MgCl2改性橘子皮对水溶液中镉镍的吸附性能
郭学益,梁莎,肖彩梅,李晓静,田庆华
(中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘要:以橘子皮(OP)为原料通过MgCl2改性制备新型橘子皮吸附剂MgOP。考察溶液pH、固液比、温度、吸附时间和金属离子质量浓度对其从水溶液中吸附Cd2+和Ni2+的吸附性能的影响。采用扫描电镜及红外光谱仪对吸附剂进行表征。MgOP对2种金属离子的吸附率随pH和固液比的增大而增大;温度对吸附率的影响较小;吸附速度很快,能在20 min内达到吸附平衡。MgOP对Cd2+和Ni2+的吸附动力学均符合准二级动力学方程;MgOP对Cd2+和Ni2+的的Langmuir最大吸附量分别为125.47和44.58 mg/g。
关键词:改性橘子皮;吸附;Cd2+;Ni2+
中图分类号:X703.1 文献标志码:A 文章编号:1672-7207(2011)07-1841-06
Adsorption of Cd2+ and Ni2+ from aqueous solutions by MgCl2 modified orange peel
GUO Xue-yi, LIANG Sha, XIAO Cai-mei, LI Xiao-jing, TIAN Qing-hua
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Orange peel (OP) was modified with MgCl2 to prepare a novel orange peel adsorbent named as MgOP. The effects of pH, ratio of solid to liquid, temperature, contact time and metal ion mass concentration on Cd2+ and Ni2+ adsorption from aqueous solutions by MgOP were investigated. SEM and FTIR were used to characterize the adsorbent. The adsorption efficiencies of Cu2+ and Cd2+ by MgOP increase with the increase of pH and the ratio of solid mass to liquid volume. The temperature has little effect on adsorption capacity, and both adsorption processes are very fast and can attain equilibrium with 20 min. Both kinetics data of Cd2+ and Ni2+ are fitted to pseudo-second-equation. The maximum adsorption capacity of Cd2+ and Ni2+ by MgOP are 125.47 and 44.58 mg/g, respectively.
Key words: modified orange peel; adsorption; Cd2+; Ni2+
近年来,随着工业化进程的发展,废水的排放量日益增加,从而导致了严重的环境污染问题。废水中溶解的重金属离子经过水体中各种生物链产生富集,最终进入人体,给人类健康带来严重的危害。如过量的镉会使肾功能受到破坏,糖、蛋白质代谢发生紊乱,引发尿蛋白症、糖尿病,进入呼吸道引发肺炎、肺气肿等;镍中毒会引发各种皮炎,慢性超量摄取或超量暴露,可导致心肌、脑、肝和肾退行性变。传统除去水中重金属离子的方法通常成本较高,且具有潜在的危害性,不宜用于所含金属离子浓度较低的情况。生物吸附法提供了一种技术可行、环境友好的方法,它利用廉价的生物材料对重金属进行吸附,具有原料来源广泛、环境友好、吸附量高、吸附速度快等优点[1-3]。