文章编号:1004-0609(2016)-01-0197-07
锌冶炼中浸渣锌还原浸出机制与动力学
张 纯1, 2,闵小波1, 3,张建强1,王 密1,李辕成1
(1. 中南大学 冶金与环境学院,长沙 410083;
2. 湖南城市学院 市政与测绘工程学院,益阳 413000;
3. 中南大学 国家重金属污染防治工程技术研究中心,长沙 410083)
摘 要:以锌冶炼中浸渣为研究对象,研究中浸渣的化学成分及锌的存在形态,锌主要以铁酸锌形式存在。采用SO2做还原剂,研究温度、初始硫酸浓度、二氧化硫分压对锌浸出效率的影响,并分析中浸渣中锌还原浸出反应机制及动力学。结果表明:H+在锌还原浸出过程中起关键作用,锌还原浸出反应活化能为31.67 kJ/mol,为化学反应控制;SO2做还原剂时,反应时间、液固比及初始酸度均大幅降低。反应最佳工艺条件:初始硫酸浓度80 g/L、温度95 ℃、液固比(L/S) 10 mL/g、二氧化硫分压200 kPa、反应时间120 min。该工艺条件下,中浸渣中锌浸出率达99%以上。XRD和ICP分析表明:中浸渣中铁酸锌分解,硫化锌在该反应条件下未完全浸出,还原浸出渣中主要化学成分为铅和锌,主要物相为PbSO4和ZnS。
关键词:中浸渣;还原浸出;二氧化硫;动力学
中图分类号:TF813 文献标志码:A
硫化铅锌矿浮选产出的锌精矿,成分一般为锌50%左右,硫30%左右,铁5%~14%,还含有少量铅、镉、铜及其他稀有金属。硫化锌精矿炼锌方法有湿法和火法。目前,由于环境保护及能耗等原因,火法炼锌基本处于停滞状态,湿法炼锌得到不断发展,目前国内运行的炼锌厂,多采用湿法[1-2]。
湿法冶金工艺主要包括焙烧-中浸-低酸浸出-高温高酸浸出-沉铁-电积等工序[3]。锌精矿焙烧产生的锌焙砂先中性浸出,控制浸出终点pH为 5.2~5.4,使铁、砷、锑等金属水解沉淀。浸出条件如下:温度55~60 ℃,时间60 min左右,液固比9:1~13:1。低酸浸出溶解浸出矿浆中残余的氧化锌,常采用1~2段酸性浸出。浸出条件:温度60~75 ℃,时间120~150 min左右,液固比7:1~9:1,锌焙砂中大部分氧化锌溶解[1-3]。由于锌精矿中含铁5%~14%,焙烧过程中部分锌不可避免转化为铁酸锌。铁酸锌具有尖晶石结构,结构稳定,中浸条件下铁酸锌难以溶解。热酸浸出-黄钾铁矾或针铁矿法先以高温高酸溶解中浸渣中铁酸锌,再以人造矿物方法除去溶液中铁,所得含锌溶液返回焙烧浸出系统,回收其中锌。此法锌回收率高,并有利于锌精矿综合利用。夏志华等[4]研究中浸渣高温高酸浸出动力学,热酸浸出条件:温度95 ℃以上,终点残酸40~60 g/L,时间3~4 h,锌浸出率可达95%以上[5-8],但此法反应条件苛刻,耗酸量大、反应时间长、能耗大。
目前,一些难溶矿物的强化浸出方法得到国内外较多研究[9-12]。SENANAYAKE等[13]研究了机械活化对铟铁酸锌的影响,机械活化虽强化了铁酸盐的浸出效果,但反应时间、酸耗量仍较大。作为锌精矿焙烧阶段副产物,二氧化硫具有强还原性,可将Fe(Ⅲ)还原为Fe(Ⅱ),从而破坏铁酸锌稳定的正八面体结构,达到锌高效还原浸出目的。本文作者以二氧化硫为还原剂,研究二氧化硫还原分解铁酸锌反应机制、温度、初始酸浓度及二氧化硫分压对中浸渣中锌浸出效率的影响,提示锌还原浸出反应动力学特征。
