文章编号:1004-0609(2015)06-1705-08
NaCl-HCl体系浸出铅渣中铅的动力学分析
杨利姣1,陈南春1,钟夏平2,解庆林3,高 军4,郎耀秀5,刘长淼6,吴照洋6
(1. 桂林理工大学 材料科学与工程学院 省部共建广西有色金属及特色材料加工国家重点实验室培育基地
广西矿冶与环境科学实验中心,桂林 541004;
2. 广西科学院 应用物理研究所,南宁 530000;
3. 桂林理工大学 环境科学与工程学院,桂林 541004;
4. 广西金山铟锗冶金化工有限公司,河池 547200;
5. 河池学院 化学与生物工程学院,宜州 546300;
6. 中国地质调查局 郑州矿产综合利用研究所,郑州 450000)
摘 要:以浸出温度、NaCl浓度、颗粒粒度和液固比对铅浸出率影响的实验条件和数据为基础,建立NaCl-HCl体系,采用液-固多相反应的收缩核模型,系统分析了铅渣中铅的浸出动力学过程。结果表明:根据实验数据求出浸出反应的宏观动力学方程,计算得到表观活化能为45.239 kJ/mol,说明该体系浸出过程受表面化学反应控制;在实验选取的参数范围内,增大NaCl浓度、浸出温度和液固比以及减小颗粒粒度均有利于提高铅的浸出率。
关键词:NaCl-HCl体系;铅渣;铅;浸出;动力学
中图分类号:TF803.21 文献标志码:A
Kinetics analysis of leaching lead from lead residue in NaCl-HCl solution
YANG Li-jiao1, CHEN Nan-chun1, ZHONG Xia-ping2, XIE Qing-lin3, GAO Jun4,
LANG Yao-xiu5, LIU Chang-miao6, WU Zhao-yang6
(1. Ministry-Province Jointly-Constructed Cultivation Base for State Key Laboratory of Processing for Non-ferrous Metal and Featured Materials, Guangxi Scientific Experiment Center of Mining, Metallurgy and Environment,
College of Materials Science and Engineering, Guilin University of Technology, Guilin 541004, China;
2. Institute of Applied Physics, Guangxi Academy of Sciences, Nanning 530000, China;
3. College of Environmental Science and Engineering, Guilin University of Technology, Guilin 541004, China;
4. Guangxi Jinshan Indium and Germanium Chemical Metallurgy Co.Ltd., Hechi 547200, China;
5. College of Chemical and Biological Engineering, Hechi University, Yizhou 546300, China;
6. Zhengzhou Institute of Multipurpose Utilization, Mineral Resources, Chinese Academy of Geological Science, Zhengzhou 450000, China)
Abstract: In order to provide a theoretical basis to improve the leaching rate of lead in lead residue, the kinetics of leaching lead in NaCl-HCl solution were investigated with the shrinking core model for the reaction of liquid-solid. The effects of temperature, brine concentration, particle size and liquid-solid ratio on the leaching lead were studied. The results show that the leaching process is controlled by the surface chemical reaction, and the apparent activation energy is determined to be 45.239 kJ/mol. The kinetics equation can be expressed by a semi-empirical equation according to the experimental data. The leaching speed and the leaching rate of lead are increased by increasing the brine concentration, temperature, liquid-solid ratio and decreasing the particle size of sample.