据资料显示,2007年全国柑橘栽培面积达 194.1亿m2,总产量达2 058.3万t。无论在栽培总面积上,还是总产量上,中国已经成为世界柑橘第一大生产 国,随之带来的则是大量的柑橘加工的副产物即柑橘皮,约占柑橘总质量的25%~40%[4]。因此,柑橘果皮的综合利用对提高柑橘加工厂的经济效益和减少污染、保护环境都是十分有利的。柑橘皮中含有丰富的果胶、纤维素、半纤维素等多糖类高分子化合物和木质素,它们可提供氨基、酰胺基、羧基、羟基等官能团与金属离子结合,因此,可用作制备生物吸附剂。但直接采用柑橘皮作吸附剂不仅存在吸附容量小、性能不稳定、不易长期存放保存的缺点,而且存在着由于一些可溶性有机物质如木质素、单宁酸、果胶质和纤维素的溶解而导致水中化学耗氧量增加等问题[5-6],因此,需通过化学改性的方法提高柑橘皮的吸附容量和化学稳定性。国内外研究者通过各种改性方法,如皂化、交联、磷酸化、接枝、硫化等,改善了其物理化学性能,制备了吸附性能良好的柑橘类生物吸附 剂[7-11]。本文作者以橘子皮为基体,经乙醇、氢氧化钠和氯化镁改性处理,制备了新型改性橘子皮生物吸附剂,并研究其对Cd2+和Ni2+ 2种金属离子的吸附性能,同时考察各种因素对吸附过程的影响,分析吸附动力学及吸附等温模型。
1 实验
1.1 仪器与试剂
仪器:3510原子吸收分光光度计,PHS-3C酸度计, SHA-C水浴恒温振荡器,DZF-300真空干燥箱, JSM-6360LV扫描电镜,Nicolet 380傅里叶变换红外光谱仪。
试剂:3CdSO4·8H2O,Ni(NO3)2·6H2O,HCl,NaOH,乙醇和MgCl2等,均为分析纯。
1.2 改性橘子皮生物吸附剂的制备
橘子皮(OP)经自来水和蒸馏水洗净后于70 ℃鼓风干燥箱中烘干24 h,粉碎至粒径小于300 μm。
取40 g的OP于500 mL的锥形瓶中,分别加入200 mL无水乙醇、100 mL NaOH(0.5 mol/L)和100 mL 的MgCl2溶液(1 mol/L),于室温浸泡24 h,随后抽滤并用蒸馏水洗涤至pH为中性,在70 ℃鼓风干燥箱中烘干24 h,粉碎至粒径小于300 μm,所得橘子皮吸附剂简写为MgOP。
1.3 吸附实验
在50 mL锥形瓶中加入一定固液比的改性橘子皮生物吸附剂及金属离子(Cd2+或Ni2+)溶液,用HCl和NaOH调节溶液pH,密封瓶口以防实验过程中体积的变化。将其放入一定温度的水浴恒温振荡器中振荡吸附一定时间后过滤,用原子吸收分光光度计测定滤液中金属离子的平衡质量浓度。用下式计算吸附量:
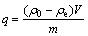 (1)
(1)
式中:V为溶液体积,mL;ρ0和ρe分别为金属溶液的初始质量浓度和平衡质量浓度,mg/L;m为所用生物吸附剂的质量,mg。
2 结果与讨论
2.1 吸附剂的表征
橘子皮改性前后表面形貌SEM像如图1所示。由图1可见:MgOP比OP表面更为粗糙,有更多的吸附位点暴露在吸附剂表面,因此,更有利于吸附过程的进行。

图1 OP和MgOP的SEM像
Fig.1 SEM images of OP and MgOP
图2所示是橘子皮改性前后的红外光谱。从图2可见:在OP的红外光谱中,3 413 cm-1附近的峰表明吸附剂表面存在大量的羟基(—OH);2 923 cm-1附近的峰来自CH,CH2和CH3中C—H键的伸缩振 动;1 741 cm-1附近的峰来自自由羧基(—COOH, —COOCH3)中的C=O键的伸缩振动;1 647和1 442 cm-1附近的峰分别来自于离子化羧基(—COO-)中C=O键的不对称和对称伸缩振动;1 275 cm-1附近的脂肪酸族振动峰可能来自于羧酸和酚类化合物中C=O键的变形振动和—OH键的伸缩振动;1 068 cm-1附近的峰来自于醇和羧酸中C—OH键的伸缩振动[12]。比较MgOP和OP的光谱可以发现:OP中3 413 cm-1和1 068 cm-1附近的羟基峰分别移动到3 386.8 cm-1和1 099 cm-1,自由羧基峰(1 741 cm-1)减弱,离子化羧基峰(1 647 cm-1和1 442 cm-1)发生移动,同时,MgOP在1 244 cm-1产生了新的峰,这些都表明改性使橘子皮上的羟基和羧基等官能团发生了变化。

