5 Column adsorption test
Mutual adsorptive separation of small concentration of Pb2+ from large excess of Zn2+ was carried out using a glass column of 8 mm in internal diameter packed with 0.10 g of SCOP. The column was conditioned by passing water of pH=2.5 overnight. The sample solution containing 10 mg/L of Pb2+ and 100 mg/L of Zn2+ whose pH was maintained at 2.5 was percolated into the column at a constant flow rate of 6.1 mL/h using a peristaltic pump (IWAKI PST-100N, Japan). Effluent samples were collected at each 1 h interval of time by using a fraction collector (BIORAD Model 2110 fraction collector) to measure the corresponding metal ion concentrations.
For the elution tests, the column was pre-washed with double distilled water so as to expel any unbound metal ions. Hydrochloric acid solution (0.1 mol/L) used as the eluent was percolated into the column at the same constant flow rate of 6.1 mL/h using the peristaltic pump. The concentration of the eluted metal ions collected at each interval in the tube of fraction collector was measured by using Shimadzu model AA-6650 atomic absorption spectrometer.
3 Results and discussion
3.1 Adsorbent characterizations
The SEM images clearly reveal the surface texture and morphology of the adsorbent (Fig. 1). The surface morphology of SCOP is different from that of OP. After treatment with sodium hydroxide and calcium chloride, SCOP has more irregular and porous structure than that of OP, and therefore more specific surface area. This surface characteristic would result in the higher adsorption capacity. The surface areas of OP and SCOP were observed to be 0.828 m2/g and 1.496 m2/g by BET method.
The FT-IR spectra of OP and SCOP are shown in Fig. 2. The broad and intense absorption peaks at 3350 cm-1 correspond to the O—H stretching vibrations of cellulose, pectin, absorbed water, hemicellulose, and lignin. The peaks observed at 2919 cm-1 can be attributed to the C—H stretching vibrations of methyl, methylene and methoxy groups. The presence of the peak at 1744 cm-1 and 1649 cm-1 in the OP spectrum indicates the ester carbonyl (C=O) groups and carboxylate ions (COO-) stretching band of pectin, respectively [21]. The vibrations at 1430-1455 cm-1 could be due to aliphatic and aromatic (C—H) groups in the plane deformation vibrations of methyl, methylene and methoxy groups. The bands in the range of 1300-1000 cm-1 can be assigned to the C—O stretching vibration of carboxylic acids and alcohols.
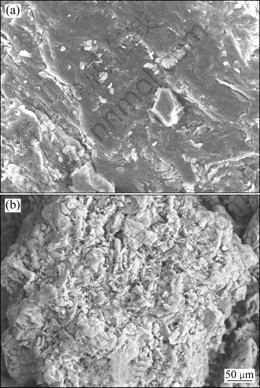
Fig. 1 SEM images of OP (a) and SCOP (b)
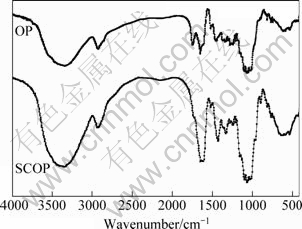
Fig. 2 FTIR analysis of OP and SCOP
The FTIR spectra of SCOP show a variation in the characteristic —COOH bands: the non-ionized (methyl- lated or protonated) —COOH stretching peaks at 1744 and 1649 cm-1 in the OP shift after the demethylation process by treating with NaOH-CaCl2 to a characteristic symmetrical and asymmetrical COO- stretching at 1648 and 1422 cm-1. This shift in the peaks confirms a decrease of the number of methylated —COOH groups [22].
