文章编号:1004-0609(2014)06-1629-08
熔池熔炼中金属硫酸盐分解过程的热力学研究
刘 群1,谭 军1,刘常青1,尹周澜1,陈启元1,廖 舟2,谢富春1,张平民1
(1. 中南大学 化学化工学院,有色金属资源化学教育部重点实验室,长沙 410083;
2. 株洲冶炼集团股份有限公司,株洲 412000)
摘 要:利用化学反应的标准平衡常数求得冶炼废渣熔池熔炼过程中5种金属硫酸盐(FeSO4, In2(SO4)3, Fe2(SO4)3, ZnSO4, PbSO4)的分解压,确定硫酸盐在高温下发生分解反应的温度;采用热重(TG)及差示扫描量热(DSC)分析各金属硫酸盐的分解过程,验证热力学计算结果。结果表明,5种硫酸盐发生分解反应由易到难的顺序为FeSO4、In2(SO4)3、Fe2(SO4)3、ZnSO4、PbSO4;热力学计算得到的相变温度、分解温度与DSC曲线吸热峰位置、TG曲线质量损失阶段相吻合,且理论计算温度比TG-DSC测定温度低7.5~283.1 K;熔炼炉温度达到1316.1 K以上可使浸锌渣、沉铁渣中的硫酸盐分解脱硫较彻底。
关键词:冶炼废渣;金属硫酸盐;熔池熔炼;热力学计算
中图分类号:TF812 文献标志码:A
Thermodynamic study of metal sulfate decomposition process in bath smelting
LIU Qun1, TAN Jun1, LIU Chang-qing1, YIN Zhou-lan1, CHEN Qi-yuan1, LIAO Zhou2,
XIE Fu-chun1, ZHANG Ping-min1
(1. Key Laboratory of Resources Chemistry of Nonferrous Metals, Ministry of Education,
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Zhuzhou Smelter Group Co., Ltd., Zhuzhou 412000, China)
Abstract: The decomposition pressures of five metal sulfates (FeSO4, In2(SO4)3, Fe2(SO4)3, ZnSO4, PbSO4) in smelting slag were calculated with the standard equilibrium constants of related chemical reactions and the sulfate decomposition temperatures were obtained by the pressures. The metal sulfates decomposition was investigated through thermogravimetric (TG) and differential scanning calorimetry (DSC), and the TG-DSC analysis confirmed the results of thermodynamic calculation. The results show that the ability of metal sulfate decomposition can be ordered as follows (from easy to difficult): FeSO4, In2(SO4)3, Fe2(SO4)3, ZnSO4, PbSO4. The phase transition and decomposition temperatures from thermodynamic calculation are consistent with TG-DSC results. The calculated temperatures are lower than those from TG-DSC, and the difference is from 7.5 to 283.1 K. The sulfates in zinc leaching residue and iron sinking slag will decompose more thoroughly when the furnace temperature is above 1316.1 K.