图2 OP和MgOP的红外光谱
Fig.2 FTIR spectra of OP and MgOP
对橘子皮的改性处理中,乙醇的作用是去除色素及一些可溶性小分子;氢氧化钠可以使果胶分子上甲酯化的羧基发生皂化,从而提高羧基官能团的数 目[13],同时氢氧化钠可以部分地与纤维素、半纤维素和木质素分子中的醇羟基或酚羟基反应,生成醇 钠[14];氯化镁的加入能起到交联作用,使吸附剂分子间结合得更紧密,减少有效物质的溶出。
2.2 pH的影响
图3所示为溶液平衡pH对MgOP吸附的影响。 由图3可见:随着pH的增大,吸附效率增大。这主要是因为当溶液pH较低时,吸附剂表面带正电荷,不利于金属阳离子吸附,且溶液中的H+会与金属阳离子进行竞争吸附,因此吸附效率较低; 随着pH的增大,吸附剂表面负电荷增多,且阳离子与表面活性位点的静电斥力和H+的竞争吸附都减弱,因此,吸附效率增大[15]。从图3可看出:MgOP对Cd2+和Ni2+的最大吸附率均出现5.0~6.0之间,且对Cd2+的吸附率较大于Ni2+的吸附率。
2.3 固液比的影响
吸附剂用量与金属离子水溶液的固液比(质量与体积比)是影响重金属离子吸附效率的又一重要因素,如果吸附剂投入太少,就不能有效地去除重金属离子;反之,如果吸附剂投入太多,又会形成资源浪费,造成不必要的经济损失。考察在一定体积M2+(Cd2+或Ni2+)溶液中投加不同质量吸附剂MgOP对其吸附效率的影响,结果如图4所示。从图4可见:随着固液比的增大,2种金属离子的吸附率均增大并逐渐趋于平缓。这可能是由于吸附剂量的增加使溶液中吸附官能团增多,吸附位点增多,因而吸附率提高;而吸附剂量的增加同时也引起了吸附剂粒子团聚,减小了吸附剂表面积。此外,在高吸附剂质量浓度下,粒子的相互作用也可能使一些在吸附剂表面结合较为松散的金属离子解 吸[16],而造成吸附率降低。

图3 pH对吸附的影响
Fig.3 Effect of pH on adsorption
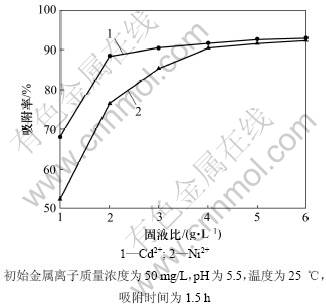
图4 固液比对吸附的影响
Fig.4 Effect of ratio of solid to liquid on adsorption
2.4 温度对吸附过程的影响
考察了不同温度对MgOP吸附Cd2+和Ni2+的影响,结果见图5。从图5可以看出:随着温度的增加,MgOP对Cd2+和Ni2+的吸附率变化较小,说明MgOP对这2种离子的吸附过程可能为化学吸附[17]。

图5 温度对吸附率的影响
Fig.5 Effect of temperature on adsorption
2.5 吸附动力学
图6所示为25 ℃时吸附时间对MgOP吸附Cd2+和Ni2+性能的影响。从图6可以看出:吸附速度很快,随着时间增加吸附量增大,在20 min时基本上达到吸附平衡。
在生物吸附动力学的研究中,通常用一级和二级动力学方程对试验数据进行模拟,以分析金属离子浓度随吸附时间的变化关系。准二级动力学方程的线性表达式为[18]:
 (2)
(2)
式中:k2为准二级速率常数,g/(mg×min);qt和qe分别为时间 t和平衡时的吸附量,mg/g。利用上述方程对试验数据进行模拟,以t/q对 t作图,可得到准二级动力学方程模拟结果,相关动力学参数如表1所示。可见:试验结果可以很好地用准二级动力学方程进行模拟,相关系数接近1,且qe的实验值与理论值相差很小。这表示吸附过程遵循准二级反应机理,吸附速率被化学吸附所控制[19]。

图6 吸附时间对吸附的影响
Fig.6 Effect of time on adsorption
表1 准二级吸附动力学参数
Table 1 Kinetic parameters of pseudo-second-order equation

2.6 等温吸附
图7所示为25 ℃时OP和MgOP对Cd2+和Ni2+(初始质量浓度为20~1 000 mg/L)的吸附等温线。由图7可知:吸附量随溶液中金属离子质量浓度的增加而增加,经改性后的橘子皮吸附剂MgOP较原始橘子皮OP对2种金属离子的吸附能力有所提高,说明改性后的橘子皮吸附剂中含有更多能与金属离子结合的官能团。同时可以看出:在高金属离子质量浓度下,吸附剂对Ni2+的吸附量要大于Cd2+的吸附量。
