DOI: 10.11817/j.ysxb.1004.0609.2021-36521
高岭石与氧化铁还原焙烧过程中二氧化硅固溶体的形成机理
王洪阳1, 2,张晓雪1,宋少先1, 2
(1. 武汉理工大学 资源与环境工程学院,武汉 430070;
2. 武汉理工大学 矿物资源加工与环境湖北省重点实验室,武汉 430070)
摘 要:采用XRD、XPS、FTIR以及SEM-EDS分析手段对高岭石和氧化铁还原焙烧的反应过程进行研究,并借助碱浸实验明确还原焙烧产物中二氧化硅固溶体的溶解性,在此基础上探讨二氧化硅固溶体的形成机理。结果表明:高岭石在1000~1150 ℃还原气氛中单独焙烧时会转变为无定型态,氧化铁的添加可将高岭石在1050~1150 ℃完全转变为铁铝尖晶石和二氧化硅固溶体-石英固溶体和方石英固溶体。此外,石英固溶体和方石英固溶体与天然的石英和方石英具有相似的结构,但在氢氧化钠溶液中溶出性能有很大的区别。还原焙烧过程为固相反应,氧化铝会选择性与氧化亚铁反应并生成铁铝尖晶石,受氧化铝迁移的影响,少量的Fe2+进入硅氧四面体中并最终形成二氧化硅固溶体。
关键词:高岭石;还原焙烧;铝酸亚铁;石英固溶体;方石英固溶体
文章编号:1004-0609(2021)-03-0756-09 中图分类号:TF11.31 文献标志码:A
引文格式:王洪阳, 张晓雪, 宋少先. 高岭石与氧化铁还原焙烧过程中二氧化硅固溶体的形成机理[J]. 中国有色金属学报, 2021, 31(3): 756-764. DOI: 10.11817/j.ysxb.1004.0609.2021-36521
WANG Hong-yang, ZHANG Xiao-xue, SONG Shao-xian. Formation mechanism of silica solid solution during reduction roasting of kaolinite and ferric oxide[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(3): 756-764. DOI: 10.11817/j.ysxb.1004.0609.2021-36521
高岭土作为一种主要的铝硅酸盐矿物被广泛应用于造纸、耐火材料、水泥和陶瓷等领域[1-4],因此,在工业应用中就涉及到高岭石的焙烧分解。普遍认为,高岭石在约550 ℃因脱水而变成无定型的偏高岭石,提高焙烧温度至约980 ℃,偏高岭石进一步分解为无定型氧化硅和含铝尖晶石。当焙烧温度超过1200 ℃,其最终焙烧产物为莫来石和方石英。目前对于含铝尖晶石是独立的γ-氧化铝还是铝硅化学物仍存在争议[5-6]。其主要原因[7-9]:1) 含铝尖晶石结晶程度较差,难以通过有效的手段进行界定;2) 高岭土中存在的杂质元素对其焙烧过程中的物相变化会产生影响。鉴于高岭石在约980 ℃分解的无定型氧化硅比含铝尖晶石更易溶于碱溶液,因此,通过碱浸结果可对含铝尖晶石的化学组成进行分析。相关研究结果表明[10-11],焙烧高岭土的碱浸最大脱硅率约为65%,且浸出渣中氧化铝和氧化硅的比值(A/S)稳定在约2.65,说明含铝尖晶石更趋向于3Al2O3·2SiO2莫来石(A/S=2.55)。
氧化铁是高岭土中常见的金属氧化物,对高岭石在还原气氛中的焙烧行为有显著影响。SEGNIT等[12]最先发现FeO与高岭石在高温焙烧时会反应生成铁铝尖晶石及石英固溶体,但是其仅对实验结果进行了描述。HUGHES[13]在中性气氛中对含氧化铁的高岭土进行高温焙烧,发现焙烧产物中出现了铁铝尖晶石。TAKEUCHI等[14]通过热力学计算,明确FeO-Al2O3-SiO2三元系进行高温反应时,FeO优先与Al2O3反应生成铁铝尖晶石,此结论被ZHOU等[15]进一步证实。LI等[16]对高岭石与氧化铁还原焙烧过程中的反应行为进行了系统的研究,通过控制生料中Fe2O3、Al2O3和C摩尔比、还原焙烧温度及时间,可将高岭石完全转变为铁铝尖晶石及二氧化硅固溶体-石英固溶体和方石英固溶体。然而,对于二氧化硅固溶体的形成机理研究较少。