Key words: NaCl-HCl system; lead residue; lead; leaching; kinetics
随着铅消耗量增长,铅矿资源日益枯竭,从二次含铅物料中回收铅引起世界各国广泛关注。含铅物料包括含铅浸出渣、含铅烟尘和铅蓄电池废渣等,既是污染环境的有害物质,同时又是综合回收有价金属的二次资源[1]。目前,从含铅物料中湿法回收铅的方法主要有氯盐法[2-4]、碱浸法[5-6]和碳酸化转化法[7-8]等。张亚莉等[9]采用氯盐一步法浸出氧化锌贫矿提锌渣中铅和银,铅的浸出率达到95%左右。韦岩松等[10]研究硝酸浸出法从锑精矿除铅渣中回收铅,铅的浸出率达到94%。刘清等[11]提出氢氧化钠浸出-两步沉淀法从贫杂氧化锌矿中制备铅锌精矿新工艺,得到铅含量为78%(质量分数)的铅精矿,达到行业标准。ZHU等[12]研究化学转化法用碳酸铵和碳酸钠从废铅渣中回收铅,然后用过热分解法得到铅氧化物,铅回收率达98%。
有关铅回收的研究已经很多,但是关于含铅浸出渣浸出动力学的研究较少。DRAGAN等[13]研究发现,硫酸铅在氯化钙-氯化镁体系中浸出同时受扩散和化学反应控制;齐美富等[14]研究废铅酸蓄电池中铅膏在HCl-NaCl-CaCl2体系中浸取铅的动力学,发现其属于固膜扩散控制过程。本文作者在大量试验研究基础上,对比其他浸出方法,发现NaCl-HCl体系对湿法炼锌过程中产生的铅渣具有很好的浸出效果;通过考察各因素对铅浸出率的影响,发现可以用液-固多相反应的收缩核模型来分析其浸出过程动力学,以寻找强化浸出过程的措施。
1 实验
1.1 实验原料
实验原料为广西某炼锌厂的冶锌酸浸渣,又称为铅渣,其主要化学成分和XRD谱分别见表1和图1。由表1可知,铅渣含铅量较高,而图1物相分析结果表明,铅的存在形式主要为硫酸铅。
1.2 实验方法
本实验中进行温度、氯盐浓度、粒度和液固比4个因素实验。实验在500 mL圆底烧瓶中进行,按一定液固体积质量比加入NaCl溶液,在磁力搅拌器上加热搅拌,达到预热温度后,加入实验原料,用15%盐酸溶液调节pH值为1.0~1.5。反应结束后,进行热过滤,滤饼用热NaCl溶液洗涤2~3次。采用EDTA滴定法测定浸出液中铅离子质量浓度。
表1 铅渣主要成分分析
Table 1 Main composition of lead slag


图1 铅渣XRD谱
Fig. 1 XRD pattern of lead slag
1.3 动力学分析
铅渣中的铅主要以PbSO4形式存在,氯盐浸出铅的反应为
PbSO4(s)+2Cl-=PbCl2(s)+SO42- (1)
PbCl2(s)+2Cl-=PbCl42- (2)
PbSO4在NaCl-HCl体系中浸出是复杂的液-固多相反应过程,浸出反应首先在固体颗粒表面发生,随着反应进行,反应逐渐向固体颗粒中心收缩,未反应核缩小。因此,氯化钠浸出铅渣过程可由未反应核收缩模型来描述。浸出过程主要由液相传质、固膜扩散以及表面化学反应3个步骤组成,在控制高速搅拌条件下,可以忽略液相传质(外扩散)对浸出速率的影响,浸出速率取决于固膜扩散或表面化学反应[15]。根据未反应核收缩模型[16],如果表面化学反应是整个浸出过程的控制步骤,那么反应速率遵循以下方程:
1-(1-α)1/3 = krt (3)
式中:α为铅浸出率,kr为化学反应速率常数,t为反应时间。
如果固膜扩散是整个浸出过程的控制步骤,那么反应速率遵循以下方程:
1-2/3α-(1-α)2/3 = kdt (4)
式中:α为铅浸出率,kd为扩散速率常数,t为反应时间。
式(3)和(4)中左边与反应时间t呈线性关系,直线的斜率为反应速率常数kr或kd,而反应速率常数与温度的关系,可根据阿累尼乌斯公式来表示:
k =A exp[-Ea/(RT)] (5)
式中:k为不同温度下反应速率常数,A为频率因子(s-1),Ea为活化能(J/mol),R为摩尔气体常数[J/(mol·K)],T为绝对温度(K)。
2 结果与讨论
2.1 温度变化对铅浸出过程的影响
固定条件为:NaCl溶液300 g/L,铅渣粒度0.106~ 0.075 mm,液固比10:1,用HCl溶液调节pH值为1.0~1.5。考察浸出温度变化对Pb浸出率的影响,其结果如图2所示。
温度对铅浸出率有很大影响,在同一温度下Pb浸出率随时间延长明显增大,最后趋于不变;在同一时间下,温度越高,铅浸出率越大。当温度从60 ℃升高到90 ℃时,铅平衡浸出率由60.50%增大为90.10%。温度升高,浸出剂向固体颗粒表面扩散速度加大,化学反应速率加快[9];PbSO4在NaCl溶液中溶解度迅速增加,避免PbCl2结晶析出,降低浸出黏度,进一步加快化学反应速率[17]。
将不同温度下铅浸出率数据分别代入式(3)、(4)进行拟合(见图3),发现1-(1-α)1/3与时间t的线性关系明显优于1-2/3α-(1-α)2/3,因此,可以认为铅在NaCl-HCl体系中的浸出受表面化学反应控制,而非固膜扩散控制。
以ln k对T-1作图(见图4)得到一条直线,直线的斜率为(-Ea/R)。由此直线方程求出浸出反应的表观活化能为Ea=45.293 kJ/mol,Ea>42 kJ/mol,进一步验证浸出铅的过程为表面化学反应控制过程[18]。

图2 温度变化对铅浸出率影响
Fig. 2 Effect of temperature on leaching rate of lead

图3 不同温度下1-(1-α)1/3和1-2/3α-(1-α)2/3与t关系
Fig. 3 Relationships between 1-(1-α)1/3(a) and 1-2/3α-(1-α)2/3(b) and time at different temperatures

图4 浸出铅Arrhenius图
Fig. 4 Arrhenius plot of reaction rate against reciprocal of temperature
2.2 NaCl浓度变化对铅浸出过程的影响
固定条件为:铅渣粒度0.106~0.075 mm,液固比10:1,温度90 ℃,用HCl溶液调节pH值为1.0~1.5。考察了NaCl浓度变化对Pb浸出率的影响,其结果如图5所示。
在同一NaCl浓度下,铅浸出率随浸出时间延长而增大;在同一浸出时间下,NaCl浓度越大铅浸出率越高,NaCl浓度从150 g/L升到300 g/L时,铅的平衡浸出率由47.54%增大到91.14%。这是由于络合剂Cl-1浓度越大,越有利于反应(2)向生成络合物[PbCl4]2-的方向进行,铅浸出率越大[19]。将不同NaCl浓度下铅浸出率数据代入式(3)进行线性拟合(见图6),其结果与表面化学反应控制模型相吻合。

图5 NaCl浓度变化对Pb浸出率影响
Fig. 5 Effect of NaCl concentration on leaching rate of lead

图6 不同NaCl浓度下1-(1-α)1/3与时间关系
Fig. 6 Relationship between 1-(1-α)1/3 and time at different NaCl concentrations
2.3 铅渣粒度变化对铅渣浸出过程的影响
固定条件为:NaCl溶液300 g/L,液固比10:1,温度90 ℃,用HCl溶液调节pH值为1.0~1.5。考察铅渣粒度变化对Pb浸出率(α)的影响,结果如图7所示。