用Langmuir和 Freundlich吸附等温模型对图7中的数据进行模拟。Langmuir模型假设金属离子在吸附剂上的吸附为单层吸附,其方程为[20]:
 (3)
(3)
式中:qm为吸附剂最大吸附量,mg/g;b为吸附常数,L/mg。qm和b可由ρe/qe对ρe作直线方程的斜率(1/qm)和截距(1/(qmb))求出。
Freundlich模型是用来描述非均相吸附体系的经验式模型,若固体表面是不均匀的,则交换吸附平衡常数与表面覆盖度有关,其方程为[21]:
 (4)
(4)
式中:KF和1/n分别为经验常数。n和KF由lg qe对 lg ρe作直线方程的斜率1/n和截距lg KF求出。

图7 OP和MgOP吸附Cd2+和Ni2+的等温线
Fig.7 Adsorption isotherm of Cd2+ and Ni2+ on OP and MgOP
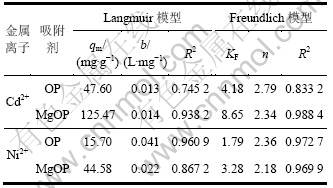
表2列出Langmuir和Freundlich方程的模拟等温吸附常数。MgOP吸附Cd2+和Ni2+的最大吸附量分别为125.47和44.58 mg/g,较OP的最大吸附量有较大提高,说明改性提高了吸附剂对金属离子的结合能力。同时通过比较相关系数R2可以看出:OP和MgOP对Cd2+和Ni2+的吸附等温线均更符合Freundlich经验模型。
表2 Langmuir和Freundlich等温吸附模型参数
Table 2 Parameters of Langmuir and Freudlich isotherm models
3 结论
(1) 以橘子皮OP为基体,通过乙醇、氢氧化钠和氯化镁改性制备了高效橘子皮生物吸附剂MgOP,用该改性橘子皮生物吸附剂处理含重金属废水,吸附效率高,是一种性能良好的吸附剂。
(2) 改性后的橘子皮表面更粗糙,更有利于吸附重金属离子;改性使橘子皮表面的有效官能团增加。
(3) 考察了溶液平衡pH、固液比、温度、吸附时间及金属离子质量浓度对MgOP吸附Cd2+和Ni2+的影响,吸附动力学实验表明吸附MgOP对2种金属离子的吸附速度很快,能在20 min内达到吸附平衡,同时吸附动力学符合准二级动力学方程。MgOP较OP对金属离子的吸附能力有所提高。
参考文献:
[1] 梁莎, 冯宁川, 郭学益. 生物吸附法处理重金属废水研究进展[J]. 水处理技术, 2009, 35(3): 13-17.
LIANG Sha, FENG Ning-chuan, GUO Xue-yi. Progress of heavy metal wastewater treatment by biosorption[J]. Technology of Water Treatment, 2009, 35(3): 13-17.
[2] Ahluwalia S S, Goyal D. Microbial and plant derived biomass for removal of heavy metals from wastewater[J]. Bioresource Technology, 2007, 98(12): 2243-2257.
[3] Bailey S E, Olin T J, Bricka R M, et al. A review of potentially low-cost sorbents for heavy metals[J]. Water Research, 1999, 33(11): 2469-2479.
[4] 臧玉红. 柑橘皮的综合利用[J]. 食品与发酵工业, 2005, 31(7): 145-146.
ZANG Yu-hong. Research on the extracting condition of useful substances from the orange peels[J]. Food and Fermentation Industries, 2005, 31(7): 145-146.