1 实验
1.1 实验原理
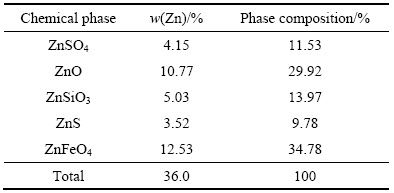
试验用锌焙砂中浸渣取自湖南衡阳某锌冶炼厂。原渣105 ℃烘干后,振磨10 min,粒径小于75 μm,得到实验用矿样。矿样粒度分析(LS-POP(6))如图1所示,绝大部分颗粒粒径在30 μm以下。中浸渣化学成分及锌物相分析分别见表1和2所示。根据表1,中浸渣中锌、铁含量分别为36.0%和15.6%。由表2可知,锌物相包括硫酸锌、氧化锌、硅酸锌、硫化锌及铁酸锌。氧化锌及铁酸锌占比最大,分别为29.92%和34.78%。中浸渣的XRD谱如图2所示,渣中晶相主要为铁酸锌、氧化锌、水合硫酸锌及硫酸铅,其中铁酸锌衍射特征峰最为明显。
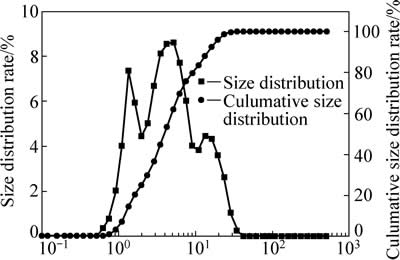
图1 中浸渣粒径分布图
Fig. 1 Size distribution rate of zinc neutral leaching residue
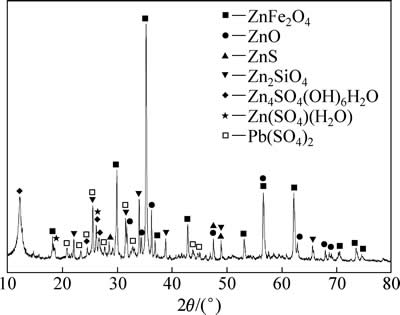
图2 中浸渣的XRD谱
Fig. 2 XRD pattern of zinc neutral leaching residue
1.2 实验及检测仪器
主要实验设备及仪器有:粒度分析仪(LS-POP(6),珠海欧美克);强磁力回转高压反应釜(KCF-1L,威海景达);电子分析天平(AUY220 ,METTLER TOLEDO Instr. LTD)、振动磨样机(XZM-100,武汉探矿机械厂生产);X射线衍射仪(D/max2550VB+,日本理学株氏会社生产);扫描电镜(Nano SEM 230,FEI公司生产)。
1.3 实验方法
称取一定质量制备好的原渣样品,倒入一定浓度硫酸,密封高压釜,通入二氧化硫气体至一定压力,加热至设定温度,反应一定时间后冷却高压釜至25℃,虹吸法取出反应混合物。过滤,分别收集滤液和滤渣,滤液定容后分析溶液中锌浓度。滤渣理化性质定性分析。锌浸出率采用下式(1)计算:
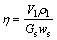 (1)
(1)
式中:Gs为原渣质量,g;ws为原渣中锌质量分数,%;Vl为浸出液定容体积,L;ρl为浸出液锌质量浓度,g/L。
表1 锌冶炼中浸渣的化学组成
Table 1 Chemical composition of zinc neutral leaching residue
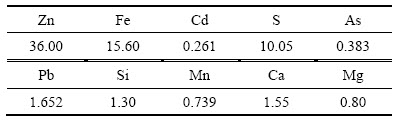
表2 锌冶炼中浸渣锌物相组成
Table 2 Phase composition of zinc in neutral leaching residue
2 结果与讨论
2.1 中浸渣锌浸出机制分析
2.1.1 二氧化硫对中浸渣锌浸出效率影响