3.2 Effect of biomass treatment on adsorption
The work done by BAIG et al [23] on the binding of Pb(II), Cu(II), Ni(II), Cd(II), Zn(II), Cr(Ⅲ) and Cr(Ⅵ) to the inactivated biomass of Solanum elaeagnifolium has suggested that carboxyl groups (—COOH) are responsible to some extent for the binding of metal ions. This means that increasing the number of carboxylate ligands in the biomass can enhance metal binding. Cellulose, pectin, hemicellulose, and lignin, which are major constituents of orange peel, contain methyl esters that do not bind metal ions significantly. However, these methyl esters can be modified to carboxylate ligands by treating the biomass with a base such as sodium hydroxide, thereby increasing the metal-binding ability of the biomass. The hydrolysis reaction of the methyl esters is as follows:
R—COOCH3+NaOH=R—COO-+CH3OH+Na+
Calcium, as a divalent metal cation, has a precipitation effect on the polysaccharides that contain carboxyl groups, such as pectin or alginate, and can be removed from solution and retained in the gel structure, according to the “egg-box model” [24]. The addition of calcium chloride makes the pectin acid in OP precipitate and reduces its solubility in solution. Therefore, chemically modifying the biomass with sodium hydroxide and calcium chloride solutions increase the number of carboxylate ligands that would enhance the binding ability of the biomass. The metal-binding capacities of the untreated and NaOH-CaCl2 treated biomass are compared in Table 1. An increase of approximately 30% in removed Pb2+ ions is observed.
Table 1 Effect of biomass treatment on Pb2+ adsorption

In addition, after adsorption by SCOP, the solution of copper ions is colorless, but after adsorption by OP, it is yellow. The fact may be due to the outflow of pigment in OP when agitated with copper ions solution, which leads to very high COD of solution. However, most pigment and other soluble organic compounds contained in the OP could be removed by treatment process [17]. So, SCOP is more stable and makes the solution have a low COD.
3.3 Effect of pH on adsorption
Figure 3 shows the adsorption behavior of SCOP for Cu2+, Pb2+and Zn2+ wherein adsorption of metal ions is plotted against equilibrium pH. The effect of pH is distinctive. Adsorption efficiencies are 83.5%, 93.2%, and 8.0% at a solution pH of 2.5 for Cu2+, Pb2+ and Zn2+, respectively, but they increase when solution pH increases from 2.5 to 6.0 (sharply for Zn2+). At pH 5.5, adsorption efficiencies are 93.7%, 99.4%, and 86.6% for Cu2+, Pb2+ and Zn2+, respectively. However, at pH value higher than 5.5, these metal ions precipitated because of the high concentration of OH- ions in the solution so that adsorption experiments at these pH values could not be performed well [25]. In order to examine the adsorption potential of the SCOP and to ensure that the heavy metals exist in their ionic states during adsorption, the pH values in subsequent experiments were controlled at 5.0 for Cu2+, 5.5 for Pb2+ and Zn2+.
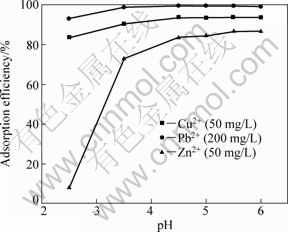
Fig. 3 Effect of pH on adsorption efficiency of Cu2+, Pb2+ and Zn2+
The order of the selectivity with respect to the width of pH range among the tested metal ions is as follows: Pb2+ > Cu2+ > Zn2+. This fact suggests the feasibility of mutual separation of metals discussed herein themselves by specifying pH values.
3.4 Effect of contact time on adsorption
The effect of contact time on adsorption was studied between 0 and 120 min. The results are presented in Fig. 4. The metal uptake is very rapid and the equilibrium is reached within 10 min. After this equilibrium period, the amount of adsorbed Cu2+, Pb2+ and Zn2+ ions does not significantly change with time. Therefore, 2 h of contact time is chosen as the adsorption time for the experimental test to ensure that equilibrium is achieved.
