
J. Cent. South Univ. (2020) 27: 2249-2258
DOI: https://doi.org/10.1007/s11771-020-4446-3
Separation of silicon and iron in copper slag by carbothermic reduction-alkaline leaching process
WANG Hong-yang(王洪阳)1, SONG Shao-xian(宋少先)1, 2
1. School of Resources and Environmental Engineering, Wuhan University of Technology,Wuhan 430070, China;
2. Hubei Key Laboratory of Mineral Resources Processing and Environment, Wuhan University of Technology, Wuhan 430070, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: Approximately 2.0-3.0 t of copper slag (CS) containing 35%-45% iron is generated for every ton of copper produced during the pyrometallurgical process from copper concentrate. Therefore, the recovery of iron from CS utilizes a valuable metal and alleviates the environmental stress caused by stockpile. In this paper, a new method has been developed to realize the enrichment of iron in CS through the selective removal of silica. The thermodynamic analyses and experimental results show that the iron in CS can be fully reduced into metallic iron by carbothermic reduction at 1473 K for 60 min. The silica was converted into free quartz solid solution (QSS) and cristobalite solid solution (CSS). QSS and CSS are readily soluble, whereas metallic iron is insoluble, in NaOH solution. Under optimal leaching conditions, a residue containing 87.32% iron is obtained by decreasing the silica content to 6.02% in the reduction roasted product. The zinc content in the residue is less than 0.05%. This study lays the foundation for the development of a new method to comprehensively extract silicon and iron in CS while avoiding the generation of secondary tailing.
Key words: copper slag; quartz solid solution; cristobalite solid solution; carbothermic reduction; alkaline leaching
Cite this article as: WANG Hong-yang, SONG Shao-xian. Separation of silicon and iron in copper slag by carbothermic reduction-alkaline leaching process [J]. Journal of Central South University, 2020, 27(8): 2249-2258. DOI: https://doi.org/10.1007/s11771-020-4446-3.
1 Introduction
Copper slag (CS) is a byproduct of the pyrometallurgical production of copper. According to statistics, approximately 40-50 million tons of CS are produced annually worldwide [1, 2]. Approximately 35%-45% iron and 25%-35% silica exist in CS, whereas the concentrates of other ingredients of aluminum, calcium, magnesium, zinc and copper are normally less than 10% [3, 4]. Therefore, about 16-20 million tons of iron can be recovered from CS if treated suitably. Unfortunately, only a small amount of CS is used to make building materials and abrasive despite its favourable physico-mechanical characteristics [5, 6]. The majority of CS is disposed by stockpile, which causes land occupation and environmental pollutions [7, 8]. Thus, iron recovery from CS would allow the comprehensive utilization of a valuable metal, decrease the number of dump sites, and alleviate the environmental burden.
The methods for iron recovery from CS can be roughly classified into physical separation and pyrometallurgical process. Slow cooling rate benefits the growth of magnetite particle in CS. Then, partial iron is recovered by magnetic separation, because more than 50% of the iron in CS exists in the form of fayalite [8-10]. By carbothermic reduction, the main iron-bearing minerals of magnetite and fayalite in CS can be reduced into metallic iron, which is suitable for recovery by magnetic separation. The addition of fluxing materials, such as CaO and Na2CO3, decreases the roasting temperature and promotes iron grain growth [11, 12]. Undoubtedly, the generation of secondary tailing is unavoidable, because only iron, copper and cobalt in CS can be recovered in the form of iron-rich alloy, and the fluxing materials are difficult to recycle.
To minimize the potential impacts, many new technologies for treating the complex ores and slags are explored based on the establishment of a closed loop cycle by recycling and reusing instead of establishing an open loop cycle [13-16]. Considering that the main chemical components in CS are iron and silicon, iron content can be upgraded through the selective removal of silicon. Relevant studies have been proved that fayalite in CS can be decomposed into amorphous silica and hematite by oxidation roasting. Then, the amorphous silica is selectively removed by alkaline leaching at temperature higher than 433 K [17, 18]. However, the obtained leaching residue cannot be used as raw material in iron-making process due to the presence of zinc and lead [19]. Therefore, carbothermic reduction is performed for the decomposition of fayaltie into metallic iron and free silica, whereas zinc and lead are enriched in dust. Previous studies on carbothermic reduction focused on the phase transformation of iron, copper, and zinc in CS for iron recovery by magnetic separation, and few studies discussed the phase transformation of silica [3, 9, 11].