二氧化硫对中浸渣中锌浸出效率的影响见图3。由图3可知,二氧化硫对中浸渣中锌浸出率影响较大。浸出系统仅二氧化硫存在时,反应2 h后,锌浸出率为23.47%,锌浸出主要来自于为硫酸锌和氧化锌溶解;仅初始浓度为80 g/L硫酸存在时,反应2 h后,锌浸出率为65.9%,主要为硫酸锌、氧化锌及硅酸锌溶解;二氧化硫和硫酸混合体系中,反应2h后,锌浸出率为85.89%,主要为硫酸锌、氧化锌、硅酸锌及部分铁酸锌溶解;反应3 h后,锌浸出率为87.84%,并无显著提高。仅SO2存在时,锌浸出率较低。加硫酸后,锌浸出率明显增加,说明SO2还原分解铁酸锌需要一定酸度下才能进行。
2.1.2 中浸渣中锌浸出反应机制分析
中浸渣原渣及30 min、60 min还原产物的O1s 光谱分析如图4所示。3种样品在533.79 eV处有共同的特征峰,而中间反应产物在531.5 eV处出现了新特征峰,说明中间产物中O原子呈现与原渣中不同的存在形态,这主要归因于—OH基团中O—H的作用。
这和图3所示的结果基本吻合,证明了在还原浸出过程中,H+起到关键作用。O—H的存在说明在高速搅拌过程中,H+取代了Zn—O中的锌原子,从而形成O-H。因此,可能反应机理如(2)~(6)所示[14]:
SO2+H2O=H++HSO3- (2)
ZnFe2O4(s)+H++HSO3-=ZnFe2O4H+·HSO3-(fast equilibration) (3)
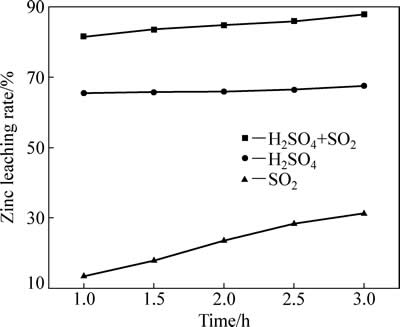
图3 二氧化硫还原气氛对锌浸出效率的影响
Fig. 3 Effect of reductive atmosphere of SO2 on zinc leaching rate
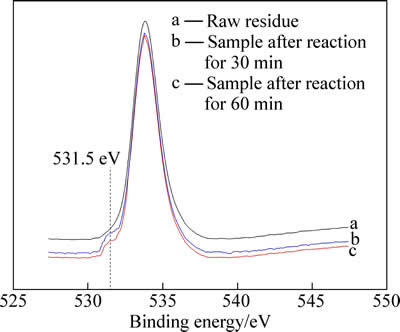
图4 中浸渣及还原产物O1s 能谱分析
Fig. 4 XPS O1s spectra analysis of raw residue and intermediate products
ZnFe2O4H+·HSO3-=ZnFe2O3OH+HSO3(fast redox reaction) (4)
2HSO3 H2S2O6 (5)
H2S2O6 (5)
ZnFe2O3OH+HSO3+2H2SO4=ZnSO4+2FeSO4+3H2O (6)
2.2 中浸渣锌浸出动力学分析
2.2.1 动力学模型
湿法冶金中浸出反应系统主要是固-液多相反应。其特点是反应发生在两相界面上,反应速度常与反应物在界面处的浓度有关,同时也与反应产物在界面的浓度及性质有关。因此,反应速度与反应物接近界面的速度、生成物离开界面的速度以及界面反应速度都有关,其中最慢步骤决定浸出反应速度[15-16]。
化学反应控制模型:
1-(1-α)1/3=krt (7)
扩散控制模型:
1-3(1-α)2/3+2(1-α)=kdt (8)
2.2.2 温度对锌浸出率的影响
