改性活性炭吸附脱除氰化氢
蒋明,宁平,王重华,田森林,庙荣荣,周键,陈炜,王磊
(昆明理工大学 环境科学与工程学院,云南 昆明,650500)
摘要:采用浸渍法制备铜金属氧化物改性活性炭吸附剂,采用同步热重-差热分析(TG/DTA)、比表面分析(N2-BET)和X线光电子能谱(XPS)分析,考察吸附剂的净化性能。研究结果表明:最佳焙烧温度为300 ℃,最优体积空速为600 h-1;铜金属氧化物改性能显著增加活性炭对HCN的吸附性能,孔径为0.74~1.98 nm的微孔对HCN的吸附贡献较大,改性活性炭在325 ℃以内保持较优的热稳定性,CuO是吸附剂的主要活性组分,吸附HCN后,HCN被催化氧化分解为NH3,吸附剂的失活可能是HCN在脱除过程中生成的CuCN填充和覆盖了活性炭的微孔。
关键词:氰化氢;改性活性炭;吸附净化;醋酸铜
中图分类号:X701.7 文献标志码:A 文章编号:1672-7207(2012)08-3294-06
Adsorption removal of hydrogen cyanide by modified activated carbon
JIANG Ming, NING Ping, WANG Zhong-hua, TIAN Sen-lin,
MIAO Rong-rong, ZHOU Jian, CHEN Wei, WANG Lei
(Faculty of Environmental Science and Engineering, Kunming University of Science and Technology, Kunming 650500, China)
Abstract: The activated carbon modified by copper oxide was prepared using impregnation method. The effects of roasting temperature and gas hourly space velocity on the purification performance were investigated by simultaneous measurements of thermogravimetry(TG) and differential thermal analysis(DTA), specific surface area (N2-BET) and X-ray photoelectron spectroscopy(XPS). The results show that the optimum roasting temperature is 300 ℃ and the optimum volume space velocity is 600 h-1. The copper oxide significantly improves the adsorption performance of activate carbon as a HCN adsorbent. The micropore with radius of 0.74-1.98 nm makes primary contribution for adsorbing removal of HCN. The modified activated carbon maintains optimum thermal stability at temperatures lower than 325 ℃. CuO is the major species on adsorbent. After adsorption, HCN is converted to NH3 by the catalytic oxidation. Moreover, deactivation of the adsorbent is due to micropore filling and covering by the formation of CuCN along with the HCN removal.
Key words: hydrogen cyanide; modified activated carbon; adsorption purification; copper acetate