在同一粒度下,随着浸出时间延长,铅浸出率逐渐增大;在同一浸出时间下,粒度越小铅浸出率越高,当粒度从180~250 μm减小至75~106 μm时,铅的平衡浸出率由70.79%升高到91.14%。这是因为浸出速率与原料比表面积成正比[20],颗粒越小,比较面积越大,铅的反应速率和浸出率越大。将不同NaCl浓度下铅浸出率数据代入式(3)进行线性拟合(见图8),1-(1-α)1/3与时间t呈很好的线性关系。
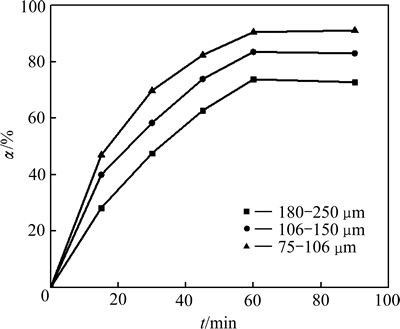
图7 铅渣粒度变化对Pb浸出率影响
Fig. 7 Effect of particle size on leaching rate of lead
2.4 液固比变化对铅渣浸出过程的影响
固定条件为:NaCl溶液300g/L,铅渣粒度75~106 μm,温度90 ℃,用HCl溶液调节pH值为1.0~1.5。考察了液固比变化对Pb浸出率的影响,其结果如图9所示。

图8 不同粒度下1-(1-α)1/3与时间关系
Fig. 8 Relationship between 1-(1-α)1/3 and time at different particle sizes

图9 不同时间下液固比变化对Pb浸出率影响
Fig. 9 Effect of liquid-solid ratio on leaching rate of lead at different time
在同一液固比下随着浸出时间延长铅的浸出率逐渐增大;在同一浸出时间下,液固比越大,铅的浸出率越高,液固比从5:1增大至10:1时,铅的平衡浸出率由56.23%升高到91.14%。液固比较大时,可以减小溶液黏度和溶剂扩散阻力,铅浸出率较高且易于过滤;而液固比较小时,因不能满足反应的化学计量比,铅浸出率较低。将不同液固比下铅浸出率数据代入式(3)进行线性拟合(见图10),1-(1-α)1/3与时间t呈很好的线性关系,验证了铅在NaCl-HCl体系中的浸出受未反应收缩核表面化学反应控制。

图10 不同液固比下1-(1-α)1/3与时间关系
Fig. 10 Relationship between 1-(1-α)1/3 and time at different liquid-solid ratios
2.5 浸出前后渣样物相组成和表面特征
图11所示为铅渣和浸出渣的XRD谱。从图11的XRD测试结果可以看出,浸出前铅渣主要物相为PbSO4、SiO2、ZnS,浸出后PbSO4相消失,表明大部分硫酸铅已被浸出。图12所示为浸出前后铅渣和浸出渣的SEM像。由图12可看出,浸出前铅渣颗粒表面较光滑、棱角分明[21];浸出后,渣样颗粒表面粗糙,且棱角被侵蚀消失,样品表面反应是表面化学反应过程,这与动力学分析结果一致。
2.6 浸出动力学方程的确定
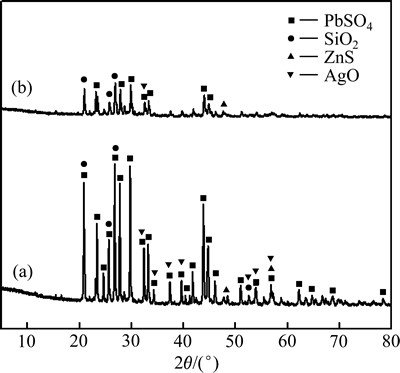
图11 铅渣和浸出渣的XRD谱
Fig. 11 XRD patterns of lead residue(a) and leaching residue(b)
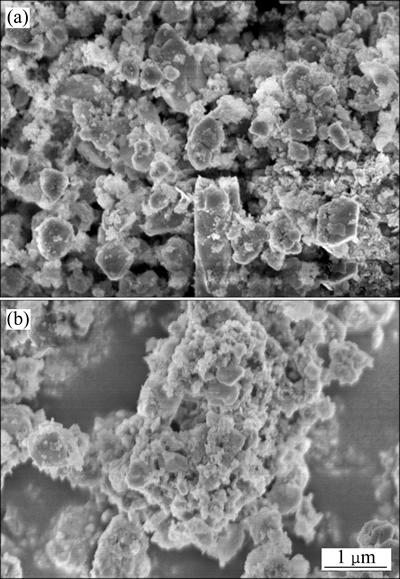
图12 铅渣和浸出渣的SEM像
Fig. 12 SEM images of lead residue(a) and leaching residue(b)
根据上述各影响因素的研究结果,对于所研究的体系,其动力学方程可表示为式(3),式中kr可以表示为[22]
 (6)
(6)
将上式代入式(3),得到半经验公式
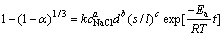 (7)