温度对中浸渣中锌还原浸出率的影响如下图5所示。反应条件如下:初始硫酸浓度80 g/L;SO2分压200 kPa,液固比10:1;反应釜内转子转速400 r/min。由图5可知,温度对锌浸出率有明显影响。95 ℃时,反应30 min后,锌浸出率达到40.7%,这主要是由于中浸渣中硫酸锌和氧化锌的溶解;反应120 min后,锌浸出率达到98.96%,说明此时铁酸锌已经基本完全浸出,同时大部分硫化锌也被浸出;而继续反应至150 min时,锌浸出率为99.45%,无明显提升。
锌浸出数据代入化学反应控制模型(式(7))和扩散控制模型(式(8)),对图5数据进行线性拟合,其结果如图6和7可知,所得直线斜率即为反应速率常数k。以lgk 对T -1作图可得中浸渣中锌浸出的阿伦尼乌斯曲线,其结果如图8所示。经计算得到根据化学反应控制模型得出的活化能为31.67 kJ/mol,扩散控制模型得出的活化能为41.64 kJ/mol。据文献[17]报道,化学反应控制的反应活化能一般为30~85 kJ/mol,扩散控制为不大于10 kJ/mol。本实验中计算得出的活化能为31.67 kJ/mol,符合化学控制特征。由图6和7可知,化学反应控制的相关因数均大于扩散控制的,因而,可以得出中浸渣中锌还原浸出更倾向于为化学反应控制。
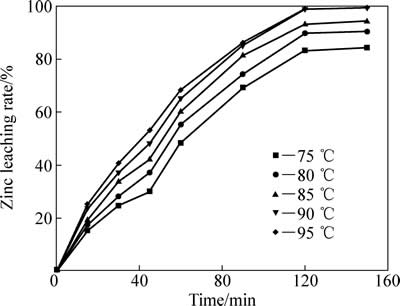
图5 温度对锌浸出效率的影响
Fig. 5 Effect of temperature on zinc leaching rate
2.2.3 硫酸初始浓度对锌浸出率的影响
硫酸初始浓度(50~90 g/L)对中浸渣中锌浸出效率的影响见图9。根据图9、图3以及铁酸锌还原分解原理分析,初始硫酸浓度对锌浸出效率影响较大。初始浓度分别为50 及80 g/L时,反应120 min后,锌浸出率分别为79.35%和98.95%。初始浓度提升至90 g/L对锌浸出率促进较小。这主要基于溶液中酸度对反应式(3)有较大影响,而锌浸出率主要受化学反应控制,当酸度提高到一定数值,其对锌浸出率影响变小。ZHANG等[7]采用热酸浸出方法进行了铁酸盐浸出实验研究,初始硫酸浓度为245 g/L,液固比为200:1,温度90 ℃,反应90 min后锌浸出率达到98%以上,而夏志华等[4]研究的高温高酸下中浸渣中锌浸出动力学的最佳反应条件为:硫酸初始浓度为245 g/L,液固比为50:1,温度90 ℃,反应300 min后,锌浸出率亦达98%以上。
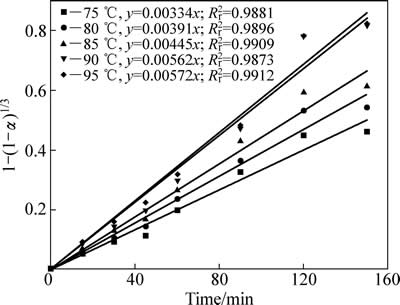
图6 不同温度下1-(1-α)1/3-t拟合曲线
Fig. 6 Variation of 1-(1-α)1/3 with time at different temperatures
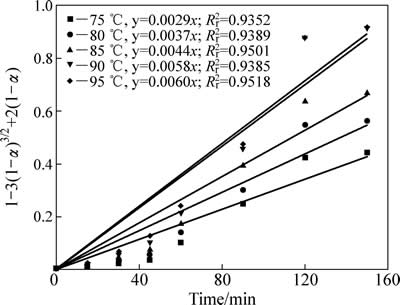
图7 不同温度下[1-3(1-α)2/3+2(1-α)]-t拟合曲线
Fig. 7 Variation of [1-3(1-α)2/3+2(1-α)] with time at different temperatures
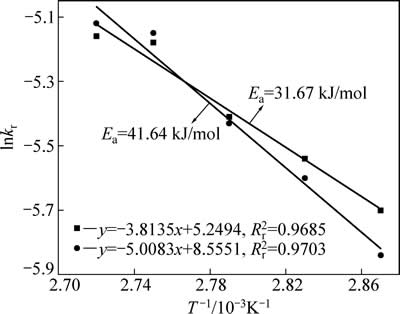