In this paper, the phase transformation of CS during carbothermic reduction was investigated through X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and energy dispersive spectroscopy (EDS). The research on silica dissolution was conducted by alkaline leaching to clarify the solubility of silica in reduction roasted product of CS.
2 Experimental
2.1 Materials
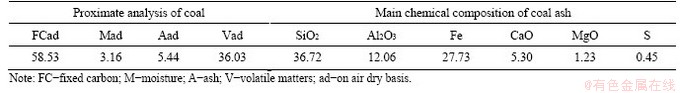
The CS (d(50)=23.96 μm) used in the experiment is a flotation tailing of converting slag from the pyrometallurgical production of copper in Tongling Nonferrous Metals Group, China. The coal (d(50)=10.40 μm) used as reducing agent during roasting is offered by Shaanxi Shenmu Energy Group Co., Ltd, China. The proximate analysis of the coal and main chemical composition of the coal ash are presented in Table 1. The fixed carbon and volatile matters in this coal are 58.53% and 36.03%, respectively, whereas the percentage of ash is as low as 5.44%. Therefore, this type of coal is suitable for use as reducing agent of iron ore [20].
2.2 Procedures
The CS was uniformly mixed with the coal using a V-mixer (WKA-10, Senli Medicale Quipment Co. Ltd, China). The amount of coal was approximately 150% more than the stoichiometric proportion needed to convert iron oxides in CS to metallic iron based on the conversion of fixed carbon into carbon monoxide. Then, the mixture of CS and coal was placed into a corundum crucible and reductively roasted in a thermostatic muffle furnace (SX-5-12, Changsha Dianlu Co., Ltd, China), which was outlined in reference [21]. When the duration was reached, the corundum crucible was taken out from the furnace and cooled to room temperature with the protection of coke powder. The obtained reduction roasted product was named as reduced copper slag (RCS).
The silica leaching experiments were conducted in an autoclave (GS-0.25, Weihai Dingda Chemical Machinery Co., Ltd, China). Certain amounts of ground RCS (-74 μm) and 100 mL NaOH solution were added into the autoclave. Then, the slurry was heated to the reaction temperature for a curtain duration. Afterward, the autoclave was immediately cooled by using tap water, and the solid in the slurry was obtained by liquid-solid separation for further analysis.
Table 1 Properties of the coal (mass fraction, %)
The phase analysis of the CS, RCS and residue was determined by X-ray diffractometer (XRD, MAX-RB, Rigaku Co., Tokyo, Japan) and FTIR 6700 spectrometer with 4 cm-1 resolution (Nicolet Co., USA). Surface microscopic morphology was performed using a scanning electron microscope (SEM, JSM-IT300, JEOL Ltd, Tokyo, Japan). The particle size distribution of the samples was measured by using a particle size analyzer (Mastersizer 2000, Malvern, UK). The chemical compositions were analyzed by atomic absorption spectrometer (CONTRAA-700, Analytik Jena AG, Germany).
2.3 Theoretical fundamental
2.3.1 Carbothermic reduction
According to relevant studies, the main phases in CS are fayaltie, magnetite, zinc ferrite, hercynite and other glass phase [6, 22]. The possible reactions that occur during carbothermic reduction are listed in Eqs. (1)-(6). The stoichiometric coefficient of C was normalized as 1 for the convenience of comparison.
1/2Fe2SiO4+C=Fe+1/2SiO2+CO(g) (1)
1/2Fe2SiO4+CO(g)=Fe+1/2SiO2+CO2(g) (2)
1/4Fe3O4+C=3/4Fe+CO(g) (3)
1/4ZnFe2O4+C=1/4Zn(g)+1/2Fe+CO(g) (4)
FeAl2O4+C=Fe+Al2O3+CO(g) (5)
3Al2O3+2SiO2=3Al2O3·2SiO2 (6)
Based on the thermodynamic data in Ref. [23], the relationships between standard Gibbs free energy change △rGΘ and temperature T in Eqs. (1)-(6) were calculated and plotted in Figure 1.
Figure 1 shows that the conversion of fayalite into metallic iron and silica can occur readily by carbothermic reduction, because the △rGΘ for Eq. (1) becomes negative with the temperature exceeding 1043 K. However, fayaltie is stable in CO atmosphere due to the positive △rGΘ for Eq. (2). Only fixed carbon affects the decomposition of fayalite during the carbothermic reduction. Moreover, the value of △rGΘ for Eqs. (3) and (4) is smaller than that for Eq. (1) at >1043 K, indicating that the reduction of magnetite and zinc ferrite by carbon occurs prior to that of fayalite. In addition, hercynite is more stable than fayalite and can be thermodynamically reduced into metallic iron and alumina through Eq. (5) at >1272 K. When the carbothermic reduction of hercynite occurs, alumina further reacts with silica to form mullite through Eq. (6) for the negative △rGΘ. Therefore, iron- bearing minerals in CS can be fully reduced into metallic iron by carbothermic reduction. The silica in RCS mainly exists in the forms of free silica and mullite.