为避免煤粉中杂质元素对还原焙烧过程的影响,本文在CO气氛下考察氧化铁和高岭石在还原焙烧过程中的反应行为。借助于XRD、XPS、FTIR以及SEM-EDS对还原焙烧熟料中的二氧化硅固溶体进行系统的研究,并进一步探讨二氧化硅固溶体的形成机理。
1 实验
1.1 实验原料
实验所用氧化铁和氢氧化钠均为分析纯的化学试剂。高岭土购买于广东省,其主要化学组成为Al2O3和SiO2,含量分别为37.69%和44.61%,此外还含有0.27% Fe和0.35% CaO。图1(a)所示物相分析结果表明,此高岭土的主要物相组成为高岭石。
1.2 实验过程
将高岭土和氧化铁按Fe2O3和Al2O3摩尔比为1.2:2.0进行配料,并在振动磨中混匀2 min以制备生料。还原焙烧过程是在带有玻璃管的卧式管炉(型号:TL 1200)中进行。首先将卧室管炉升至一定的焙烧温度,通入N2以保证玻璃管中为中性气氛。然后将装有5 g高岭土或者生料的刚玉坩埚置于玻璃管中,此时通入CO并控制CO和N2的体积比为5:95。焙烧时间60 min结束后,将玻璃管从卧式管炉中取出并在N2保护下冷却至室温,以获得焙烧高岭土或还原焙烧熟料。
还原焙烧熟料中的二氧化硅固溶体的溶出实验是在GS -0.25型微量反应釜中进行。一定量的还原焙烧熟料和100 mL氢氧化钠溶液加入反应釜中,然后加热至反应温度并计时。待反应时间结束,立即往冷却管中通入自来水,使反应釜内温度快速降低至50 ℃以下。经固液分离以获得浸出渣,并在105 ℃烘箱中干燥5 h以用于后续分析和检测。
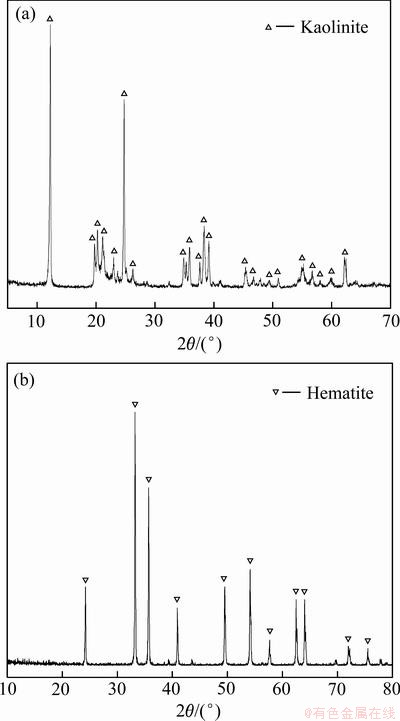
图1 高岭土和赤铁矿的XRD谱
Fig. 1 XRD patterns of kaolin (a) and hematite (b)
1.3 分析和检测
焙烧高岭土及还原焙烧熟料的物相组成采用MAX-RB型X射线衍射仪(XRD)进行分析,Cu Kα射线(λ=1.5406 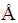 ),扫描角度5°<2θ<70°,扫描速度10 (°)/min。还原焙烧熟料中的无定型相采用ESCALAB 250XI型X射线光电子能谱仪(XPS)进行分析,数据选用C 1s峰的284.5 eV进行能量矫正。采用型号Nicolet 6700型红外光谱仪(FTIR)对还原焙烧熟料中二氧化硅固溶体进行分析,其分析采用KBr压片法。还原焙烧熟料中的元素分布采用JEM-7500F型扫描电子显微镜(SEM)进行分析,还原焙烧熟料经煮胶、磨片和抛光后进行喷金处理,以实现样品具有良好的导电性。此外,浸出渣中的二氧化硅含量采用重量法进行分析。
),扫描角度5°<2θ<70°,扫描速度10 (°)/min。还原焙烧熟料中的无定型相采用ESCALAB 250XI型X射线光电子能谱仪(XPS)进行分析,数据选用C 1s峰的284.5 eV进行能量矫正。采用型号Nicolet 6700型红外光谱仪(FTIR)对还原焙烧熟料中二氧化硅固溶体进行分析,其分析采用KBr压片法。还原焙烧熟料中的元素分布采用JEM-7500F型扫描电子显微镜(SEM)进行分析,还原焙烧熟料经煮胶、磨片和抛光后进行喷金处理,以实现样品具有良好的导电性。此外,浸出渣中的二氧化硅含量采用重量法进行分析。
2 结果与讨论
2.1 高岭石和氧化铁的还原焙烧反应行为
高岭土与氧化铁混合生料或者高岭土在不同温度下还原焙烧熟料的XRD谱如图2所示。由图2(a)可知,高岭土单独还原焙烧时,高岭石在1000~1150 ℃转变为无定型态。提高焙烧温度至1200 ℃,此时焙烧产物中仅发现莫来石的存在。说明高岭石在1000~1150 ℃会分解为无定型的铝硅尖晶石(莫来石)和二氧化硅[11],提高焙烧温度至1200 ℃会促使莫来石晶体结构的完善。