图8 lnkr-T-1的关系曲线
Fig. 8 Arrhenius plots of lnkr-T-1
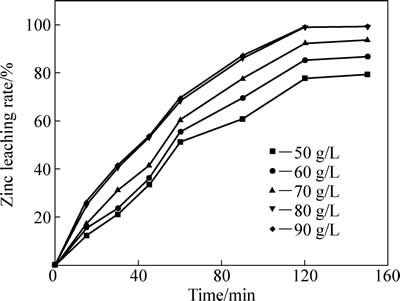
图9 不同初始硫酸浓度对锌浸出率的影响
Fig. 9 Effect of initial sulfuric acid concentration on zinc leaching rate
不同硫酸初始浓度中浸渣锌浸出过程1-(1-α)1/3与时间t关系曲线见图10。对图8中曲线作线性回归求出每条曲线反应速率常数kr,以lnkr对lnρH2SO4作图得图11,硫酸初始浓度相关因数为0.53。
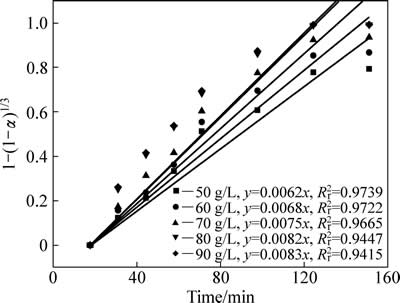
图10 不同初始硫酸浓度下1-(1-α)1/3-t拟合曲线
Fig. 10 Variation of 1-(1-α)1/3 with time at different initial sulfuric acid concentrations
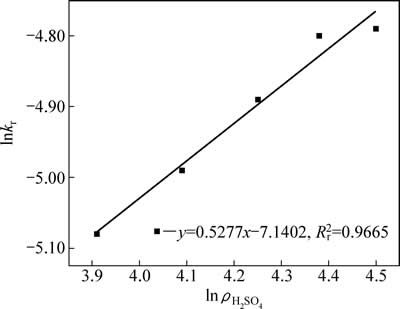
图11 lnkr-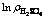 的关系曲线
的关系曲线
Fig. 11 Plots of lnkr-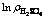
2.2.4 二氧化硫分压对锌浸出效率的影响
中浸渣中锌浸出系统引入SO2后,SO2具有较强还原性,可将渣中Fe (Ⅲ) 还原为Fe (Ⅱ),促进中浸渣中铁酸锌溶解。二氧化硫分压对锌浸出效率的影响如图12所示。反应条件如下:温度95 ℃,初始硫酸浓度80 g/L,液固比10:1,搅拌速度400 r/min。二氧化硫分压对锌浸出效率有较大影响,二氧化硫分压越高,化学反应推动力大,锌浸出率也越高。二氧化硫分压为50 kPa 时,反应2 h后,锌浸出率为73.64%;分压为200 kPa时,锌浸出率达到98.95%,反应时间和二氧化硫分压继续增加对锌浸出率作用较小,200 kPa分压可满足锌浸出率的要求。
不同二氧化硫分压中浸渣锌浸出率1-(1-α)1/3与时间t的关系曲线见图13。1-(1-α)1/3与时间t基本呈线性相关关系。对图13中曲线作线性回归求出每条曲线的反应速率常数kp,以lnkp对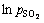 作图得图14,二氧化硫分压相关因数为0.58。
作图得图14,二氧化硫分压相关因数为0.58。
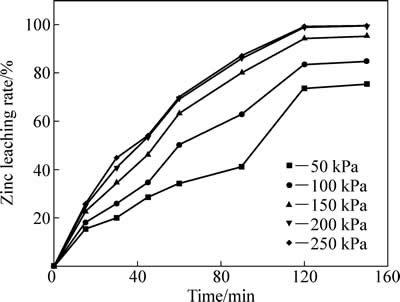
图12 二氧化硫分压对锌浸出效率的影响
Fig. 12 Effect of partial pressure of sulfur dioxide on zinc leaching rate
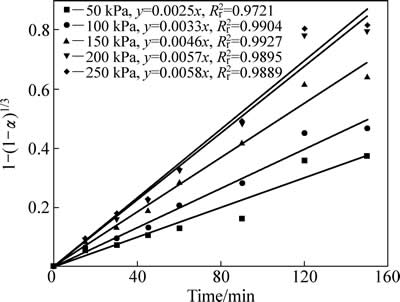
图13 不同二氧化硫分压下1-(1-α)1/3-t拟合曲线