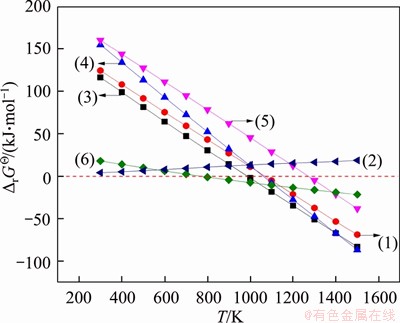
Figure 1 Relationship between △rGΘ and T for Eqs. (1)-(6)
2.3.2 Alkaline leaching of silica
The silica can be dissolved into NaOH solution through Eq. (7) as shown below. However, the dissolution temperature strongly depends on the crystallization of silica. The amorphous silica from the decomposition of kaolinite at >1253 K is readily soluble in NaOH solution at atmospheric pressure [24], while the quick dissolution temperature of quartz into NaOH solution occurs at >433 K [25]. Thus, the iron in RCS can undergo beneficiation through the alkaline leaching of silica.
SiO2(s)+2NaOH(aq)=Na2H2SiO4(aq) (7)
3 Results and discussion
3.1 Characteristics of CS
The chemical composition of the CS is listed in Table 2. The main ingredients in the CS are iron and silica, and their contents are 48.55% and 26.05%, respectively. The mineralogical analysis as present in Figure 2 indicates that the main minerals in the CS are fayalite and magnetite, whereas the other minerals are not observed by XRD because of their low content or poor crystallinity. The diffraction apex at 2θ of 31.0° in the XRD pattern of CS is still an exception [26].
Table 2 Chemical composition of CS (mass fraction, %)
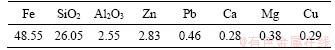
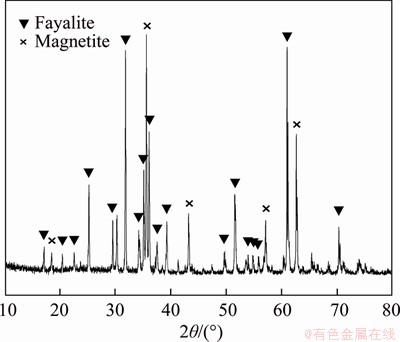
Figure 2 XRD pattern of CS
3.2 Phase transformation of CS during carbothermic reduction
3.2.1 XRD analysis
The influence of temperature and duration on the phase transformation of CS during carbothermic reduction were investigated experimentally. As shown in Figure 3(a), with duration of 60 min, the typical diffraction peaks of magnetite vanish, whereas the diffraction apices of fayalite decrease in the XRD pattern of RCS-1273K compared with those in Figure 2, indicating that the reduction of magnetite occurs prior to that of fayalite during carbothermic are reduction. These results agree with those discussed in Section 2.3.1. The typical diffraction peaks of quartz and cristobalite are found in the XRD pattern of RCS-1273K. However, the quartz and cristobalite found in RCS are defined as quartz solid solution (QSS) and cristobalite solid solution (CSS), respectively, due to their solubility in NaOH solution under atmospheric pressure [14]. With increasing temperature, the diffraction apices of fayalite gradually weaken in the XRD pattern of RCS-1373K, whereas those of both QSS and CSS strengthen. When the roasting temperature reaches 1473 K, only the typical diffraction peaks of metallic iron, QSS and CSS can be found in the XRD pattern of corresponding RCS. Thus, 1473 K is chosen as the appropriate roasting temperature for the full decomposition of fayalite.