Key words: smelting slag; metal sulfates; bath smelting; thermodynamic calculation
我国铅锌冶炼企业数量多、分布广。铅冶炼几乎都采用火法冶炼,锌冶炼以湿法冶炼为主[1-2]。当前可用于冶炼金属的富矿日趋减少,而冶金、化工、材料等领域经常产生各种固体废渣。因此,开展经济与环境友好的冶炼新工艺,回收渣中的有价金属势在必行[3]。
沉铁渣和浸锌渣是湿法炼锌过程产生的湿固体渣,含有铁、锌、铅、铜、银、镉、钴、镍、铋、铟和铊等多种有价金属[4-5],并且多数金属以硫酸盐的形式赋存于渣中。熔池熔炼是炉料在液态熔池(熔渣和熔锍)中迅速完成气-液-固相间主要反应的熔炼方法[6],广泛用于炼铜、炼镍、炼铅和炼铁等[7-8]。目前,很多冶金工作者采用熔池熔炼法搭配浸锌渣、沉铁渣等废渣炼铅[9],且Ausmelt熔池熔炼法处理有色金属冶炼废料废渣的优势明显[10-11]。因此,研究熔池熔炼过程中各类硫酸盐在熔炼炉内的分解行为有重要意义。
近些年来,很多研究者对金属硫酸盐分解过程的动力学及热力学进行了探索。TOMASZEWICA等[12]研究了在空气和氦气气氛中NiSO4·6H2O的热分解过程,并在TG、DTA及XRD分析的基础上提出了NiSO4·6H2O的分解机理。TAGAWA等[13-14]对Fe、Co、Ni、Cu、Zn的硫酸盐在氮气及空气气氛中分解过程的动力学进行了探索,确定了不同分解速率下中间产物及硫酸盐的分解温度。KOLTA等[15]讨论了几种金属硫酸盐的热分解机理及影响因素,采用TG和DTA热分析法确定了硫酸盐分解的温度范围。SIRIWARDANE等[16]采用XPS、SEM/EDS、TG、XRD和DRIFTS分析方法对Ni、Cu、Zn、Fe(Ⅱ)和Fe(Ⅲ)硫酸盐的分解过程进行研究,分析了硫酸盐表面形态的变化及在真空、空气条件下分解温度的关系。PERLMUTTER等[17]研究了气氛环境、颗粒大小以及加热速率对Zn、Mn、Co、Ni和Fe等硫酸盐分解的影响。PETKOVA等[18]采用DSC热分析方法对Fe(Ⅱ)和Fe(Ⅲ)的相关硫酸盐在氩气、空气和氧气条件下的分解过程进行研究,证实了气体组成分压对亚铁盐热分解的影响。
这些研究者虽讨论了硫酸盐分解反应发生的机理,并通过热分析方法考察了硫酸盐分解的温度范围及影响分解的因素,但缺少对硫酸盐分解过程的热力学计算方法及硫酸盐分解压的探索。姚腾猛等[19]利用平衡反应的热力学计算确定了Pb-S-O体系优势区图的两种类型,解释了Kivcet炉闪速区直接出铅的现象并获得了PbSO4稳定存在的温度区域,但没有讨论PbSO4的分解压,且缺少与其他硫酸盐的比较分析。
本文作者通过化学反应标准平衡常数的热力学计算方法研究了沉铁渣和浸锌渣中的主要金属硫酸盐PbSO4、ZnSO4、FeSO4、In2(SO4)3和Fe2(SO4)3的分解压、分解温度及分解过程,并利用热重(TG)及差示扫描量热(DSC)的热分析方法对计算结果进行验证。这对于实现熔池熔炼新工艺回收冶炼渣中的有价金属具有重要理论意义,是一项具有很高应用价值的研究工作。
1 热力学计算公式
基于化学热力学原理,对于任一化学反应
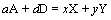 (1)
(1)
在等温等压条件下有
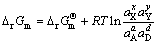 (2)
(2)
式中: 是化学反应的标准吉布斯自由能变化;
是化学反应的标准吉布斯自由能变化; 是温度为T时化学反应的吉布斯自由能变化;
是温度为T时化学反应的吉布斯自由能变化; 是反应物X的活度(当X为气体时,用压力表示)。高温下任一反应的
是反应物X的活度(当X为气体时,用压力表示)。高温下任一反应的 都可由下式计算
都可由下式计算
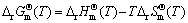 (3)
(3)