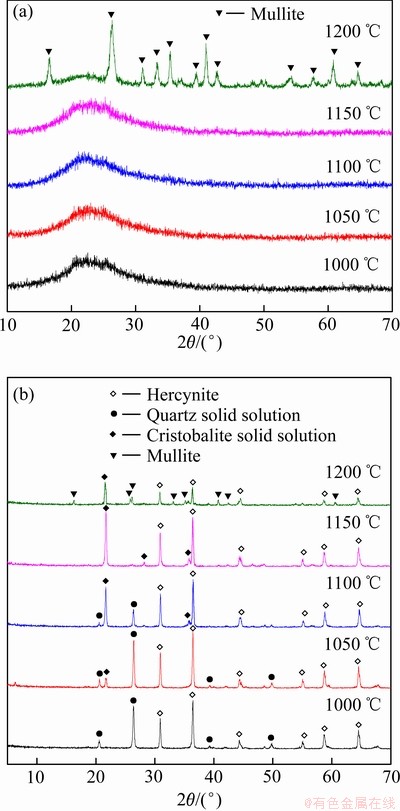
图2 不同温度下焙烧高岭土和还原焙烧熟料的XRD谱
Fig. 2 XRD patterns of roasted kaolin (a) and clinkers (b) obtained at different temperatures
图2(b)可知,控制焙烧温度在1000~1150℃,还原焙烧熟料中的含硅物相有石英固溶体和方石英固溶体,而含铝物相只有铁铝尖晶石。焙烧温度的升高会促使石英固溶体转变为方石英固溶体,此时铁铝尖晶石结晶形态完善并无明显变化。1150 ℃还原焙烧熟料中的主要物相为方石英固溶体和铁铝尖晶石。提高温度至1200 ℃,除方石英固溶体和铁铝尖晶石以外,还原焙烧熟料中也发现了莫来石的存在。结合图2(a)分析可知,高岭土和氧化铁混合生料在还原焙烧过程中,高岭石的热分解以及氧化铁的还原同时进行。在1000~1150 ℃下高岭石分解的非晶态产物具有很高的反应活性,可以与氧化亚铁快速反应生成铝酸亚铁和二氧化硅固溶体。而1200 ℃下莫来石结晶形态完善,使其与氧化亚铁的反应速率降低[17],因此,在相同的焙烧条件下获得的还原焙烧熟料中仍能发现莫来石的存在。需要指出的是,由于高岭石在1000~1150 ℃热分解产物呈无定型态,因此通过XRD难以确定在还原焙烧熟料中是否有无定型相的存在。
为明确还原焙烧熟料中是否有无定型相存在,采用XPS对1000~1150 ℃还原焙烧熟料进行分析,实验结果见图3。由图3(a)所示Al 2p的分析结果可知,在1000 ℃还原焙烧熟料中,除铝酸亚铁外,还存在少量的莫来石相。在还原焙烧过程中,高岭石热分解产物中的无定型莫来石与氧化亚铁结合生成铁铝尖晶石。在动力学方面,焙烧温度越高越有利于反应的进行,而未反应的无定型莫来石则以独立相存在于还原焙烧熟料中。在1050~1150 ℃还原焙烧熟料中的含铝物相为铁铝尖晶石,意味着焙烧温度的升高有利于铁铝尖晶石的形成。
由图3(b)所示Si 2p的分析结果可知,除了图2(b)中已发现的石英固溶体外,1000 ℃还原焙烧熟料中也发现了莫来石和无定型氧化硅的存在。进一步说明高岭土和氧化铁混合生料在此焙烧温度下并未完全反应,未参与反应的高岭石在此温度下分解为莫来石和无定型二氧化硅。在1050~1150 ℃还原焙烧熟料中能发现石英固溶体和方石英固溶体的存在,且焙烧温度的升高会促使石英固溶体转变为方石英固溶体。在1150 ℃还原焙烧熟料中仅发现方石英固溶体的存在。需要指出的是,XPS图谱中二氧化硅的特征峰与石英和方石英的特征峰一致,此结果类似于图2(b)的XRD分析结果。
图3 还原焙烧熟料中Al 2p和Si 2p的XPS谱
Fig. 3 XPS spectra for Al 2p(a) and Si 2p(b) in clinkers
综上所述,控制高岭土和氧化铁混合生料中Fe2O3和Al2O3摩尔比为1.2:2.0,在1050~1150 ℃还原焙烧60 min可将高岭石完全转变为铁铝尖晶石和二氧化硅固溶体-石英固溶体和方石英固溶体。此外,焙烧温度升高会促使石英固溶体转变为方石英固溶体。
进一步采用FTIR对还原焙烧熟料中二氧化硅固溶体进行研究,实验结果见图4。为便于对比,天然石英和硅胶的FTIR图谱也列于图4中。石英具有良好的结晶形态,其FTIR图谱中的1082 cm-1为硅氧四面体中Si—O—Si反对称伸缩振动峰,796 cm-1和778 cm-1为分裂的对称伸缩振动峰。硅胶为无定型氧化硅,其对称伸缩振动峰为单峰,位于807 cm-1[18]。因此,通过FTIR中Si—O—Si对称伸缩振动峰的形状可以推测结晶二氧化硅和无定型二氧化硅。由图2(b)和图3(b)分析结果可知,1000 ℃和1050 ℃还原焙烧熟料中的主要含硅物相为石英固溶体,然而其FTIR图谱中仅存在795 cm-1处一个特征峰。说明还原焙烧熟料中的石英固溶体晶体具有长程有序结构,但是在短程上仍保持无序结构,致使其在XRD图谱中显示为石英,而在FTIR图谱中显示为无定型二氧化硅。随着焙烧温度的升高,硅氧四面体中Si—O—Si反对称伸缩振动峰逐渐往高波数偏移,说明氧化硅固溶体结构趋于稳定,此结果与图2(b)中石英固溶体转变为方石英固溶体结果一致。