(7)
就不同的NaCl浓度而言,当其他条件固定时,式(7)可以改写成式(8)所示形式。
 (8)
(8)
对式(8)两边进行微分并取对数可得
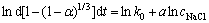 (9)
(9)
以ln cNaCl为横坐标,ln d[1-(1-α)1/3]/dt为纵坐标作线性拟合,如图13所示,该直线斜率即为NaCl的反应级数a,a=1.469。同理,分别以lnd、ln(s/l)为横坐标,ln d[1-(1-α)1/3]/dt为纵坐标作线性拟合(见图14和15),可分别求出铅渣粒度的反应级数b=-0.469,液固比的反应级数c=-2.112。
将上述求出的a、b、c和Ea代入式(7),再用式(7)拟合图3、5、7、8中各直线,可求得k0的统计值为1.73×10-2。由以上可以确定在NaCl-HCl体系中铅渣中铅的浸出动力学方程可表示为
1-(1-α)1/3 = 1.73×10-2cNaCl1.469d -0.469 (s/l)-2.112·exp{[-45293/(RT)]t} (10)

图13 ln d[1-(1-α)1/3]/dt与ln cNaCl关系图
Fig. 13 Plot of ln d[1-(1-α)1/3]/dt against ln cNaCl

图14 ln d[1-(1-α)1/3]/dt与ln d关系图
Fig. 14 Plot of ln d[1-(1-α)1/3]/dt against ln d

图15 ln d[1-(1-α)1/3]/dt与ln (s/l)关系图
Fig. 15 Plot of ln d[1-(1-α)1/3]/dt against ln (s/l)
3 结论
1) 在NaCl浓度300 g/L、液固比10:1、浸出温度80 ℃条件下,浸出反应60 min,铅浸出率达到91.14%。在实验选取的参数范围内,增大NaCl浓度、提高浸出温度、增大液固比、减小铅渣粒度和延长浸出时间均有利于提高铅的浸出率。
2) 在NaCl-HCl体系中,铅渣中铅的浸出过程可用未反应收缩核模型来表示,反应速率受表面化学反应控制,表观活化能为45.293 kJ/mol,其宏观动力学方程可表示为1-(1-α)1/3=1.73×10-2cNaCl1.469d -0.469· (s/l)-2.112exp{[-45293E/(RT)] t}。
REFERENCES
[1] 焦志良, 陈为亮, 张旭. 从二次含铅物料中湿法回收铅的研究现状[J]. 湿法冶金, 2014, 33(2): 90-93.
JIAO Zhi-liang, CHEN Wei-liang, ZHANG Xu. Current situation of recovery lead from secondary lead-containing materials by hydrometallurgical technology[J]. Hydrometallurgy of China, 2014, 33(2): 90-93.
[2] 唐秋香. 从锌烟灰中浸出锌和铅的试验研究[J]. 湿法冶金, 2013, 32(5): 302-304.
TANG Qiu-xiang. Test study on leaching of zinc and lead from zinc fume dust[J]. Hydrometallurgy of China, 2013, 32(5): 302-304.
[3] 徐 辉, 颜文斌, 张传宝, 高 峰, 易 静, 华 骏. 从冶锌酸浸渣中回收铅、锌的工艺研究[J]. 矿冶工程, 2012, 32(6): 78-81.