[5] Noeline B F, Manohar D M, Anirudhan T S. Kinetic and equilibrium modelling of lead(Ⅱ) sorption from water and wastewater by polymerized banana stem in a batch reactor[J]. Separation and Purification Technology, 2005, 45(2): 131-140.
[6] Gaballah I, Goy D, Allain E, et al. Recovery of copper through decontamination of synthetic solutions using modified barks[J]. Metallurgical and Materials Transactions B, 1997, 28(1): 13-23.
[7] LIANG Sha, GUO Xue-yi, FENG Ning-chuan, et al. Application of orange peel xanthate for the adsorption of Pb2+ from aqueous solutions[J]. Journal of Hazardous Materials, 2009, 170(1): 425-429.
[8] LIANG Sha, GUO Xue-yi, FENG Ning-chuan, et al. Adsorption of Cu2+ and Cd2+ from aqueous solution by mercapto-acetic acid modified orange peel[J]. Colloids and Surfaces B: Biointerfaces, 2009, 73(1): 10-14.
[9] Ajmal M, Rao R A K, Ahmad R, et al. Adsorption studies on Citrus reticulata (fruit peel of orange): Removal and recovery of Ni(Ⅱ) from electroplating wastewater[J]. Journal of Hazardous Materials, 2000, 79(1/2): 117-131.
[10] Li X M, Tang Y R, Xuan Z X, et al. Study on the preparation of orange peel cellulose adsorbents and biosorption of Cd2+ from aqueous solution[J]. Separation Purification Technology, 2007, 55(1): 69-75.
[11] Pérez-Marín A B, Zapata V M, Ortu?o J F, et al. Removal of cadmium from aqueous solutions by adsorption onto orange waste[J]. Journal of Hazardous Materials, 2007, 139(1): 122-131.
[12] Iqbal M, Saeed A, Iqbal-Zafar S. FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste[J]. Journal of Hazardous Materials, 2009, 164(1): 161-171.
[13] Baig T H, Garcia A E, Tiemann K J, et al. Adsorption of heavy metal ions by the biomass of Solanum elaeagnifolium (Silverleaf night-shade)[C]//Erickson L E, ed. Proceedings of the 10th Annual EPA Conference on Hazardous Waste Research. Washington DC: US Environmental Protection Agency, 1999: 131-139.
[14] Li X, Tang Y, Cao X, et al. Preparation and evaluation of orange peel cellulose adsorbents for effective removal of cadmium, zinc, cobalt and nickel[J]. Colloids and Surfaces A: Physicochem Eng Aspects, 2008, 317(1/3): 512-521
[15] Khormaei M, Nasernejad B, Edrisi M, et al. Copper biosorption from aqueous solutions by sour orange residues[J]. Journal of Hazardous Materials,2007, 149(2): 269-274.
[16] Monahar D M, Anoop Krishnan K, Anirudhan T S. Removal of mercury(Ⅱ) from aqueous solutions and chlor-alkal industry wastewater using 2-mercaptobenzimidazole-clay[J]. Water Research, 2002, 36(6): 1609-1619.
[17] Dang V B H, Doan H D, Dang-Vu T, et al. Equilibrium and kinetics of biosorption of cadmium (Ⅱ) and copper (Ⅱ) ions by wheat straw[J]. Bioresource Technology, 2009, 100(1): 211-219.
[18] Ho Y S, Mckay G. Pseudo-second order model for sorption processes[J]. Process Biochem, 1999, 34(5): 451-465.
[19] Kim H, Lee K. Application of cellulose xanthate for the removal of nickel ion from aqueous solution[J]. J Kor Soc Eng, 1998, 20: 247-254.
[20] Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum[J]. Journal of American Chemistry Society, 1918, 40(9): 1361-1403.
[21] Freundlich H M F. Uber die adsorption in Losungen[J]. Z Phys Chem, 1906, 57: 385-470.
(编辑 陈爱华)
收稿日期:2010-05-22;修回日期:2010-08-28
基金项目:国家自然科学基金资助项目(50774100)
通信作者:郭学益(1966-),男,湖南长沙人,教授,从事资源循环与环境材料研究;电话:0731-88877863;E-mail: xyguo@csu.edu.cn