Fig. 13 Variation of 1-(1-α)1/3 with time for partial pressure of sulfur dioxide
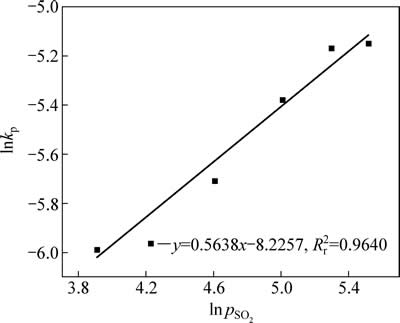
图14 lnkp-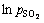 的关系曲线
的关系曲线
Fig. 14 Plots of lnpkp-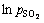
2.2.5 浸出动力学方程
根据中浸渣锌浸出动力学分析,锌还原浸出更符合化学反应控制过程。因此,中浸渣中锌还原浸出宏观动力学模型可用下式表达:
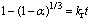
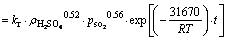 (9)
(9)
锌浸出率在研究的初始浓度及二氧化硫分压范围内,随着其浓度和分压的增大而逐渐增大。
2.3 还原浸出渣定性分析
对最佳反应条件下(温度:95 ℃;初始硫酸浓度:80 g/L;二氧化硫分压:200 kPa;液固比:10:1;转速:400 r/min)的还原浸出渣采用ICP-MS和XRD进行定性分析。ICP-MS化学元素分析结果见表3,还原浸出渣中Zn含量为10.5%,含量最高金属为Pb,为22.5%。XRD分析结果见图15,中浸渣中主要晶相为硫酸铅和硫化锌,说明渣中铅主要以硫酸铅形式存在,铅难以浸出。未浸出锌主要为硫化锌,说明还原浸出系统中部分硫化锌未完全浸出,锌浸出率仍可达99%以上。
表3 还原浸出渣化学组成
Table 3 Chemical composition of reductive leaching residue
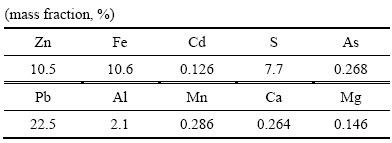
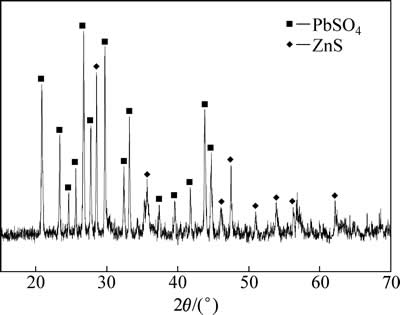
图15 还原浸出渣XRD谱
Fig. 15 XRD pattern of reductive leaching residue
3 结论
1) 锌冶炼中浸渣中锌还原浸出的最佳反应条件:温度为95 ℃,初始硫酸浓度为80 g/L,二氧化硫分压为200 kPa,液固比为10:1,转速为400 r/min,反应120 min后,锌浸出率可达99%以上。
2) 二氧化硫还原浸出铁酸锌表现出高效性,硫酸初始浓度、液固比及反应时间与传统高温高酸法相比均大幅降低,减少了能耗。
3) 锌焙砂中浸渣中锌还原浸出宏观动力学方程:
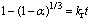
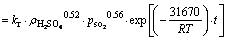
锌浸出率随着初始硫酸浓度及二氧化硫分压增大而增大。
REFERENCES
[1] 张向阳, 王吉坤, 巨 佳, 徐 静, 谢红艳, 贺山明, 氧压酸浸处理锌焙砂中浸渣的新工艺研究[J]. 中国材料进展, 2012, 31(8): 52-56.