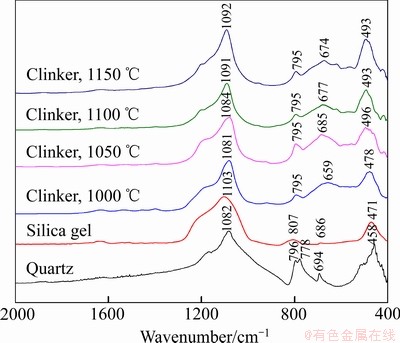
图4 还原焙烧熟料的FTIR谱
Fig. 4 FTIR spectra of clinkers
2.2 二氧化硅固溶体在碱溶液中的溶解行为
通过添加氧化铁还原焙烧,可将高岭石完全转变为铝酸亚铁和二氧化硅固溶体-石英固溶体和方石英固溶体。天然的石英和方石英在氢氧化钠溶液中比较稳定,其中石英在>180 ℃才与氢氧化钠溶液剧烈反应[19]。鉴于铁铝尖晶石具有稳定的尖晶石结构,难溶于氢氧化钠溶液,将1100 ℃还原焙烧熟料在氢氧化钠溶液中进行溶出,以明确二氧化硅固溶体的溶解性。碱浸溶出实验结果见图5。在液固比(L/S)10:1、氢氧化钠浓度(ρ(NaOH)) 160 g/L、浸出时间120 min的条件下,还原焙烧熟料在不同温度下的浸出结果如图5(a)所示。还原焙烧熟料中二氧化硅固溶体易溶于碱溶液,在80 ℃下其溶出率即达到50%以上,且随着浸出温度的升高而急剧增加。当浸出温度提高至110 ℃时,此时浸出渣中二氧化硅含量降低至3.80%,二氧化硅溶出率达到94.19%。浸出温度的升高会提高二氧化硅固溶体与氢氧化钠溶液的反应速率,加快二氧化硅的溶解。继续提高浸出温度至120 ℃对二氧化硅的溶出率并无明显影响,因此,适宜的浸出温度为110 ℃。此外,浸出结果也表明,还原焙烧熟料中的石英固溶体和方石英固溶体均有别于天然的石英和方石英,在常压下即易溶于氢氧化钠溶液。
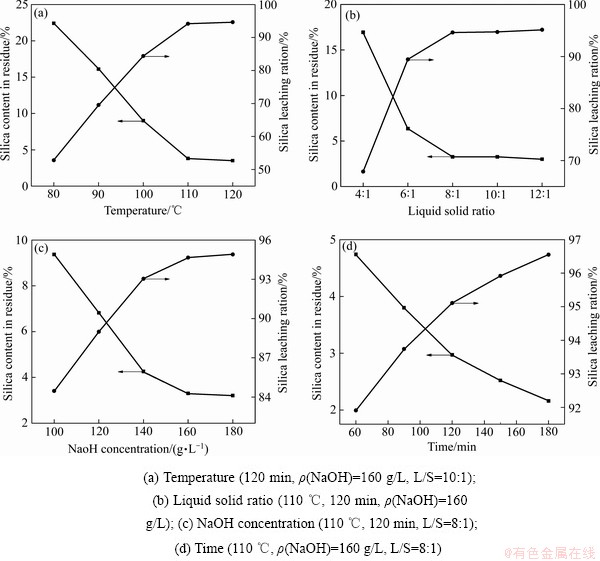
图5 实验条件对1100 ℃还原焙烧熟料的碱浸溶硅结果的影响
Fig. 5 Effect of experimental conditions on silica leaching results in clinker obtained at 1100 ℃ with different leaching conditions
在浸出温度110 ℃、氢氧化钠浓度160 g/L、浸出时间120 min的条件下,还原焙烧熟料在不同液固比下的浸出结果如图5(b)所示。当液固比由10:1降低至8:1时,还原焙烧熟料中二氧化硅溶出率并无明显变化,仍保持在94%以上。此后,二氧化硅的溶出率随液固比减小而急剧降低。在相同浸出条件下,液固比的减少降低了溶出过程中二氧化硅与氢氧化钠溶液的接触机会,从而不利于二氧化硅的浸出。为保证较高的二氧化硅溶出率,适宜的浸出液固比为8:1。
改变浸出条件为浸出温度110 ℃、浸出时间120 min、液固比8:1,氢氧化钠浓度对还原焙烧熟料浸出结果的影响见图5(c)。由图5(c)可知,二氧化硅溶出率与氢氧化钠浓度密切相关。氢氧化钠浓度低于160 g/L时,二氧化硅溶出率急剧降低。溶液中氢氧化钠浓度的降低意味着浸出过程中游离的OH-减少,二氧化硅的溶解速率降低。提高氢氧化钠浓度至180 g/L时,二氧化硅浸出率的增加幅度较小。因此,控制氢氧化钠浓度为160 g/L即可获得较好的浸出结果。
以160 g/L的氢氧化钠溶液为浸出剂,在浸出温度110 ℃和液固比8:1条件下考察浸出时间对二氧化硅溶出率的影响,实验结果见图5(d)。浸出时间为60 min时,二氧化硅溶出率即达到90%以上,且二氧化硅的溶出率随浸出时间的延长进一步提高。在180 min时,浸出渣中二氧化硅含量可降低至2.16%,此时二氧化硅溶出率达到96.56%。在碱浸溶出二氧化硅过程中,还原焙烧熟料中的铁铝尖晶石比较稳定而形成未反应层,致使颗粒内部的二氧化硅难以被溶解。因此,二氧化硅的溶出率随浸出时间的延长而提高。