式中: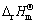 为化学反应的标准反应热效应;
为化学反应的标准反应热效应; 为化学反应的标准反应熵变。
为化学反应的标准反应熵变。
参加反应的各物质随温度的变化可能出现相变,这时应将相变热和相变熵计算进去;同时,由于物质的聚集状态不同,恒压热容与温度的函数关系式也不相同。故在出现相变的情况下,计算反应的热效应及熵变时,要根据相变温度划分温度区间进行分段计算。
当化学反应有相变时,在为温度T时的反应热效应和反应熵变的计算公式分别为
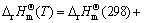
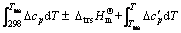 (4)
(4)
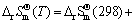
 (5)
(5)
式中:Ttrs为发生相变时的温度; 和
和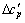 分别为相变前后反应体系的恒压比热容的差值;
分别为相变前后反应体系的恒压比热容的差值; 为反应物/生成物的摩尔相变热。当反应物发生相变时
为反应物/生成物的摩尔相变热。当反应物发生相变时 前取负号;生成物发生相变时
前取负号;生成物发生相变时 前取正号。
前取正号。
将式(4)和(5)代入式(3),可求出反应在任一温度下的标准吉布斯自由能变化,进而可以算出化学反应在相应温度下的标准平衡常数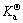 。
。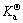 只是温度的函数,它与化学反应的标准吉布斯自由能之间的关系为
只是温度的函数,它与化学反应的标准吉布斯自由能之间的关系为
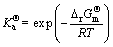 (6)
(6)
利用式(2),根据过程发生的条件可以确定化学反应能否自发进行。
在冶金和化工等行业中,所用到的金属氧化物、硫化物、碳酸盐及金属硫酸盐等化合物,都是能发生加热分解产生气体的反应,也都属于凝聚相为纯物质的多相反应。当某种物质的分解反应达到平衡时,所产生的某气体物质的平衡分压或气体物质的平衡总压称为该种物质的分解压。物质的分解压与温度有关,分解压的大小可以反映物质的稳定程度,分解压越大,物质越易分解。
要确定某种金属硫酸盐在高温下的分解反应能否进行,使用物质分解压的概念和数据更为方便。各种物质的分解压数据可以通过化学反应标准平衡常数的热力学计算方法求得。
2 硫酸铅等硫酸盐分解压的热力学计算
利用文献[20]中所提供的各反应物和生成物的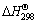 、
、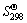 和cp数据,根据式(2) ~ (6)进行硫酸盐分解过程的热力学计算[21]。
和cp数据,根据式(2) ~ (6)进行硫酸盐分解过程的热力学计算[21]。
硫酸铅的分解反应有两种表示法,即
PbSO4(s)=PbO(s or l)+SO3(g) (7)
PbSO4(s)=PbO(s or l)+SO2(g)+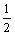 O2(g) (8)
O2(g) (8)
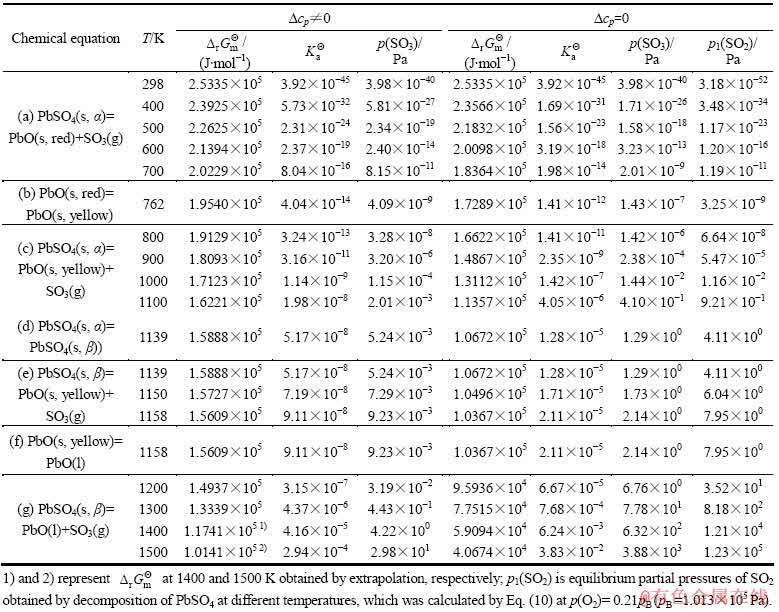
通常依据前一种表示法来定义和求算硫酸铅的分解压。在这种形式下硫酸铅的分解压实际上就是分解反应的标准平衡常数,即硫酸铅的分解压(p(SO3))等于式(7)的标准平衡常数。根据文献[20]数据计算得出不同温度下PbSO4分解的热力学数据,如表1所列。
由表1可知,根据前述热力学计算公式及文献数据,如果取 进行精确计算可以得到相应的热力学数据,或取
进行精确计算可以得到相应的热力学数据,或取 的情况下进行近似计算也可以得到相应的热力学数据。将二者进行比较可知,反应的吉布斯自由能变化量随温度的变化趋势和变化率很相近,且两种情况下得到的SO3平衡分压在相同温度下有很好的对应性,因此,在