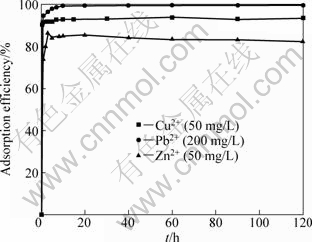
Fig. 4 Effect of contact time on adsorption efficiency of Cu2+, Pb2+ and Zn2+
3.5 Effect of initial concentration of metal ions: Adsorption isotherms
Figure 5 shows the adsorption isotherms of Cu2+, Pb2+ and Zn2+. The adsorption increases with the increase in equilibrium concentration at a low metal ion concentration and tends to approach constant values for each metal ion at their high concentration, suggesting that these metal ions are adsorbed onto SCOP according to the Langmuir adsorption. The fixed number of active sites eventually limits the adsorption of metal ions and a resulting plateau can be observed. The maximum adsorption capacity may well be described by Langmuir parameter, qmax, as shown in Table 2. The Langmuir parameters (qmax and b) provide useful information regarding the equilibrium sorption process behavior. As it can be seen from Table 2, equilibrium data agree well with the Langmuir model, which illustrates that the adsorption on the surface of SCOP is a monolayer adsorption. The maximum adsorption capacities evaluated are 70.73 mg/g for Cu2+, 209 mg/g for Pb2+, 56.18 mg/g for Zn2+, respectively and are higher than those with OP (Table 3). A comparison of adsorption capacities (qmax) of SCOP and some other adsorbents reported in literatures are listed in Table 3. The adsorption capacities of SCOP for Cu2+, Pb2+ and Zn2+ are higher than those of the majority of other biomasses mentioned. Therefore, it can be noteworthy that the SCOP has an important potential for the removal of Cu2+, Pb2+ and Zn2+ ions from aqueous solution.
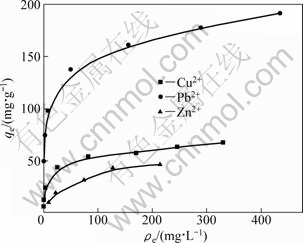
Fig. 5 Adsorption isotherms of Cu2+, Pb2+ and Zn2+
Table 2 Langmuir model parameters for adsorption isotherm

3.6 Adsorptive separation of binary metal ion mixture by using packed column
Based on the batchwise adsorption tests of various metal ions on SCOP as shown in Fig. 3 and a remarkable adsorption capacity for Pb2+ observed in Fig. 5, a mutual chromatographic separation test was carried out between Pb2+ and Zn2+, namely, a small amount of Pb2+ was separated from a large excess of Zn2+ using a column packed with the SCOP. Figure 6(a) shows the breakthrough profiles of Pb2+ and Zn2+ under the conditions described in the figure legend. From the figure it is clear that zinc breaks through immediately after the feed flow is started, since Pb2+ is much more selectively adsorbed than Zn2+, as shown in Fig. 3. Figure 6(b) shows the elution profiles of both metal ions with 0.1 mol/L hydrochloric acid solutions from the loaded column after the breakthrough of Pb2+. It is clearly seen that Pb2+ is eluted at a very high concentration, as high as 37 times compared with the feed concentration. Also, almost equal areas were observed in both breakthrough and elution curves of Pb2+. This means that 100% elution was achieved by using 0.1 mol/L hydrochloric acid solutions. These results suggest that effective mutual separation and pre-concentration of Pb2+ away from Zn2+ using SCOP can be satisfactorily achieved.
Table 3 Adsorption capacities of various adsorbents
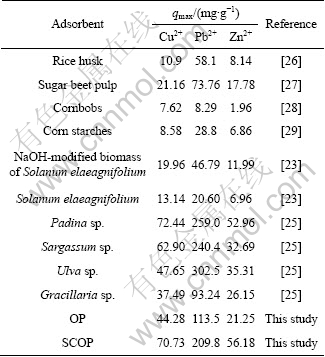
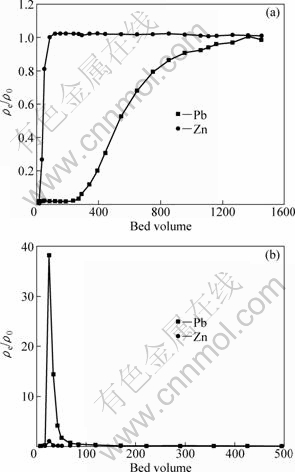
Fig. 6 Breakthrough profiles of Pb2+ and Zn2+ from column packed with SCOP under conditions of feed concentration of Pb2+=10 mg/L and Zn2+=100 mg/L, pH 2.5, mass of adsorbent packed in bed=0.10 g, feed rate=6.1 mL/h (a) and elution profiles of Pb2+ and Zn2+ from loaded column of SCOP by 0.1 mol/L HCl solution, feed rate=6.1 mL/h (b)
3.7 Mechanism of adsorption
The SCOP can adsorb the metal ions through electrostatic interaction and/or ion exchange, complexation, or by a combination of all the processes. Determination of calcium release during adsorption in combination with energy dispersive X-ray analysis was carried out to understand the mechanistic principle involved in the present adsorption process.