工业排放气态污染物是大气污染的重要来源。近年来,我国常规气态污染物控制方面已经取得了一定的成效,但仍然不能满足具有多样性、复合性等特点的有毒有害工业废气污染控制的技术需求。HCN为第一类A级无机剧毒品,是含氮矿物燃料燃烧所产生的工业废气中最为典型的有毒有害污染物之一[1],通过皮肤、呼吸道或消化道进入人体后,迅速分解出的游离氰在人体内易与各种细胞内呼吸酶中的铁、铜、铝等结合,阻止金属离子的还原,导致该酶失活,使细胞不能利用氧,从而产生细胞内窒息性缺氧[2]。在我国,HCN主要产生于焦炉煤气[3-4]、黄磷尾气[5]、密闭电石炉尾气[6-7]和聚丙烯腈基炭纤维(PANCF)[8-9]等典型工业废气中,其存在不仅阻碍典型工业废气的净化和资源化,还严重影响周围环境和人类健康。目前,国内外主要通过吸收[2]、吸附[10]、燃烧[11]、催化氧 化[12]、催化水解[12-13]等手段脱除HCN,而以吸附力强、比表面大、价格低廉的活性炭为载体的吸附剂对低浓度HCN进行吸附净化则能起到优良的脱除效果。为此,本文作者以过量溶液浸渍法改性活性炭吸附脱除低浓度HCN,并对吸附剂的净化性能和脱除机理进行研究。
1 材料与方法
1.1 主要材料及仪器
主要材料及仪器有:市售ZP-2活性炭,其粒径为2 mm,孔容为0.41 cm3/g,装填密度为500 g/L、比表面积≥800 m2/g;购自大连大特气体有限公司质量浓度约为112 mg/m3的HCN钢瓶气(N2为平衡气); PHS-3C型数显酸度计、ES-3072磁力搅拌器、PCN-1Q9氰离子选择电极、217型双盐桥参比电极;美国康塔公司生产的NOVA2000e氮气吸附仪,分析吸附温度为77.350 K;美国PHI5600型X线光电子能谱(XPS),能谱数据校正C标准结合能为248.8 eV,数据通过Gaussian-Lorentzian进行拟合;日本岛津公司DTG-60H同步热重-差热(TG-DTA)分析仪。
1.2 改性炭的制备
将10 g空白活性炭用蒸馏水洗涤3次,待干燥后备用;然后用100 mL浓度为0.02 mol/L的Cu(CH3COO)2溶液浸渍活性炭24 h,再经干燥、焙烧即得用于吸附HCN的改性炭。
1.3 实验方法
实验流程如文献[14]中所示,先将HCN气体与空气经混合器进行混合使氧体积分数为1%,混合器出来的气体有2个出口:一个出口用来测定吸附反应之前的HCN浓度;另一出口的混合气体首先进入浸没在恒温水浴箱里的气体预热装置,预热后的混合气体再进入填充有5 g改性炭的吸附柱进行吸附净化反应,净化后的HCN气体通入装有NaOH溶液的吸收瓶(瓶内放入用于调节离子强度的少许KNO3固体)吸收并用氰离子选择电极法测定吸收液中氰离子浓度,从而换算成HCN气体实际浓度;最后,HCN尾气经NaOH尾气吸收瓶吸收后放空,实验过后尾气吸收瓶中的NaCN废液用一定浓度的Na2S2O3溶液进行处理,将氰离子转化为无毒的硫氰酸盐(SCN-)后排放。反应在60 ℃,氧体积分数为1%条件下进行。当净化后气体浓度为净化前的10%时可视为吸附剂穿透。
2 结果与讨论
2.1 焙烧温度的影响
焙烧是制备改性活性炭吸附剂的重要步骤之一,焙烧后活性组分以高度分散的形式存在于载体表面,在焙烧过程中,活性组分晶粒变化导致活性表面积的变化。焙烧温度对HCN吸附性能的影响如图1所示,其中,C0和C分别为净化前后气体浓度。由图1可知:当改性活性炭在300 ℃的温度下焙烧成型时,其表面活性最高,说明该温度下Cu(CH3COO)2分解后的活性组分最有利于对HCN的吸附。虽然200 ℃和400 ℃时改性炭对HCN的吸附性能相当,但吸附机理可能并不相同。200 ℃时,该温度不足以使活性炭表面的Cu(CH3COO)2分解,此时仅为HCN与活性炭的物理吸附使HCN得以脱除,故脱除效率不高;当焙烧温度在400 ℃以上时,活性炭对HCN的吸附性能急剧下降,虽然活性炭表面仍分散着Cu(CH3COO)2分解后的活性组分,但此时活性炭载体已有部分分解,温度继续升高,炭分解越多,造成孔道进一步破坏和塌陷,此刻便失去了载体的功能。因此,300 ℃是本实验条件下较适宜的焙烧温度。
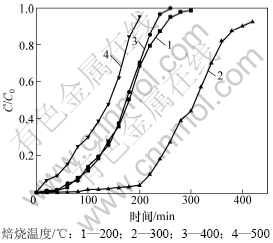
图1 焙烧温度对HCN吸附性能的影响
Fig.1 Influence of roasting temperature on HCN removal
2.2 体积空速的影响
实验所用改性炭质量为5 g,按照文献[15]中的方法测得其堆体积为9.8 mL,并通过改变进气流量来改变空速,因此考察流量分别为100,150,200和250 mL/min,即空速分别为600,900,1200和1 500 h-1时改性炭对HCN吸附性能的影响,结果如图2所示。