的情况下进行近似计算也可以得到相应的热力学数据。将二者进行比较可知,反应的吉布斯自由能变化量随温度的变化趋势和变化率很相近,且两种情况下得到的SO3平衡分压在相同温度下有很好的对应性,因此,在 的条件下来计算硫酸盐的分解压是可行的。
的条件下来计算硫酸盐的分解压是可行的。
表1 不同温度下PbSO4分解的热力学数据
Table 1 Thermodynamic data of decomposition of PbSO4 at different temperatures
在氧势较高的升温氧化段冶炼炉气中,通常以SO2的形态计量表征元素硫的气态化合物,而不是以SO3的形态表征。在熔池熔炼高温炉气中,可将硫酸铅的分解压转换为一定氧分压条件下的二氧化硫平衡分压。SO2和SO3之间存在如下反应及热力学计算关系,即
SO2+1/2O2=SO3 (9)
 (10)
(10)
式中: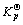 为反应的标准平衡常数。取氧分压为p(O2)=0.21pB(pB=1.013×105 Pa),将p(O2)及不同温度下硫酸铅分解得到的p(SO3)代入式(10)中,可计算出不同温度下PbSO4分解气氛中SO2平衡分压的数据,如表1所列。
为反应的标准平衡常数。取氧分压为p(O2)=0.21pB(pB=1.013×105 Pa),将p(O2)及不同温度下硫酸铅分解得到的p(SO3)代入式(10)中,可计算出不同温度下PbSO4分解气氛中SO2平衡分压的数据,如表1所列。
由表1可知,当温度为298~1100 K时,SO2平衡分压很低,仅为3.18×10-52~9.21×10-1 Pa,PbSO4不发生分解反应。当温度为1139 K(表1中的(d)段)时,SO2平衡分压为4.11×100 Pa,PbSO4发生相变且开始分解;当温度为1158 K(表1中的(f)段)时,SO2平衡分压为7.95×100 Pa,PbSO4分解且产物PbO发生相变;当温度为1300 K时,SO2平衡分压为8.18×102 Pa,PbSO4发生了分解反应;当温度为1500 K时,SO2平衡分压为1.23×105 Pa(超过了标准大气压的值),PbSO4分解反应剧烈。
除PbSO4外,浸锌渣和沉铁渣中还含有Fe、In、Zn、Cu、Al和Mg等硫酸盐,在此对FeSO4、In2(SO4)3、Fe2(SO4)3和ZnSO4的分解反应在氧分压p(O2)=0.21pB下进行相似的热力学计算。
ZnSO4分解反应的热力学计算结果如表2所列。由表2可知,当温度为298~900 K时,SO2平衡分压为1.54×10-39~3.08×100 Pa,ZnSO4几乎不分解。当温度为1000 K时,SO2平衡分压为2.59×102 Pa,ZnSO4发生了分解反应;当温度为1027 K(表2中的(b)段)时,SO2平衡分压为7.39×102 Pa,ZnSO4分解且产物ZnO发生了相变;当温度为1200 K时,SO2平衡分压为1.40×105 Pa(超过了标准大气压值),ZnSO4的分解反应剧烈。
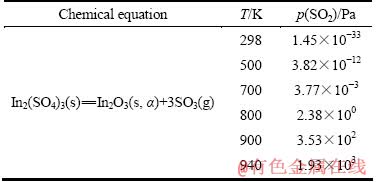
In2(SO4)3分解反应的热力学计算结果如表3所列。由表3可知,当温度为900 K时,SO2平衡分压为3.53×102 Pa,In2(SO4)3发生了分解反应;当温度为940 K时,SO2平衡分压为1.93×103 Pa,In2(SO4)3分解反应明显。
表2 不同温度下ZnSO4分解气氛中SO2平衡分压
Table 2 Equilibrium partial pressures of SO2 in decomposition atmosphere of ZnSO4 at different temperatures
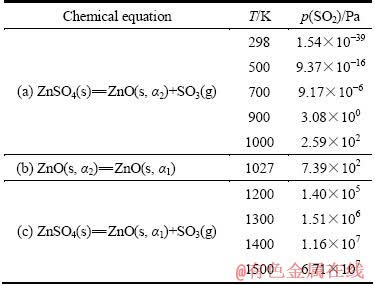
表3 不同温度下In2(SO4)3分解气氛中SO2平衡分压
Table 3 Equilibrium partial pressures of SO2 in decomposition atmosphere of In2(SO4)3 at different temperatures
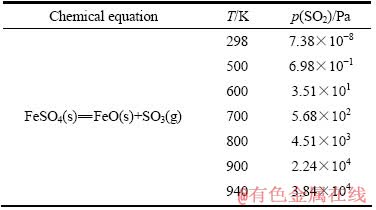