ZHANG Xiang-yang, WANG Ji-kun, JU Jia, XU Jing, XIE Hong-yan, HE Shan-ming. Research on oxidizing pressure leaching the residues from the neutral leaching process[J]. Chinese Materials Research Society, 2012, 31(8): 52-56.
[2] 李诚国, 唐谟堂, 唐朝波, 杨声海, 李鸿飞, 巨少华, 陈永明. 氯盐体系中锌焙砂中浸渣高温高酸还原浸出研究[J]. 湿法冶金, 2005, 24(8): 52-56.
LI Cheng-guo, TANG Mo-tang, TANG Chao-bo, YANG Sheng-hai, LI Hong-fei, JU Shao-hua, CHEN Yong-ming. Study on reductive leaching of neutral leached residue in chloride system by high concentration acid and reductive agent at high temperature[J]. Hydrometallurgy of China, 2005, 24(8): 52-56.
[3] 巨 佳, 王吉坤, 张向阳, 徐 静, 谢红艳, 贺山明. 锌焙砂中浸渣氧压酸浸新工艺探讨[J]. 有色金属, 2011, 63(2): 159-162.
JU Jia, WANG Ji-kun, ZHANG Xiang-yang, XU Jing, XIE Hong-yan, HE Shan-ming. Discussion on oxidizing pressure leaching of residues from zinc neutral leaching process[J]. Nonferrous Metals, 2011, 63(2): 159-162.
[4] 夏志华, 唐谟堂, 李仕庆, 罗 艳, 唐朝波. 锌焙砂中浸渣高温高酸浸出动力学研究[J]. 矿冶工程, 2005, 25(2): 53-57.
XIA Zhi-hua, TANG Mo-tang, LI Shi-qing, LUO Yan, TANG Chao-bo. A Study on the kinetics of leaching the residues from the neutral leaching process with high concentration of sulfuric acid at high temperature[J]. Mining Metallurgical Engineering, 2005, 25 (2): 53-57.
[5] LECLERC N, MEUX E, LECUIRE J. Hydrometallurgical extraction of zinc from zinc ferrites[J]. Hydrometallurgy, 2003,70(1): 175-183.
[6] HOLLAGH A R E, ALAMDARI E K, MORADKHANI D, SALARDINI A A. Kinetic analysis of isothermal leaching of zinc from zinc plant residue[J]. International Journal of Nonferrous Metallurgy, 2013, 39( 2): 10-20.
[7] ZHANG Yan-juan, LI Xuan-hai, PAN Liu-ping, LIANG Xin-yuan, LI Xue-ping. Studies on the kinetics of zinc and indium extraction from indium-bearing zinc ferrite[J]. Hydrometallurgy, 2010, 100(13): 172-176.
[8] LU Z Y, JEFFREY M I, LAWSON F. The effect of chloride ions on the dissolution of chalcopyrite in acidic solutions[J]. Hydrometallurgy, 2000, 56(2): 189-202.
[9] ALEX T C, KUMAR R, ROY S K, MEHROTRA S P. Anomalous reduction in surface area during mechanical activation of boehmite synthesized by thermal decomposition of gibbsite[J]. Powder Technonolygy, 2011, 208(1): 128-136.
[10] SENANAYAKE G, DAS G K. A comparative study of leaching kinetics of limonitic laterite and synthetic iron oxides in sulphuric acid containing sulphur dioxide[J]. Hydrometallurgy, 2004, 72(1/2): 59-72.
[11] MCDONALD R G, MUIR D M. Pressure oxidation leaching of chalcopyrite. PartⅠ. Comparison of high and low temperature reaction kinetics and products[J]. Hydrometallurgy, 2007, 86(3/4): 191-205.
[12] DAS G K, DE LANGE J A B. Reductive atmospheric acid leaching of West Australian smectitic nickel laterite in the presence of sulphur dioxide and copper (Ⅱ)[J]. Hydrometallurgy, 2011, 105(3/4): 264-269.