XU Hui, YAN Wen-bin, ZHANG Chuan-bao, GAO Feng, YI Jing, HUA Jun. Study on recovery of Pb and Zn from acid-leaching residue[J]. Mining and Metallurgical Engineering, 2012, 32(6): 78-81.
[4] 彭国敏, 俎小凤, 张福元. 酸浸渣综合回收浸铅工艺研究[J]. 无机盐工业, 2012, 44(1): 52-54.
PENG Guo-min, ZU Xiao-feng, ZHANG Fu-yuan. Study on leaching process of lead from acid residue [J]. Inorganic Chemicals Industry, 2012, 44(1): 52-54.
[5] AMIN A S, ABDALLAH M, OMAR A A. Hydrometallurgical treatment of Egyptian zinc-lead oxide ore[J]. Modelling, Measurement & Control, 1995, 49(1/3): 1-12.
[6] FERRACIN L C, CHACON-SANHUEZA A E, DAVOGLIO R A, ROCHA L O, CAFFEU D J, FONTANETTI A R, ROCHA-FILHO R C, BIAGGIO S R, BOCCHI N. Lead recovery from a typical Brazilian sludge of exhausted lead-acid batteries using an electro hydrometallurgical process[J]. Hydrometallurgy, 2002, 65(2/3): 137-144.
[7] 张福元, 俎小凤, 彭国敏. 沉淀转化法综合回收酸浸渣中铅的工艺研究[J]. 无机盐工业, 2013, 45(1): 40-59.
ZHANG Fu-yuan, ZU Xiao-feng, PENG Guo-min. Study on process of recovering lead from acid leaching residue by precipitation transformation method[J]. Inorganic Chemicals Industry, 2013, 45(1): 40-59.
[8] 任兴丽, 蒋兴荣, 朱复跃. 回收铅渣中碳酸铅工艺条件研究[J]. 河北化工, 2012, 35(9): 43-44.
REN Xing-li, JIANG Xing-rong, ZHU Fu-yue. Research on process conditions of recovering lead carbonate from lead slag[J]. Hebei Chemical Engineering and Industry, 2012, 35(9): 43-44.
[9] 张亚莉, 于先进, 李小斌. 氧化锌贫矿提锌渣中铅和银的氯盐一步浸出[J]. 中国有色金属学报, 2012, 22(1): 296-330.
ZHANG Ya-li, YU Xian-jin, LI Xiao-bin. Leaching of silver and lead by chloride simultaneously from residue after zinc extraction of low-grade zinc oxide ores[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(1): 296-330.
[10] 韦岩松, 黎铉海, 马 宸. 硫化铟氧化酸浸与常规酸浸的动力学比较[J]. 金属矿山, 2014, 43(3): 165-170.
WEI Yan-song, LI Xuan-hai, MA Chen. Kinetics comparison of oxidizing acid leading and conventional acid leading of indium sulfide[J]. Metal Mine, 2014, 43(3): 165-170.
[11] 刘 清, 赵由才, 招国栋. 氢氧化钠浸出-两步沉淀法制备铅锌精矿新工艺[J]. 湿法冶金, 2010, 29(1): 32-36.
LIU Qing, ZHAO You-cai, ZHAO Guo-dong. A novel process for preparation of zinc and lead concentrates by alkaline leaching and precipitation[J]. Hydrometallurgy of China, 2010, 29(1): 32-36.
[12] ZHU X F, YANG J K, GAO L X, LIU J W, YANG D N, SUN X J, ZHANG W, WANG Q, LI L, HE D S, KUMAR R V. Preparation of lead carbonate from spent lead paste via chemical conversion[J]. Hydrometallurgy, 2013, 134: 47-53.