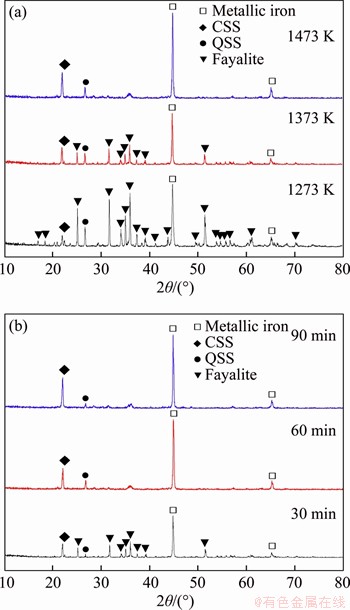
Figure 3 Influence of temperature (a) and duration (b) on phase transformation in CS
The mixtures of CS and coal were respectively roasted at 1473 K for 30, 60 and 90 min. XRD patterns of the obtained RCS are present in Figure 3(b). Shortening the duration from 60 to 30 min cannot realize the full decomposition of fayalite in CS due to the presence of typical diffraction peaks of fayalite in the RCS. However, prolonging the duration to 90 min benefits the conversion of QSS to CSS in terms of the variation of their diffraction apices in RCS.
In summary, adopting proper temperature and duration can lead to the full conversion of CS into metallic iron and free silica in the forms of QSS and CSS by carbothermic reduction. Thus, the optimal conditions include a temperature of 1473 K and a duration of 60 min.
3.2.2 SEM-EDS analysis
To determine the variation of silicon and iron during carbothermic reduction, the CS and RCS were analyzed by SEM-EDS. The SEM-EDS images of CS indicate that iron and silicon disperse in the form of fine grain dissemination, together with small amounts of independent silica and iron oxide as shown in Figure 4(a). After carbothermic reduction, the particles of metallic iron and silica exist independently in RCS as shown in Figure 4(b), which indicates that CS has been totally reduced. However, the particle of metallic iron in RCS is wrapped by free silica, and its size is less than 10 μm, resulting in the low recovery of iron by means of grinding and magnetic separation [7]. Thus, the separation of silicon and iron in RCS should be examined by chemical methods.
3.2.3 FTIR analysis
To determine the characteristics of silica solid solutions, the RCS was analyzed by FTIR. The results are shown in Figure 5. The infrared spectrum of pure silica gel is also exhibited in Figure 5 for comparison. There are three characteristic peaks in the infrared spectrum of silica gel, as follows: 471 and 806 cm-1 belong to Si—O—Si stretching vibration; and 1104 cm-1 belongs to Si—O—Si asymmetric stretching vibration [27]. QSS and CSS are the main silicon-bearing minerals in the XRD pattern of RCS, as shown in Figure 3. Compared with silica gel, the infrared spectrum of RCS shows the three characteristic peaks at 480,792 and 1093 cm-1, respectively, and does not split into two peaks at about 800 cm-1 for the characteristic of quartz [28]. Therefore, the structures of QSS and CSS are similar to those of natural quartz and cristobalite in XRD, but show dissimilar Si—O—Si bonds in FTIR.
Figure 4 SEM-EDS images of CS (a, b, c) and RCS (d, e, f)

Figure 5 Infrared spectra of silica gel and RCS
3.3 Removal of silica by alkaline leaching
Alkaline leaching was conducted to investigate the solubility of silica in RCS, and the results are present in Figure 6. The influence of temperature on silica leaching was first studied under the following conditions: leaching time of 180 min, liquid/solid (L/S) ratio of 10:1 and NaOH concentration (ρ(NaOH)) of 160 g/L. The results shown in Figure 6(a) reveal that the silica content in residue strongly depends on the leaching temperature. The silica content decreases from 18.34% to 5.78% with increasing leaching temperature from 353 to 383 K. The silica content is 5.35% after leaching at 393 K.
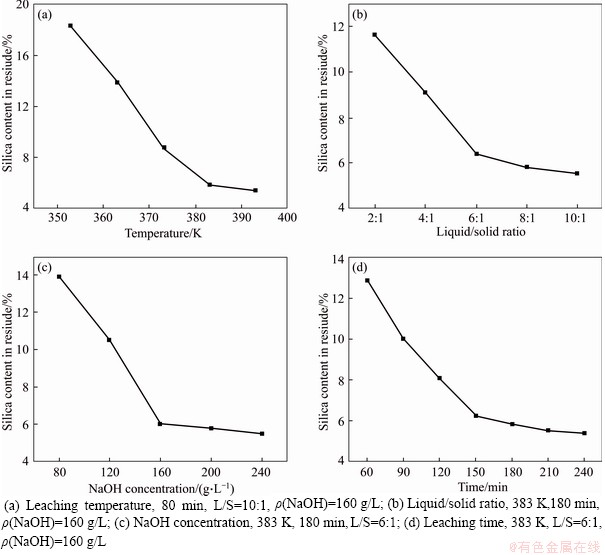
Figure 6 Effect of conditions on leaching results of silica in RCS:
From dynamic aspects, elevated temperature benefits the increase of chemical reaction rate and diffusion process, accelerating the reaction between NaOH solution and silica. The silica content in residue slightly decreases when the leaching temperature exceeds 383 K. Thus, 383 K is chosen as the optimum leaching temperature.