浸出渣的SEM和XRD图谱如图6所示。经碱浸脱硅后,浸出渣颗粒表面呈疏松多孔结构。相比于图2(b),浸出渣中仅能发现铁铝尖晶石的存在,而石英固溶体和方石英固溶体均已消失。溶出结果表明,铁铝尖晶石具有稳定的尖晶石结构,在此浸出条件下难与氢氧化钠溶液反应。而石英固溶体和方石英固溶体均有别于天然的石英和方石英,在低温(约110 ℃)即与氢氧化钠溶液剧烈反应。此溶出结果与FTIR分析结果一致,即石英固溶体和方石英固溶体在短程上仍保持无序结构,具有较高的反应活性。
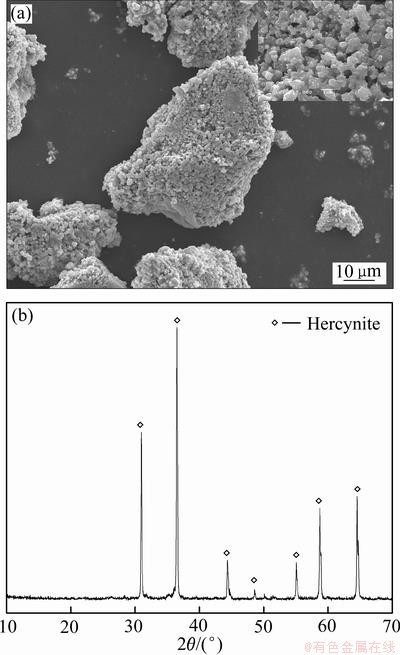
图6 浸出渣的SEM像和XRD谱
Fig. 6 SEM image(a) and XRD pattern(b) of leaching residue (Leaching conditions: 110 ℃, 180 min, ρ(NaOH)= 160 g/L, L/S=8:1)
2.3 二氧化硅固溶体的形成机理
为明确高岭石与氧化铁还原焙烧过程中的二氧化硅固溶体的形成机理,首先从热力学角度对还原焙烧过程进行讨论。高岭石的热分解和氧化铁的还原首先发生,其次是生成铁铝尖晶石和二氧化硅固溶体的过程。高岭石在>1000 ℃的热分解产物为莫来石和二氧化硅,其与氧化亚铁可能发生的反应如反应式(1)~(5)所示。反应式(1)~(5)的反应吉布斯自由能(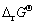 )与温度(t)的关系如图7所示,其中的热力学数据取自文献[20]。为便于比较分析,各反应式中的FeO和FeAl2O4系数均统一为1。
)与温度(t)的关系如图7所示,其中的热力学数据取自文献[20]。为便于比较分析,各反应式中的FeO和FeAl2O4系数均统一为1。
FeO+1/3(3Al2O3·2SiO2)=FeAl2O4+2/3SiO2 (1)
FeO+1/2SiO2=1/2(2FeO·SiO2) (2)
1/2(2FeO·SiO2)+1/3(3Al2O3·2SiO2)=FeAl2O4+7/6SiO2 (3)
FeAl2O4+CO(g)=Al2O3+Fe+CO2(g) (4)
FeAl2O4+2/3SiO2+CO(g)=1/3(3Al2O3·2SiO2)+Fe+CO2(g) (5)
由图7可知,反应式(1)和反应式(2)的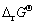 均小于0,意味着在还原焙烧过程中氧化亚铁会按照反应式(1)与莫来石反应生成铁铝尖晶石和二氧化硅,也会按照反应式(2)与二氧化硅反应而生成铁橄榄石。由于反应式(1)的
均小于0,意味着在还原焙烧过程中氧化亚铁会按照反应式(1)与莫来石反应生成铁铝尖晶石和二氧化硅,也会按照反应式(2)与二氧化硅反应而生成铁橄榄石。由于反应式(1)的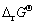 小于反应式(2)的
小于反应式(2)的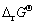 ,在热力学上反应式(1)比反应式(2)更易发生。由于反应式(3)的
,在热力学上反应式(1)比反应式(2)更易发生。由于反应式(3)的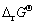 值为负,即使反应式(2)发生,铁橄榄石会进一步与莫来石反应生成铁铝尖晶石和二氧化硅。相反,反应式(4)和反应式(5)的
值为负,即使反应式(2)发生,铁橄榄石会进一步与莫来石反应生成铁铝尖晶石和二氧化硅。相反,反应式(4)和反应式(5)的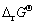 均大于0,说明生成的铁铝尖晶石在还原气氛比较稳定。在热力学上,高岭石和氧化铁混合生料的最终还原焙烧产物为铁铝尖晶石和二氧化硅。考虑到高岭石和氧化铁还原焙烧过程为固相反应[14],意味着氧化亚铁与氧化铝结合生成铁铝尖晶石时需要穿过焙烧高岭石的二氧化硅层。