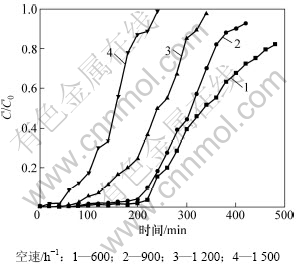
图2 空速对HCN吸附性能的影响
Fig.2 Influence of space velocity on HCN removal
由图2可知:600 h-1为实验条件下的较优空速。随着空速的增加,改性炭的脱氰性能逐渐下降,这是因为空速的增加缩短了气体分子在吸附剂床层表面的接触和停留时间,使改性炭的平均吸附速率降低,影响了对HCN的净化性能。另一方面,空速如果太小,不利于消除吸附剂表面的外扩散阻力,使气流无法与吸附剂表面和内部的活性物质发生接触并反应,从图2还可知:当空速由900 h-1降至600 h-1时,虽然吸附剂对HCN的脱除效率有所增加,但增加趋势不明显。
2.3 吸附剂物性表征
2.3.1 热重-差热分析
为了探讨改性活性炭吸附剂制备时的最佳焙烧温度和活性炭的热稳定性,将浸渍铜盐的活性炭(该活性炭仅经过110 ℃干燥,并未焙烧)粉碎、研磨成一定粒度的活性炭粉,取4 mg左右的样品放入Al2O3坩埚,在N2保护下进行热重(TG)和差热(DTA)分析,N2流量为50 mL/min,升温速率为10 ℃/min,热解终温设定为800 ℃,结果如图3所示。
由图3可知,浸渍活性炭在68~325 ℃的范围内出现了缓慢质量损失,其质量损失率为7.65%,在274 ℃时出现了明显的放热峰,这可能是:(1) 纯Cu(CH3COO)2晶体在N2气氛及300 ℃左右的温度下热解时,主要发生的是以吸热为主的分解反应[16-18];(2) 在相同气氛和温度时,Cu(CH3COO)2在活性炭的作用下,发生以放热为主的晶型转变反应,从而形成活性组分;(3) 由于仪器的不致密性,虽有N2保护,也难免有极少量空气进入炉体内,或炉内也存在N2吹扫不到的死体积空气区域,此时,活性炭表面的低燃点的有机物和杂质就会与极少量空气发生放热的氧化反应。故本实验条件下在274 ℃时出现的放热峰很可能是以上3种原因综合的结果,但可以肯定的是,在274 ℃左右改性活性炭得以成型和活化,这与300 ℃的最佳焙烧温度是一致的。由图3还可以看出:活性炭在325~800 ℃范围内出现急剧质量损失,质量损失率达到87.41%,并出现了强放热峰。这主要是因为活性炭中炭组分的热分解和燃烧所致,同时,该强烈的放热效应也掩盖了Cu(CH3COO)2在高温下进一步分解的吸热效应。故实验所用改性活性炭在325 ℃以下有较高的稳定性。
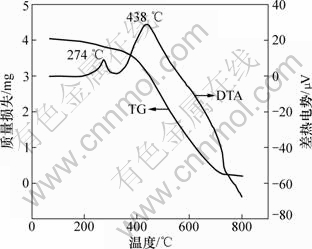
图3 浸渍活性炭的热重-差热曲线
Fig.3 TG curve and DTA curve of impregnated activated carbon
2.3.2 比表面与孔径分布
用N2吸附仪分析吸附HCN前后改性活性炭样的物性参数,结果见表1。
表1 不同活性炭样品物性参数
Table 1 Physical parameters of AC samples
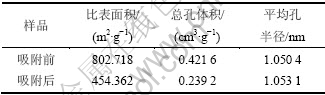
由表1可知,改性活性炭吸附HCN后,比表面积和总孔容积分别降低了43.40%和43.26%,从而说明HCN气体分子较强的吸附到改性活性炭的孔道内。图4所示为用密度函数理论(DFT)表征的各样品的孔径分布图。由图4(a)可以看出:吸附HCN前后改性炭的孔径分布主要集中分布在0.74~1.98 nm;当孔径>1.98 nm以后,二者的孔体积已接近为0,说明改性后的活性炭为典型的微孔活性炭(孔半径<2 nm[19]),HCN也主要吸附于活性炭的微孔范围内。从图4(b)可以看出:在整个微孔范围内,吸附HCN后的改性炭其孔体积总低于未吸附的孔体积,表明在该范围内活性炭对HCN的吸附贡献最大,吸附HCN后改性炭的比表面积和总孔体积的急剧减小说明HCN分子以微孔填充的方式进入到改性炭的微孔孔道内,并与炭表面的活性组分发生化学吸附,通过覆盖和堵塞炭孔道而使活性炭失活。
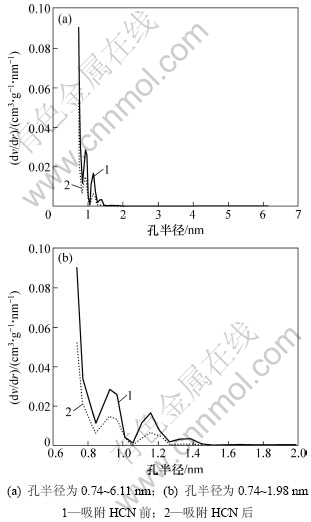
图4 不同活性炭样品的孔径分布曲线
Fig.4 Pore size distribution of AC samples
2.3.3 XPS分析
将空白活性炭、吸附HCN的空白活性炭、改性活性炭、吸附HCN的改性活性炭分别用1~4表示,4组样品的宽扫描图和活性炭上各物质原子数分数变化分别见图5和表2。