Under the conditions of 383 K temperature, 180 min leaching time and ρ(NaOH) at 160 g/L, the influence of L/S on silica leaching is determined, and the results are displayes in Figure 6(b). Silica content in the residue decreased from 11.67% to 6.41% with increasing L/S from 2:1 to 6:1, which can be attributed to the increased contact chances between NaOH solution and silica. However, when the L/S exceeds 6:1, the silica content in residue slightly decreases. Therefore, the silica in RCS has been efficiently leached at the L/S of 6:1.
The influence of ρ(NaOH) on silica leaching is illustrated in Figure 6(c) under the conditions of 383 K temperature, 180 min leaching time and L/S of 6:1. When the ρ(NaOH) increases from 80 to 160 g/L, the percentage of silica content in the residue sharply decreases from 13.92% to 6.02%. Afterward, the silica content in the residue does not significantly change with further increase of ρ(NaOH). The increase of ρ(NaOH) indicates that the reaction of free OH- with silica increases during leaching, resulting in the increase of chemical reaction rate. Considering the recycling of NaOH solution, 160 g/L was selected as appropriate ρ(NaOH) concentration.
The effect of reaction time on silica leaching is investigated at 383 K temperature with L/S of 6:1 and ρ(NaOH) at 160 g/L, and the results are shown in Figure 6(d). The silica content in the residue rapidly decreases within the first 150 min. The silica content in the residue is 6.02% after 150 min of alkaline leaching, and a slight decrease is observed with increasing time. During the alkaline leaching of silica, the metallic iron in RCS is stable and forms an unreacted layer. Therefore, the silica in the interior is more difficult to dissolve in NaOH solution than that in the surface of RCS particles, and a certain amount of time is needed for the complete reaction of silica.
The silica in RCS is readily soluble in NaOH solution and decreases to 6.02% by leaching at 383 K for 150 min with L/S of 6:1 and at a ρ(NaOH) concentration of 160 g/L.
3.4 Analysis of leaching residue
3.4.1 XRD analysis
Compared with Figure 3, only the typical diffraction peaks of metallic iron can be found in the XRD pattern of leaching residue (Figure 7), whereas those of QSS and CSS vanish after alkaline leaching of silica. These results are in accordance with those obtained for alkaline leaching, confirming that QSS and CSS can be dissolved in NaOH solution at low temperature.
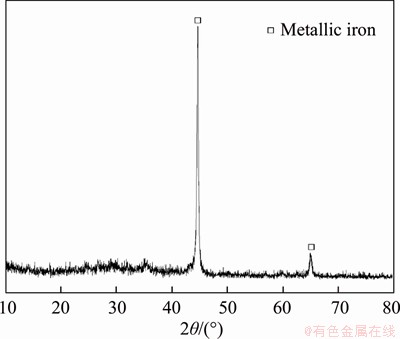
Figure 7 XRD pattern of leaching residue (Leaching conditions: 383 K, 150 min, L/S=6:1, ρ(NaOH)=160 g/L)
3.4.2 SEM-EDS analysis
The SEM-EDS images of leaching residue are shown in Figure 8. Compared with Figure 4(b), the silicon content in leaching residue has a significant decrease after alkaline leaching, whereas the metallic iron particle in leaching residue is independent and its size is less than 10 μm. Therefore, the iron content in the leaching residue can be further increased by magnetic separation.
3.4.3 Chemical compositions
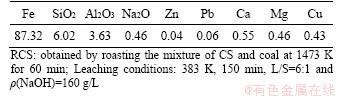
The chemical compositions of leaching residue were analyzed with the results shown in Table 3. The iron content in leaching residue reaches 87.32%, whereas the alumina content is 3.63%. The enrichment of alumina in the leaching residue can be attributed to the formation of mullite during carbothermic reduction through Eq. (6). The content of zinc in the leaching residue is less than 0.05%, which can be explained by the volatilization that occurs during carbothermic reduction, as discussed in Section 2.3.1. In addition, the copper in CS is reduced and reacts with metallic iron to form insoluble Fe-Cu alloy during carbothermic reduction [12], thereby increasing the copper content in the residue. The leaching residue is a decent raw material for iron extraction in the steelmaking industry.