均大于0,说明生成的铁铝尖晶石在还原气氛比较稳定。在热力学上,高岭石和氧化铁混合生料的最终还原焙烧产物为铁铝尖晶石和二氧化硅。考虑到高岭石和氧化铁还原焙烧过程为固相反应[14],意味着氧化亚铁与氧化铝结合生成铁铝尖晶石时需要穿过焙烧高岭石的二氧化硅层。
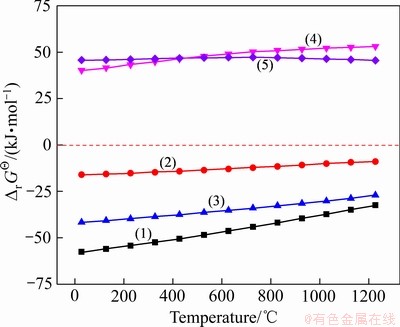
图7 反应式(1)~(5)的反应吉布斯自由能与温度的关系
Fig. 7 Relationship between 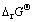 and temperature for Eqs.(1)-(5)
and temperature for Eqs.(1)-(5)
为更好地了解高岭石与氧化铁的还原焙烧过程,对1100 ℃还原焙烧熟料进行SEM像及元素面扫描分析,实验结果见图8。还原焙烧熟料中的铁元素和铝元素分布一致,均集中在白色区域,而硅元素则相对集中分布在灰色区域,且铁铝尖晶石与二氧化硅均具有一定的粒度分布。考虑到焙烧高岭石仍保持着Al—O和Si—O的非稳态的层状结构[21],说明在以固相反应为主的还原焙烧过程中,焙烧产物中氧化铝会选择性地往氧化亚铁迁移并最终生成铁铝尖晶石,受氧化铝迁移的影响,少量的Fe2+会进入硅氧四面体中。因此,还原焙烧熟料中的二氧化硅以固溶体的形式存在,在XRD和XPS中显示出长程有序结构,而在FTIR中则显示出短程无序结构。
3 结论
1) 控制高岭土和氧化铁混合生料中Fe2O3和Al2O3摩尔比为1.2:2.0,在1050~1150 ℃还原焙烧60 min,可将高岭石完全转变为铁铝尖晶石和二氧化硅固溶体-石英固溶体和方石英固溶体。焙烧温度的升高会促使石英固溶体转变为方石英固溶体。
2) 石英固溶体和方石英固溶体在XRD和XPS中显示出长程有序结构,但是在FTIR中则表现出短程无序结构,致使其在低温(约110 ℃)下即与氢氧化钠溶液剧烈反应。
3) 还原焙烧过程为固相反应,氧化铝会选择性与氧化亚铁反应并生成铁铝尖晶石。受氧化铝迁移的影响,少量的Fe2+进入硅氧四面体中并最终形成二氧化硅固溶体。
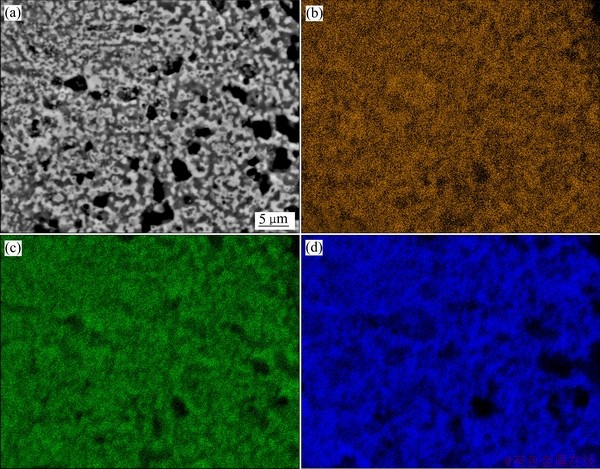
图8 1100 ℃还原焙烧熟料的SEM像和Fe、Al、Si元素面扫描图谱
Fig. 8 SEM image(a) and elements Fe(b), Al(c), Si(d) scanning mapping of clinker obtained at 1100 ℃
REFERENCES
[1] LIEW Y M, KAMARUDIN H, AL BAKRI A M M, et al. Processing and characterization of calcined kaolin cement powder[J]. Construction and Building Materials, 2012, 30: 794-802.
[2] HUBADILLAH S K, OTHMAN M H D, MATSUURA T, et al. Fabrications and applications of low cost ceramic membrane from kaolin: A comprehensive review[J]. Ceramics International, 2018, 44(5): 4538-4560.
[3] ZHANG Yun-sheng, SUN Wei, LI Zong-jin. Composition design and microstructural characterization of calcined kaolin-based geopolymer cement[J]. Applied Clay Science, 2010, 47(3/4): 271-275.