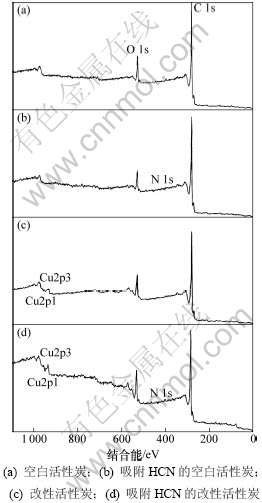
图5 不同活性炭样品的XPS全谱
Fig.5 XPS survey spectra of AC samples
表2 活性炭上各物质原子数分数变化
Table 2 Concentration of elements of AC samples %
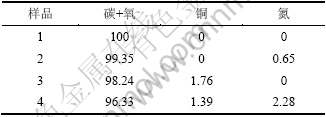
由图5可知:空白活性炭本身对HCN有一定的物理吸附作用,但吸附性能极为有限,由于吸附量较少,使氮元素的分峰拟合无法实现。结合表2还可以看出:改性后增加了活性炭表面的铜原子数分数,使改性炭对HCN的吸附能力极大增强,其表面氮元素的原子百分含量约为空白活性炭吸附HCN后的3.5倍。与此同时,吸附HCN后的改性炭表面铜含量较吸附前有所降低,这可能是由于发生吸附反应时铜与载体炭的相互作用加强,反应后部分铜离子进入了载体炭的次表层中,而反应后的生成物吸附在炭表面,极有可能将部分铜离子覆盖,从而降低了炭表面的铜原子数分数。
图6所示为改性活性炭吸附HCN前后铜元素的局部分峰拟合图。由图6可知:改性活性炭Cu2p的主峰Cu2p3/2和伴峰Cu2p1/2的结合能分别为933.46 eV和952.24 eV,但铜元素的结合态仍主要归属于其主峰Cu2p3/2的结合能。由于Cu(CH3COO)2.H2O在不同气分下分解或氧化时产物也不同,其在不同的条件下主要转变为CuO,Cu2O,Cu3O4和Cu[17-18],但在940~945 eV范围内出现了明显的二价铜的小卫星伴峰,此时,主峰Cu2p3/2的933.46 eV的结合能不仅与文献[20]中CuO的结合能基本一致,还与XPS化学状态数据库[21]中CuO的结合能最为接近,由此可知:CuO为本实验条件下改性活性炭吸附脱除HCN的活性组分。活性炭吸附HCN后,其Cu2p的结合能分别为933.29 eV和953.9 eV,且XPS谱图上没有2价铜物种的小卫星伴峰,说明在实验条件下活性炭表面的活性CuO与HCN发生了化学吸附,HCN被吸附脱除的同时也使CuO失活而转变为其他价态的铜。Brown等[22]研究表明:同时填充有Cu2+与Cr6+的木质基活性炭在吸附HCN时,Cu2+将转变为Cu+(CuCN),同时生成(CN)2,然后(CN)2在Cr6+催化下生成(NH2CO)2,形成HCN-(CN)2-Cu(II)O-Cr(VI)系统,有利的增加了活性炭的吸附容量,因此,图6(b)中933.29 eV的结合能很可能为CuCN的结合能。结合XPS化学状态数据库,该结合能与CuCN的标准结合能(933.1 eV)较为接近,故判定改性炭上活性CuO吸附HCN后的失活产物为CuCN。
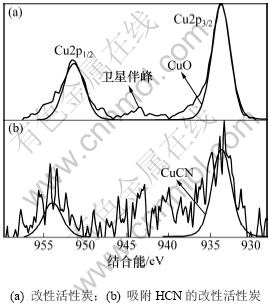
图6 不同活性炭样品Cu2p的XPS谱图
Fig.6 Cu2p XPS spectra of AC samples
同时,改性活性炭吸附HCN后氮元素的XPS分析见图7(空白活性炭对HCN的吸附量较小,实验条件下无法对其氮元素进行分峰拟合)。由图7可知:氮元素的N1s谱峰只在结合能为399.65 eV处有拟合峰,这与NH3的标准结合能(399.6 eV)基本一致。且Brown等[22-23]指出:HCN和废气中极少量的水蒸气在活性炭吸附剂上可能最终水解-催化氧化分解为NH3。为此,可认为通过负载铜离子浸渍改性活性炭在焙烧后所形成的活性CuO将气流中的微量氧气转变为活性氧原子(O*)后,与HCN发生催化氧化反应,使其转变为无毒或低毒的物质,达到HCN解毒的目的;同时,活性物质CuO最终转变为CuCN而失活。与文献[22-23]分别利用Cu-Cr和Cu-Zn混合浸渍活性炭(活性炭未进行焙烧)相比,此改性方法不仅避免了使用毒性较大的重金属离子Cr6+,还简化了吸附剂制备的工艺流程,同时,经过焙烧后得到的大量活性CuO能更均匀的负载和分散到活性炭表面,有利地增强了改性炭对HCN的吸附性能。故推测可能的反应式为:
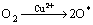 (1)
(1)
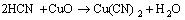 (2)
(2)
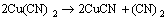 (3)
(3)