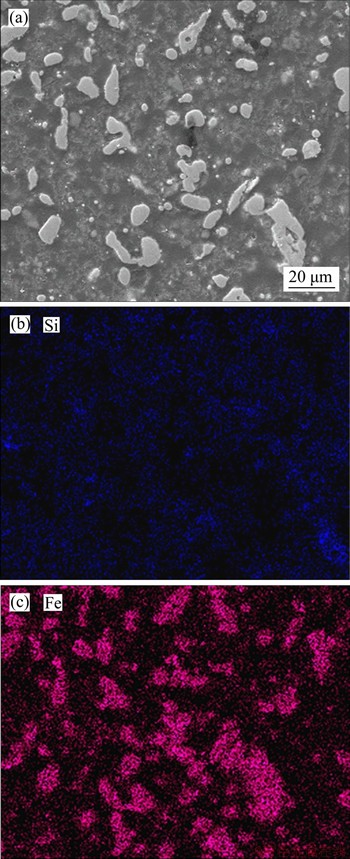
Figure 8 SEM-EDS images of leaching residue (Leaching conditions: 383 K, 150 min, L/S=6:1 and ρ(NaOH)=160 g/L)
Table 3 Chemical compositions of leaching residue of RCS (mass fraction, %)
A new method for comprehensive recovery of silicon and iron from CS is shown in Figure 9. By carbothermic reduction, the CS can be totally converted into metallic iron and readily soluble silica as QSS and CSS, whereas the zinc is enriched in dust. Then, the efficient separation of silicon and iron in RCS is achieved by alkaline leaching. In addition, the silica in alkaline silicate solution can be removed by forming a zeolite molecular sieve or wollastonite for the recycle of the NaOH solution [29, 30], whereas the residue is used for iron extraction in the steelmaking industry with or without magnetic separation. The generation of secondary tailing is very limited due to the comprehensive extraction of silicon and iron in CS. Although this new method shows potential benefits in terms of the resource utilization and low tailing generation, future work should focus on extracting zinc from dust.
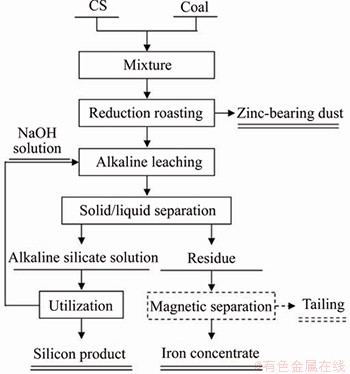
Figure 9 Flowsheet of comprehensive recovery of silicon and iron from CS
4 Conclusions
1) Thermodynamic analyses and experiments show that CS can be fully reduced into metallic iron and free silica in the forms of QSS and CSS by carbothermic reduction, and the increase of temperature and duration facilitates the conversion of QSS into CSS.
2) QSS and CSS structures are similar to those of natural quartz and cristobalite in XRD, but QSS and CSS differ from them in terms of solubility in NaOH solution.
3) A residue containing 87.32% iron and 6.02% silica is obtained by leaching the RCS at 383 K for 150 min with L/S=6:1 and ρ(NaOH)= 160 g/L. This residue can be used as raw material for iron extraction in steelmaking industry.
References
[1] FENG Yan, YANG Qi-xing, CHEN Qiu-song, KERO J, ANDERSSON A, AHMED H, ENGSTROM F, SAMUELSSON C. Characterization and evaluation of the pozzolanic activity of granulated copper slag modified with CaO [J]. Journal of Cleaner Production, 2019, 232: 1112-1120. DOI: 10.1016/j.jclepro.2019.06.062.
[2] HOLLAND K, ERIC R H, TASKINEN P, JOKILAAKSO A. Upgrading copper slag cleaning tailings for re-use [J]. Minerals Engineering, 2019, 133: 35-42. DOI: 10.1016/j.mineng.2018.12.026.
[3] CHUN T, MU G, DI Z, LONG H, NING C, LI D. Recovery of iron from copper slag by carbothermic reduction and magnetic separation in the presence of CaO [J]. Archives of Metallurgy and Materials, 2018, 63(1): 299-305. DOI: 10.24425/118941.
[4] SHEN Hui-ting, FORSSBERG E. An overview of recovery of metals from slags [J]. Waste Management, 2003, 23(10): 933-949.
[5] ALP I, DEVECI H, SUNGUN H. Utilization of flotation wastes of copper slag as raw material in cement production [J]. Journal of Hazardous Materials, 2008, 159: 390-395.