[4] 李国栋, 殷尧禹, 卢 瑞, 等. 高岭土提纯工艺及其应用研究进展[J]. 矿产保护与利用, 2018(4): 142-150.
LI Guo-dong, YIN Yao-yu, HAN Cong, et al. Purification process and application progress of kaolin[J]. Conservation and Utilization of Mineral Resources, 2018(4): 142-150.
[5] QIU Guan-zhou, JIANG Tao, LI Guang-hui, et al. Activation and removal of silicon in kaolinite by thermochemical process[J]. Scandinavian Journal of Metallurgy, 2004, 33(2): 121-128.
[6] SRIKRISHNA K, THOMAS G, MARTINEZ R, et al. Kaolinite-mullite reaction series: A TEM study[J]. Journal of Materials Science, 1990, 25(1): 607-612.
[7] BRINDLEY G W, NAKAHIRA M. The kaolinite-mullite reaction series: Ⅰ. A survey of outstanding problems[J]. Journal of the American Ceramic Society, 1959, 42(7): 311-314.
[8] CHANDRASEKHAR S, RAMASWAMY S. Influence of mineral impurities on the properties of kaolin and its thermally treated products[J]. Applied Clay Science, 2002, 21(3/4): 133-142.
[9] 周 舟, 周汉文, 崔潇妹, 等. 高岭土热分析中相变的影响因素: 以广西合浦高岭土为例[J]. 矿物学报, 2015, 35(3): 384-394.
ZHOU Zhou, ZHOU Han-wen, CUI Xiao-mei, et al. Effect of mineralogical characteristics on phase transformation of calcined kaolin: A case study on Hepu kaolin[J]. Acta Mineralogica Sinica, 2015, 35(3): 384-394.
[10] CHAKRABORTY A K, GHOSH D K. Reexamination of the kaolinite-to-mullite reaction series[J]. Journal of the American Ceramic Society, 1978, 61(3/4): 170-173.
[11] LI Xiao-bin, WANG Hong-yang, ZHOU Qiu-sheng, et al. Efficient separation of alumina and silica in reduction- roasted kaolin by alkali leaching[J]. Transactions of Nonferrous Metals Society of China, 2019, 29(2): 416-423.