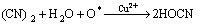 (4)
(4)
 (5)
(5)
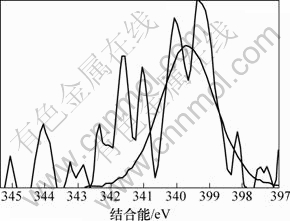
图7 改性活性炭吸附HCN后N1s的XPS谱图
Fig.7 N1s XPS spectra for exhausted modified activated carbon
3 结论
(1) 在改性活性炭制备过程和吸附净化过程中,焙烧温度和体积空速是影响净化效率的关键因素。在实验范围内,最佳焙烧温度为300 ℃,体积空速为 600 h-1。
(2) 改性活性炭在325 ℃以下有较强的热稳定性,且在孔半径0.74~1.98 nm的微孔范围内,改性炭对HCN的吸附贡献最大;在改性过程中分解得到的CuO是活性炭吸附脱除HCN的活性组分,炭表面的CuO催化活化O2使HCN与CuO和气流中极少量水蒸气发生复杂的水解-催化氧化反应,最终可能使HCN转变为NH3,而CuCN的生成是活性CuO失活的主要原因。
参考文献:
[1] Dagaut P, Glarborg P, Alzueta M U. The oxidation of hydrogen cyanide and related chemistry[J]. Progress in Energy and Combustion Science, 2008, 34(1): 1-46.
[2] 陈冠荣. 化工百科全书[M]. 北京: 化学工业出版社, 1997: 143-161.
CHEN Guan-rong. Chemical encyclopedia[M]. Beijing: ChemicalIndustryPress, 1997: 143-161.
[3] TAN Hou-zhang, WANG Xue-bin, WANG Cong-ling, et al. Characteristics of HCN removal using CaO at high temperatures[J]. Energy & Fuels 2009, 23(3): 1545-1550.
[4] 李军, 袁帅, 梁钦峰, 等. 煤及其模型化合物快速热解过程中HCN和NH3逸出规律的研究[J]. 高校化学工程学报, 2011, 25(1): 55-60.
LI Jun, YUAN Shuai, LIANG Qin-feng, et al. Formation of HCN and NH3 during the rapid pyrolysis of coal and model compounds[J]. Journal of Chemical Engineering of Chinese Universities, 2011, 25(1): 55-60.
[5] 蒋明, 宁平, 王重华, 等. 黄磷尾气中氰化氢的测定[J]. 现代化工, 2011, 31(4): 92-95.
JIANG Ming, NING Ping, WANG Zhong-hua, et al. Determination of hydrogen cyanide in yellow phosphorus tail gas[J]. Modern Chemical Industry, 2011, 31(4): 92-95.
[6] 汪乐丰. 密闭电石炉尾气利用新途径[J]. 中国环保产业, 2009(10): 51-54.
WANG Le-feng. New approach for tail gas utilization of obturated calcium carbide furnace[J]. China Environmental Protection Industry, 2009(10): 51-54.
[7] 余琼粉, 易红宏, 唐晓龙, 等. 磷化氢在改性活性炭纤维上的吸附等温过程[J]. 中南大学学报: 自然科学版, 2010, 41(1): 381-386.
YU Qiong-fen, YI Hong-hong, TANG Xiao-long, et al. Adsorption isotherm of phosphine onto CoCl2-modified activated carbon fiber[J]. Journal of Central South University: Science and Technology, 2010, 41(1): 381-386.