[6] GORAI B, JANA R K. Characteristics and utilisation of copper slag―A review [J]. Resources, Conservation and Recycling, 2003, 39: 299-313.
[7] KIM B S, JO S K, SHIN D Y, LEE J C, JEONG S B. A physico-chemical separation process for upgrading iron from waste copper slag [J]. International Journal of Mineral Processing, 2013, 124: 124-127.
[8] TSUNAZAWA Y, LIU Chang-zhi, TOI R, OKURA T, TOKORO C. Crystal formation and growth by slow cooling for recovery of magnetite particles from copper smelting slag [J]. Mineral Processing and Extractive Metallurgy, 2019, 128(4): 248-255. DOI: 10.1080/25726641. 2018.1483793.
[9] GUO Zheng-qi, ZHU De-qing, PAN Jian, WU Teng-jiao, ZHANG Feng. Improving beneficiation of copper and iron from copper slag by modifying the molten copper slag [J]. Metals, 2016, 6(4): 86. DOI: 10.3390/met6040086.
[10] NADIROV R, SYZDYKOVA L, ZHUSSUPOVA A. Copper smelter slag treatment by ammonia solution: Leaching process optimization [J]. Journal of Central South University, 2017, 24(1): 2799-2804. DOI: 10.1007/s11771-017-3694-3.
[11] HEO J H, KIM B S, PARK J H. Effect of CaO addition on iron recovery from copper smelting slags by solid carbon [J]. Metallurgical and Materials Transactions B, 2013, 44(6): 1352-1363.
[12] LI Si-wei, PAN Jian, ZHU De-qing, GUO Zheng-qi, XU Ji-wei, CHOU Jian-lei. A novel process to upgrade the copper slag by direct reduction-magnetic separation with the addition of Na2CO3 and CaO [J]. Powder Technology, 2019, 347: 159-169. DOI: 10.1016/j.powtec.2019.02.046.
[13] HEO J H, CHUNG Y S, PARK J H. Recovery of iron and removal of hazardous elements from waste copper slag via a novel aluminothermic smelting reduction (ASR) process [J]. Journal of Cleaner Production, 2016, 137: 777-787.
[14] LI Xiao-bin, WANG Hong-yang, ZHOU Qiu-sheng, QI Tian-gui, LIU Gui-hua, PENG Zhi-hong. Efficient separation of silica and alumina in simulated CFB slag by reduction roasting-alkaline leaching process [J]. Waste Management, 2019, 87: 798-804. DOI: 10.1016/j.wasman.2019.03.020
[15] SHEN Lei-ting, LI Xiao-bin, ZHOU Qiu-sheng, PENG Zhi-hong, LIU Gui-hua, QI Tian-gui, TASKINEN P. Sustainable and efficient leaching of tungsten in ammoniacal ammonium carbonate solution from the sulfuric acid converted product of scheelite [J]. Journal of Cleaner Production, 2018, 197: 690-698. DOI: 10.1016/j.jclepro. 2018.06.256.
[16] ROMCHAT C F, KATSUYA M, TAKAHIRO M, TETSUYA N. The selective alkaline leaching of zinc oxide from electric arc furnace dust pre-treated with calcium oxide [J]. Hydrometallurgy, 2016, 159: 120-125.
[17] CHEN J H, MI W J, CHEN H Y, LI B, CHOU K C, HOU X M. Iron oxide recovery from fayalite in water vapor at high temperature [J]. Journal of Mining and Metallurgy, Section B: Metallurgy, 2018, 54(1): 1-8. DOI: 10.2298/ JMMB160926011C.
[18] GYUROV S, MARINKOV N, KOSTOVA Y, RABADJIEVA D, KOVACHEVA D, TZVETKOVA C, GENTSCHEVA G, PENKOV I. Technological scheme for copper slag processing [J]. International Journal of Mineral Processing, 2017, 158: 1-7. DOI: 10.1016/j.minpro.2016.11. 008.
[19] JIAO K X, ZHANG J L, LIU Z J, CHEN C L, LIU F. Circulation and accumulation of harmful elements in blast furnace and their impact on the fuel consumption [J]. Ironmaking & Steelmaking, 2017, 44(5): 344-350. DOI: 10.1080/03019233.2016.1210913.