[13] DRAGAN S, ZELJKO K, ALEKSANDAR S. Leaching kinetics of lead from lead (Ⅱ) sulphate in aqueous calcium chloride and magnesium chloride solutions[J]. Hydrometallurgy, 1997, 47: 137-147.
[14] 齐美富, 郑园芳, 桂双林. 废铅酸蓄电池中铅膏氯盐体系浸取铅的动力学研究[J]. 矿冶工程, 2010, 30(6): 61-64.
QI Mei-fu, ZHENG Guo-fang, GUI Shuang-lin. Kinetic study on leaching lead from waste lead-acid batteries for lead plaster chloride system[J]. Mining and Metallurgical Engineering, 2010, 30(6): 61-64.
[15] 朱炳辰. 化学反应工程[M]. 北京: 化学工业出版社, 2001: 360-364.
ZHU Bing-chen. Chemical reaction engineering[M]. Beijing: Chemistry Industry Press, 2001: 360-364.
[16] MOHAMMAD S S, DAVOOD M, MEHDI O I. Kinetics of sulfuric acid leaching of cadmium from Cd-Ni zinc plant residues[J]. Journal of Hazardous Materials, 2009, 163: 880-890.
[17] 张传宝, 颜文斌, 徐 辉, 高 峰, 华 骏. 难处理铅锌矿酸浸渣回收硫酸铅的工艺研究[J]. 应用化工, 2012, 41(7): 1188-1192.
ZHANG Chuan-bao, YAN Wen-bin, XU Hui, GAO Feng, HUA Jun. Study on recovery lead sulfate from complex acid leaching residue of lead-zinc mine[J]. Applied Chemical Industry, 2012, 41(7): 1188-1192.
[18] 李洪桂. 冶金原理[M]. 北京: 科学出版社, 2005: 191-316.
LI Hong-gui. Principles of metallurgy[M]. Beijing: Science Press, 2005: 191-316.
[19] RUSEN A, SUNKAR A S, TOPKAYA Y A. Zinc and lead extraction from  leach residues by using hydrometallurgical method[J]. Hydrometallurgy, 2008, 93: 45-50.
leach residues by using hydrometallurgical method[J]. Hydrometallurgy, 2008, 93: 45-50.
[20] LI Q, ZHANG B, MIN X B, SHEN W Q. Acid leaching kinetics of zinc plant purification residue[J]. Transaction of Nonferrous Metals Society of China, 2013, 23(9): 2786-2791.
[21] 徐志峰, 朱 辉, 王成彦. 富氧硫酸体系中硫化锌精矿的常压直接浸出动力学[J]. 中国有色金属学报, 2013, 23(12): 3440-3447.
XU Zhi-feng, ZHU Hui, WANG Cheng-yan. Atmospheric direct leaching kinetics of zinc sulfide concentrate in oxygen-rich sulfuric acid system[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(12): 3440-3447.
[22] 何 静, 王小能, 刘明海, 王继民, 唐谟堂, 鲁君乐, 王 涛, 罗 超, 郭 瑞, 蓝明艳. 含锗真空炉渣在HCl-CaCl2-H2O体系中浸出锗的动力学研究[J]. 稀有金属材料与工程, 2013, 42(6): 1258-1263.
HE Jing, WANG Xiao-neng, LIU Ming-hai, WANG Ji-min, TANG Mo-tang, LU Jun-le, WANG Tao, LUO Chao, GUO Rui, LAN Ming-yan. Leaching kinetics of germanium in vacuum slag in HCl-CaCl2-H2O solution[J]. Rare Metal Materials and Engineering, 2013, 42(6): 1258-1263.
(编辑 李艳红)
基金项目:矿产资源高效综合利用技术与实用化研究(12120113088200);广西科技攻关项目(11107003-4,14124004-5-5);广西矿冶与环境科学实验中心资助项目(KH2011YB001)
收稿日期:2014-08-16;修订日期:2015-01-16
通信作者:陈南春,教授;电话:0773-5896972;E-mail: cnc@glut.edu.cn