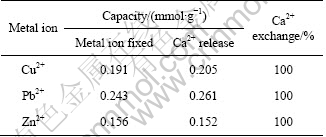
Figure 7 represents simultaneously the isotherm of Cu2+ adsorption and the isotherm of Ca2+ desorption in solution. Because the isotherms of Cu2+ adsorption and Ca2+ desorption are practically similar, Cu2+ ions seem to be exclusively adsorbed by an ion exchange mechanism. Table 4 shows the amount of Ca2+ released with respect to the amount of metal removed during Cu2+, Pb2+ and Zn2+ adsorption on SCOP at the optimum pH values (5.0 for Cu2+ and 5.5 for Pb2+, and Zn2+). According to Table 4, Ca2+ release is related to the amount of Cu2+, Pb2+ and Zn2+ adsorbed per gram of biomass, confirming that Ca2+ is exchanged with metal ions in solution. The SCOP itself contains enough Ca2+ to neutralize most of the carboxylic moieties of the galacturonic acid. CHEN et al [30] also observed this exchange between alginate calcium and adsorbed copper and lead using FTIR and XPS. The exchange between cadmium, lead and copper with calcium has also been documented by DRONNET et al [31] during the biosorption with sugar-beet pulp and non-gelled sugar-beet and citrus pectin using potentiometric and spectrophotometric methods.
The EDX spectra of the SCOP before and after adsorption of Cu2+, Pb2+ and Zn2+ are shown in Fig. 8. It is noted that due to the metal ion adsorption by the SCOP the peaks of Ca weaken with concomitant appearance of the Cu, Pb or Zn peaks. The results of an X-ray fluorescence analysis for SCOP show that the calcium and copper concentrations were approximately 7.271% and 0.007% before the adsorption and 1.908% and 11.610% after the copper adsorption, respectively. These also indicate that the adsorption process follows an ion exchange mechanism between calcium from the SCOP and metals in solution [32].
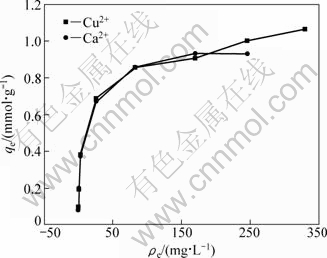
Fig. 7 Adsorption isotherms of Cu2+ and its respective Ca2+ desorption isotherms
Table 4 The maximum adsorption capacities and corresponding Ca2+ release from SCOP with initial metal concentration of 50 mg/L (except Pb2+ 200 mg/L)
4 Conclusions
1) The adsorption performance of Cu2+, Pb2+ and Zn2+ on SCOP is significantly affected by pH and metal concentrations. The adsorption equilibrium data fit well with the Langmuir isotherm. The maximum adsorption capacities for Cu2+, Pb2+ and Zn2+ are found to be 70.73, 209.8 and 56.18 mg/g, respectively.
2) The adsorbent also exhibits favorable separation for some metal ions like Pb2+ and Zn2+ due to its high selectivity.
3) The adsorption mechanism is mainly based on ion exchange between divalent cations in solution (Cu2+, Pb2+, and Zn2+) and calcium (Ca2+) chelated or linked to carboxylic groups in the polymeric structure of pectin.
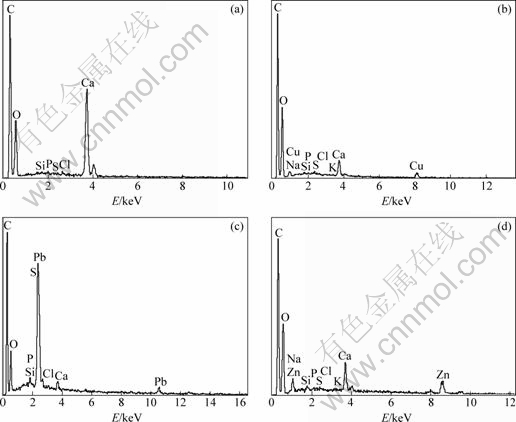
Fig. 8 EDX spectra of SCOP before (a) and after adsorption of Cu2+(b), Pb2+(c) and Zn2+(d)
References
[1] LIANG S, GUO X Y, FENG N C, TIAN Q. Application of orange peel xanthate for the adsorption of Pb2+ from aqueous solutions [J]. J Hazard Mater, 2009, 170: 425-429.