[20] ZHANG Qing-chen, QIU Guan-zhou, XIAO Qi. The effect of coal-kind on coal-based direct reduction of low grade iron ore [J]. Journal of Central South University of Technology, 1997, 28(2): 126-129. (in Chinese)
[21] LI Xiao-bin, WANG Hong-yang, ZHOU Qiu-sheng, QI Tian-gui, LIU Gui-hua, PENG Zhi-hong, WANG Yi-lin. Reaction behavior of kaolinite with ferric oxide during reduction roasting [J]. Transactions of Nonferrous Metals Society of China, 2019, 29(1): 186-193. DOI: 10.1016/ S1003-6326(18)64927-1.
[22] HAN Bin, TONG Xiong, ZHANG Guo-hao, XIE Xian, LV Hao-zi. Process mineralogy study on a copper slag [J]. Conservation and Utilization of Mineral Resources, 2015(1): 63-68. (in Chinese)
[23] BARI I. Thermochemical data of pure substances [M]. VCH Verlagsgesellschaft mbH, Germany, 1995.
[24] LUO Jun, LI Guang-hui, JIANG Tao, PENG Zhi-wei, RAO Ming-jun, ZHANG Yuan-bo. Conversion of coal gangue into alumina, tobermorite and TiO2-rich material [J]. Journal of Central South University, 2016, 23: 1883-1889. DOI: 10.1007/s11771-016-3243-5.
[25] CROKER D, LOAN M, HODNETT B. Desilication reactions at digestion conditions: an in situ X-ray diffraction study [J]. Crystal Growth & Design, 2008, 8(12): 4499-4505.
[26] MIHAILOVA I, MEHANDJIEV D. Characterization of fayalite from copper slags [J]. Journal of the University of Chemical Technology and Metallurgy, 2010, 45(3): 317-326.
[27] MING C Q, GREISH Y, El-GHANNAM A. Crystallization behavior of silica-calcium phosphate biocomposites: XRD and FTIR studies [J]. Journal of Materials Science: Materials in Medicine, 2004, 15(11): 1227-1235.
[28] REIG F B, ADELANTADO J V G, MORENO M C M M. FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples [J]. Talanta, 2002, 58(4): 811-821.
[29] MARINKOV N, MARKOVA-VELICHKOVA M, GYUROV S, KOSTOVA Y, SPASSOVA I, RABADJIEVA D, KOVACHEVA D, PENKOV I, TZVETKOVA C, GENTSCHEVA G. Preparation and characterization of silicagel from silicate solution obtained by autoclave treatment of copper slag [J]. Journal of Sol-Gel Science and Technology, 2018, 87(2): 331-339. DOI: 10.1007/s10971- 018-4741-8.
[30] YAO Cong, FANG Li, GUO Yan-xia, LIU Dan-dan, CHENG Fang-qin. Modulus control of pre-desilication solution by alumina-extracted residues of coal fly ash [J]. Journal of North University of China (Natural Science Edition), 2018, 39(3): 328-333. (in Chinese)
(Edited by ZHENG Yu-tong)
中文导读
碳热还原-碱浸工艺实现铜渣中硅和铁的分离
摘要:以铜精矿为原料,采用火法冶炼工艺每生产1.0 t金属铜将产生2.0~3.0 t含铁35%~45%的铜渣。因此,从铜渣中回收铁不仅能实现有价金属的提取,还能减少其因堆存而带来的环境问题。本文提出了一种通过选择性脱硅而实现铜渣中铁富集的新方法。热力学计算及实验结果表明,铜渣中的铁在1473 K碳热还原60 min可被完全还原为金属铁,此时氧化硅转变为游离的石英固溶体和方石英固溶体。石英固溶体和方石英固溶体均易溶于碱溶液而金属铁不溶于碱溶液。在最佳浸出条件下,铜渣还原焙烧产物中的氧化硅可降低至6.02%,同时获得含铁87.32%的浸出渣。浸出渣中锌含量低于0.05%。本研究为铜渣中硅和铁综合提取新技术的开发奠定基础,并避免二次尾矿的产生。
关键词:铜渣;石英固溶体;方石英固溶体;碳热还原;碱浸
Foundation item: Project(WUT: 2019IVA096) supported by the Fundamental Research Funds for the Central Universities, China; Project(2019M662733) supported by China Postdoctoral Science Foundation; Project(2018YFC1901502) supported by National Key Research and Development Program of China
Received date: 2019-12-11; Accepted date: 2020-05-07
Corresponding author: WANG Hong-yang, PhD, Research Assistant; Tel: +86-18229790554; E-mail: hywang3@whut.edu.cn; ORCID: https://orcid.org/0000-0002-6197-6133