[13] SENANAYAKE G, CHILDS J, AKERSTROM B D, PUGAEV D. Reductive acid leaching of laterite and metal oxides-A review with new data for Fe(Ni, Co)OOH and a limonitic ore[J]. Hydrometallurgy, 2011, 110(1/4): 13-32.
[14] JANKOVIC B, STOPIC S, GUVEN A, FRIEDRICH B. Kinetic modeling of thermal decomposition of zinc ferrite from neutral leach residues based on stochastic geometric model[J]. Journal of Magnetism and Magnetic Materials, 2014, 358/359: 105-118.
[15] 张燕娟, 黎铉海, 潘柳萍, 韦岩松. 机械活化对铟铁酸锌溶解动力学及物化性质的影响[J]. 中国有色金属学报, 2012, 22(1): 315-323.
ZHANG Yan-juan, LI Xuan-hai, PAN Liu-ping, WEI Yan-song. Influence of mechanical activation on dissolution kinetics and physicochemical properties of indium-bearing zinc ferrite[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(1): 315-323.
[16] SENANAYAKE G, DAD G K. A comparative study of leaching kinetics of limonitic laterite and synthetic iron oxides in sulfuric acid containing sulfur dioxide[J]. Hydrometallurgy, 2004, 72(5): 59-72.
[17] WANG X, WANG Xin, SRINIVASAKANNAN C, DUAN Xin-hui, PENG Jin-hui, YANG Da-jin, JU Shao-hua. Leaching kinetics of zinc residues augmented with ultrasound[J]. Separation and Purification Technology, 2013, 115(7): 66-72.
Mechanisms and kinetics on reductive leaching of zinc from zinc neutral leaching residue
ZHANG Chun1, 2, MIN Xiao-bo1, 3, ZHANG Jian-qiang1, WANG Mi1, LI Yuan-cheng1
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. School of Municipal and Mapping Engineering, Hunan City University, Yiyang 413000, China;
3. Chinese National Engineering Research Center for Control and Treatment of
Heavy Metal Pollution, Central South University, Changsha 410083, China)
Abstract: The chemical composition and zinc phases were studied by XRD, XPS, ICP and phase analysis. The effects of temperature, initial sulfuric acid concentration and partial pressure of sulfur dioxide on the Zn leaching rate were also studied when sulfur dioxide was used as reductant. The mechanism and kinetics of reductive decomposition of zinc ferrite were also studied. The results show that H+ plays a key role during the reductive leaching process. The activity energy is 31.67 kJ/mol and the kinetic equation is established based on the chemical reaction controlled model. The optimum technological conditions are as follows: initial sulfuric acid concentration 80 g/L; temperature 95 ℃; liquid-to-solid 10; sulfur dioxide partial pressure 200 kPa; reactive time 120 min. Under the optimum condition, zinc leaching efficiency reached more than 99% and the main phase in the reductive residue are lead sulfate and zinc sulfide.
Key words: zinc neutral leaching residue; reductive leaching; sulfur dioxide; kinetics
Foundation item: Project (2012FJ1010, 2014FJ1011) supported the Key Projects of Science and Technology of Hunan Province, China; Project (51474247) supported by the Authors Gratefully Acknowledge the Natural Science Foundation of China; Project (2015-40) supported by the Science and Technology Project of Yiyang, China; Project (13B009) supported by the Outstanding Youth Project of Hunan Provincial Department of Education, China
Received date: 2015-05-29; Accepted: 2015-10-16
Corresponding author: MIN Xiao-bo; Tel: +86-731-88830875; E-mail:mxbcsu@163.com
(编辑 龙怀中)
基金项目:湖南省科技重大专项(2012FJ1010);国家自然科学基金资助项目(51474247);益阳市科技计划项目(益科字[2015]40号);湖南省教育厅优秀青年项目(13B009)
收稿日期:2015-05-29;修订日期:2015-10-16
通信作者:闵小波,博士;电话:0731-88830875;E-mail:mxbcsu@163.com