[8] WANG Yan-zhi, ZHU Bo, WANG Yan-xiang, et al. Study on the preparing of plyacrylonitrile-based carbon fibers[J]. New Carbon Materials, 2001, 16(4): 12-17.
[9] Rahaman M S A, Ismail A F, Mustafa A. A review of heat treatment on polyacrylonitrile fiber[J]. Polymer Degradation and Stability, 2007, 92(8): 1421-1432.
[10] Hudson M J, Knowles J P, Harris P J F, et al. The trapping and decomposition of toxic gases such as hydrogen cyanide using modified mesoporous silicates[J]. Microporous and Mesoporous Materials, 2004, 75(1/2): 121-128.
[11] Schafer S, Bonn B. Hydrolysis of HCN as an important step in nitrogen oxide formation in fluidized combustion: Part II. Heterogeneous reactions involving limestone[J]. Fuel, 2002, 81(13): 1641-1646.
[12] Kr?cher O, Elsener M. Hydrolysis and oxidation of gaseous HCN over heterogeneous catalysts[J]. Applied Catalysis B: Environmental, 2009, 92(1/2): 75-89.
[13] Kr?cher O, Elsener M, Jacob E. A model gas study of ammonium formate, methanamide and guanidinium formate as alternative ammonia precursor compounds for the selective catalytic reduction of nitrogen oxides in diesel exhaust gas[J]. Applied Catalysis B: Environmental, 2009, 88(1/2): 66-82.
[14] 宁平, 蒋明, 王学谦, 等. 低浓度氰化氢在浸渍活性炭上的吸附净化研究[J]. 高校化学工程学报, 2010, 24(6): 1038-1045.
NING Ping, JIANG Ming, WANG Xue-qian, et al. Adsorption purification of low-concentration HCN on impregnated activated carbon[J]. Journal of Chemical Engineering of Chinese Universities, 2010, 24(6): 1038-1045.
[15] 甄开吉, 王国甲, 毕颖丽, 等. 催化作用基础[M]. 北京: 科学出版社, 2005: 44.
ZHEN Kai-ji, WANG Guo-jia, BI Ying-li, et al. Fundamentals of catalysis[M]. Beijing: SciencePress, 2005: 44.
[16] Mansour S A A. Thermoanalytical investigations of the decomposition course of copper oxysalts III. Copper(II) acetate monohydrate[J]. Journal of Thermal Analysis and Calorimetry, 1996, 46(1): 263-274.
[17] Obaid A Y, Alyoubi A O, Samarkandy A A, et al. Kinetics of thermal decomposition of Copper(II) acetate monohydrate[J]. Journal of Thermal Analysis and Calorimetry, 2000, 61(3): 985-994.
[18] Bellini J V, Machado R, Morelli M R, et al. Thermal, structural and morphological characterisation of freeze-dried Copper(II) acetate monohydrate and its solid decomposition products[J]. Materials Research, 2002, 5(4): 453-457.
[19] Sing K S W, Everett D H, Haul R A W, et al. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity[J]. Pure and Applied Chemistry, 1985, 57(4): 603-619.
[20] Li W C, Bai H , Hsu J N, et al. Metal loaded zeolite zdsorbents for phosphine removal[J]. Industrial and Engineering Chemistry Research, 2008, 47(5): 1501-1505.
[21] Moulder J F, Stickle W F, Sobol P E. Handbook of X-ray photoelectron spectroscopy[M]. Montreal: Perkin-Elmer Corporation Publisher, 1992: 220-221.
[22] Brown P N, Jayson G G, Thompson G, et al. Adsorption characteristics of impregnated activated charcoal cloth for hydrogen cyanide[J]. Journal of Colloid and Interface Science, 1987, 116(1): 211-220.
[23] Nickolov R N, Mehandjiev D R. Comparative study on removal efficiency of impregnated carbons for hydrogen cyanide vapors in air depending on their phase composition and porous textures[J]. Journal of Colloid and Interface Science, 2004, 273(1): 87-94.
(编辑 赵俊)
收稿日期:2011-09-14;修回日期:2011-12-10
基金项目:国家高技术研究发展(“863”计划)项目(2008AA062602);云南省自然科学基金资助项目(14051184);教育部科学技术研究重点项目(210202);昆明理工大学分析测试基金资助项目(2010136)
通信作者:宁平(1958-),男,山西太原人,博士,教授,博士生导师,从事环境污染控制与化工过程数值模拟研究;电话:0871-5186313;E-mail:ningping58@126.com