[2] MIRETZKY P, SARALEGUI A, CIRELLI A F. Simultaneous heavy metal removal mechanism by dead macrophytes [J]. Chemosphere, 2006, 62: 247-254.
[3] RICOU-HOEFFER P, LECUYER I, le CLOIREC P. Experimental design methodology applied to adsorption of metallic ions onto fly ash [J]. Water Res, 2001, 35: 965-976.
[4] BROWN P A, GILL S A, ALLEN S J. Metal removal from wastewater using peat [J]. Water Res, 2000, 34: 3907-3916.
[5] HO Y S, MCKAY G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat [J]. Water Res, 2000, 34: 735-742.
[6] AHLUWALIA S S, GOYAL D. Microbial and plant derived biomass for removal of heavy metals from wastewater [J]. Bioresour Technol, 2007, 98: 2243-2257.
[7] JARAMILLO J, G?MEZ-SERRANO V, ?LVAREZA P M. Enhanced adsorption of metal ions onto functionalized granular activated carbons prepared from cherry stones [J]. J Hazard Mater, 2009, 161: 670-676.
[8] CONRAD K, HANSEN H C B. Sorption of zinc and lead on coir [J]. Bioresour Technol, 2007, 98: 89-97.
[9] FEBRIANTO J, KOSASIH A N, SUNARSO J, JU Y H, INDRAWATI N, ISMADJI S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies [J]. J Hazard Mater, 2009, 162: 616-645.
[10] AJMAL M, RAO R A K, AHMAD R, AHMAD J. Adsorption studies on Citrus reticulata (fruit peel of orange): Removal and recovery of Ni (II) from electroplating wastewater [J]. J Hazard Mater, 2000, 79: 117-131.
[11] FENG Ning-chuan, GUO Xue-yi, LIANG Sha. Kinetic and thermodynamic studies on biosorption of Cu(Ⅱ) by chemically modified orange peel [J]. Transaction of Nonferrous Metals Society of China, 2009, 19: 1365-1370.
[12] SCHIEWER S, PATIL S B. Pectin-rich fruit wastes as biosorbents for heavy metal removal: Equilibrium and kinetics [J]. Bioresour Technol, 2008, 99: 1896-1903.
[13] MATHEICKAL J T, YU Q, WOODBURN G M. Biosorption of cadmium (II) from aqueous solutions by pre-treated biomass of marine alga DurvillAea potatorum [J]. Water Res, 1999, 33: 335-342.
[14] TING Y P, PRINCE I G, LAWSON F. Uptake of cadmium and zinc by the alga chlorella vulgaris: II Multi-ion situation [J]. Biotechnol Bioeng, 1991, 37: 445-455.
[15] GABALLAH I, GEY D, KILBERTUS G, THAURONT J. Decontamination of industrial effluents for environment protection and recycling of metals [J]. Resources, Conservation and Recycling, 1994, 10: 97-106.
[16] WAN NGAH W S, HANAFIAH M A K M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review [J]. Bioresour Technol, 2008, 99: 3935-3948.
[17] NOELINE B F, MANOHAR D M, ANIRUDHAN T S. Kinetic and equilibrium modelling of lead(II) sorption from water and wastewater by polymerized banana stem in a batch reactor [J]. Sep Purif Technol, 2005, 45: 131-140.
[18] FENG N C, GUO X Y, LIANG S. Adsorption study of copper (II) by chemically modified orange peel [J]. J Hazard Mater, 2009, 164: 1286-1292.
[19] ANIRUDHAN T S, NOELINE B F, MANOHAR D M. Phosphate removal from wastewaters using a weak anion exchanger prepared from a lignocellulosic residue [J]. Environ Sci Technol, 2006, 40: 2740-2745.
[20] UNNITHAN M R, ANIRUDHAN T S. Synthesis, characterization and application as a chromium (VI) adsorbent of amine-modified polyacrylamide-grafted coconut coir pith [J]. Ind Eng Chem Res, 2004, 43: 2247-2255.