[12] SEGNIT E R, GELB T. Metastable quartz-type structures formed from kaolinite by solid state reaction[J]. American Mineralogist, 1972, 57: 1505-1514.
[13] HUGHES J C. The effects of experimental conditions on the 950 ℃ kaolinite exotherm in some tropical soil clays[J]. Clay Minerals, 1979, 21(14): 21-28.
[14] TAKEUCHI N, TAKAHASHI H, ISHIDA S, et al. Mechanistic study of solid-state reaction between kaolinite and ferrous oxide at high temperatures[J]. Journal of the Ceramic Society of Japan, 2000, 108(10): 876-881.
[15] ZHOU Qiu-sheng, LI Chuang, LI Xiao-bin, et al. Reaction behavior of ferric oxide in system Fe2O3-SiO2-Al2O3 during reductive sintering process[J]. Transactions of Nonferrous Metals Society of China, 2016, 26(3): 842-848.
[16] LI Xiao-bin, WANG Hong-yang, ZHOU Qiu-sheng, et al. Reaction behavior of kaolinite with ferric oxide during reduction roasting[J]. Transactions of Nonferrous Metals Society of China, 2019, 29(1): 186-193.
[17] LI Xiao-bin, WANG Hong-yang, ZHOU Qiu-sheng, et al. Efficient separation of silica and alumina in simulated CFB slag by reduction roasting-alkaline leaching process[J]. Waste Management, 2019, 87: 798-804.
[18] 翁诗甫, 许怡庄. 傅里叶变换红外光谱分析[M]. 3版. 北京: 化学工业出版社, 2016.
WENG Shi-fu, XU Yi-zhuang. Analysis of Fourier transform infrared spectra[M]. 3rd ed. Beijing: Chemical Industry Press, 2016.
[19] CROKERD, LOAN M, HODNETT B K. Desilication reactions at digestion conditions: an in situ X-ray diffraction study[J]. Crystal Growth and Design, 2008, 8(12): 4499-4505.
[20] BARIN I. Thermochemical data of pure substances[M]. Weinheim: VCH Verlagsgesellschaft mbH, 1995.
[21] SPERINCK S, RAITERI P, MARKS N, et al. Dehydroxylation of kaolinite to metakaolin-a molecular dynamics study[J]. Journal of Materials Chemistry, 2011, 21(7): 2118-2125.
Formation mechanism of silica solid solution during reduction roasting of kaolinite and ferric oxide
WANG Hong-yang1, 2, ZHANG Xiao-xue1, SONG Shao-xian1, 2
(1. School of Resources and Environmental Engineering, Wuhan University of Technology, Wuhan 430070, China;
2. Hubei Key Laboratory of Mineral Resources Processing and Environment, Wuhan University of Technology, Wuhan 430070, China)
Abstract: The reaction behavior of kaolinite and ferric oxide during reduction roasting was systematically studied through the analysis methods of XRD, XPS, FTIR and SEM-EDS, meanwhile, the solubility of free silica in the reduction product was tested by leaching in NaOH solution. Based on the above studies, the formation mechanism of silica solid solution was discussed. The results show that kaolinite is converted into amorphous state during roasting alone at 1000-1150 ℃, while converted into hercynite and free silica, in the forms of quartz solid solution and cristobalite solid solution, during reductively roasting with hematite at 1050-1150 ℃. In addition, quartz solid solution and cristobalite solid solution have the similarity of structure with natural quartz and cristobalite, but have the dissimilarity of solubility in NaOH solution. Due to the solid-state reaction in reduction roasting, the alumina can selectively react with ferrous oxide to form hercynite, meanwhile, small amount of Fe2+ enters into silica tetrahedra, resulting in the formation of silica solid solution.
Key words: kaolinite; reduction roasting; hercynite; quartz solid solution; cristobalite solid solution
Foundation item: Project(2019M662733) supported by the China Postdoctoral Science Foundation; Project (2019IVA096) supported by the Fundamental Research Funds for the Central Universities, China; Project(2018YFC1901502) supported by the National Key Research and Development Program of China
Received date: 2020-02-19; Accepted date: 2020-12-24
Corresponding author: WANG Hong-yang; Tel: +86-18229790554; E-mail: hywang3@whut.edu.cn
(编辑 王 超)
基金项目:中国博士后科学基金资助项目(2019M662733);中央高校基本科研业务费专项资金资助项目(2019IVA096);国家重点研发计划资助项目(2018YFC1901502)
收稿日期:2020-02-19;修订日期:2020-12-24
通信作者:王洪阳,助理研究员,博士;电话:18229790554;E-mail:hywang3@whut.edu.cn