[21] GNANASAMBANDAM R, PROTOR A. Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy [J]. Food Chem, 2000, 68: 327-332.
[22] MATA Y N, BL?ZQUEZ M L, BALLESTER F A, GONZ?LEZ, MU?OZ J A. Sugar-beet pulp pectin gels as biosorbent for heavy metals: Preparation and determination of biosorption and desorption characteristics [J]. Chem Eng J, 2009, 150: 289-301.
[23] BAIG T H, GARCIA A E, TIEMANN K J, GARDEA-TORRESDEY J L. Adsorption of heavy metal ions by the biomass of Solanum elaeagnifolium (Silverleaf night-shade) [C]// ERICKSON L E. Proceedings of the 10th Annual EPA Conference on Hazardous Waste Research. Washington DC: US Environmental Protection Agency, 1999: 131-139.
[24] RENDLEMAN J A. Metal polysaccharide complexes—Part II [J]. Food Chem, 1978, 3: 127-162.
[25] SHENG P X, TING Y, CHEN J P, HONG L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of Mechanisms [J]. J Colloid Interface Sci, 2004, 275: 131-141.
[26] KRISHNANI K K, MENG X, CHRISTODOULATOS C, BODDU V M. Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk [J]. J Hazard Mater, 2008, 153: 1222-1234.
[27] REDDAD Z, GERENTE C, ANDRES Y, CLOIREC P L. Adsorption of several metal ions onto a low-cost biosorbent: Kinetic and equilibrium studies [J]. Environ Sci Technol, 2002, 36: 2067-2073.
[28] VAUGHAN T, SEO C W, MARSHALL W E. Removal of selected metal ions from aqueous solution using modified cornbobs [J]. Bioresour Technol, 2001, 78: 133-139.
[29] KWEON D K, CHOI J K, KIM E K, LIM S T. Adsorption of divalent metal ions by succinylated and oxidized corn starches [J]. Carbohydr Polym, 2001, 46: 171-177.
[30] CHEN J P, HONG L, WU S N, WANG L. Elucidation of interactions between metal ions and Ca alginate-based ion exchange resin by spectroscopic analysis and modelling simulation [J]. Langmuir, 2002, 18: 9413-9421.
[31] DRONNET V M, RENARD C M C G, AXELOS M A V, THIBAULT J F. Characterisation and selectivity of divalent metal ions binding by citrus and sugar-beet pectins [J]. Carbohydr Polym, 1996, 30: 253-263.
[32] PANDA G C, DAS S K, GUHA A K. Biosorption of cadmium and nickel by functionalized husk of Lathyrus sativus [J]. Colloids Surf B, 2008, 62: 173-179.
改性橘子皮对铜、铅和锌的吸附特性及吸附机制
冯宁川1,2,郭学益1
1. 中南大学 冶金科学与工程学院,长沙 410083;
2. 宁夏医科大学 基础医学院,银川 750004
摘 要:将橘子皮经氢氧化钠和氯化钙处理,得到改性橘子皮生物吸附剂(SCOP)。用红外光谱(IR)、扫描电镜(SEM)和N2-吸附法对其形貌和特性进行表征;通过静态吸附实验,研究pH、起始金属离子浓度等因素对改性橘子皮SCOP吸附Cu2+、Pb2+和Zn2+的影响。等温吸附结果表明,SCOP对Cu2+、Pb2+和Zn2+的吸附符合Langmuir方程,根据Langmuir方程计算的SCOP对Cu2+、Pb2+和Zn2+的饱和吸附量分别为70.73, 209.8和56.18 mg/g。根据静态吸附实验结果,用动态柱吸附实现了水溶液中Pb2+和Zn2+的分离。吸附过程中离子交换发生了重要作用,重金属离子与吸附剂中的Ca2+离子发生离子交换。
关键词:橘子皮;化学改性;吸附容量;吸附机制
(Edited by YANG Hua)
Foundation item: Project (50774100) supported by the National Natural Science Foundation of China
Corresponding author: GUO Xue-yi; Tel/Fax: +86-731-88836207; E-mail: xyguo@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(11)61309-5