FeSO4分解反应的热力学计算结果如表4所列。由表4可知,当温度为600 K时,SO2平衡分压为3.51×101 Pa,FeSO4分解不明显;当温度为700 K时,SO2平衡分压为5.68×102 Pa,FeSO4发生了分解反应;温度为800 K时,SO2平衡分压为4.51×103 Pa,FeSO4分解反应明显。
表4 不同温度下FeSO4分解气氛中SO2平衡分压
Table 4 Equilibrium partial pressures of SO2 in decomposition atmosphere of FeSO4 at different temperatures
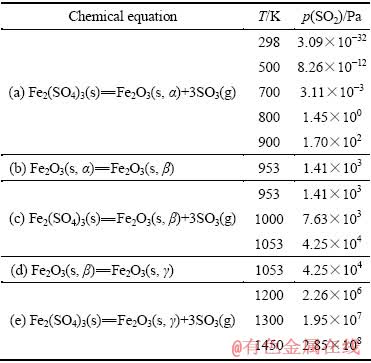
Fe2(SO4)3分解反应的热力学计算结果如表5所列。由表5可知,当温度为900 K时,SO2平衡分压为1.70×102 Pa,Fe2(SO4)3发生了分解反应;当温度为953 K(表5中的(b)段)时,SO2平衡分压为1.41×103 Pa,Fe2(SO4)3分解且产物Fe2O3发生了相变;当温度为1053 K(表5中的(d)段)时,SO2平衡分压为4.25×104 Pa,Fe2(SO4)3分解且产物Fe2O3发生了相变;当温度为1200 K时,SO2平衡分压为2.26×106 Pa(超过了标准大气压的值),Fe2(SO4)3分解剧烈。
表5 不同温度下Fe2(SO4)3分解气氛中SO2的平衡分压
Table 5 Equilibrium partial pressures of SO2 in decomposition atmosphere of Fe2(SO4)3 at different temperatures
结合表1~5可知,当温度为900 K时,PbSO4、ZnSO4、In2(SO4)3、FeSO4和Fe2(SO4)3的SO2平衡分压值分别为5.47×10-5、3.08×100、3.53×102、2.24× 104和1.70×102 Pa。因此,这5种金属硫酸盐发生分解反应由易到难的顺序为FeSO4、In2(SO4)3、Fe2(SO4)3、ZnSO4、PbSO4。PbSO4最难分解,但当氧分压为0.21pB且温度为1500 K时,PbSO4分解气氛中的SO2平衡分压值为1.23×105 Pa,超过标准大气压值。
在考虑温度对金属硫酸盐热分解反应的影响时,要保持熔池熔炼炉内的SO2分压不能过高,这不仅有利于热分解反应的进行(即利于浸锌渣与沉铁渣的处理)而且有助于对炉体的保护。
3 5种硫酸盐分解的热分析
采用德国NETZSCH STA 449 C热分析仪对实验样品进行热重-差示扫描量热(TG-DSC)分析。将PbSO4、ZnSO4·7H2O、In2(SO4)3、FeSO4·7H2O和Fe2(SO4)3·xH2O这5种物质在空气气氛下分别进行TG-DSC分析。反应容器为Al2O3坩埚;载气为空气,流量为20 mL/min;升温速率为20 K/min;试样质量为15.0~19.0 mg。
PbSO4的TG-DSC分析曲线如图1所示。由图1可知,热解的TG曲线在1146.5 K之前没有明显质量损失,且DSC曲线没有吸热峰和放热峰,与表1中(a)、(b)和(c)段的SO2平衡分压较低(3.18×10-52~ 9.21×10-1 Pa)相吻合。TG曲线在1146.5 K后有一个明显的质量损失阶段,且DSC曲线在1146.5 K附近有明显的吸热峰,与表1中的(d)段(1139 K)分析结果相吻合;DSC曲线在1235.2 K附近有吸热峰,与表1中的(f)段(1158 K)的分析结果相吻合;DSC曲线在1316.1 K处有明显的吸热峰,与表1中的(g)段(1300 K)的分析结果相吻合。TG-DSC热分析的温度比热力学计算在相应段(表1中的(d),(f)和(g)段)的温度分别高7.5、77.2和16.1 K。TG-DSC热分析结果与KOLTA等[15]的TG、DTA分析结果一致,出现质量损失阶段及吸热峰主要是由于PbSO4的分解和PbO的升华。
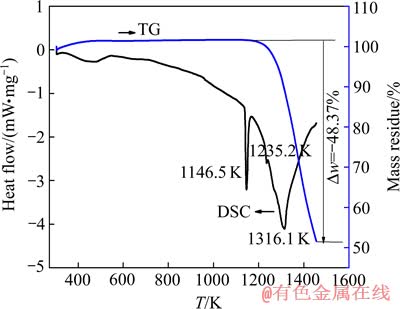
图1 PbSO4分解的TG-DSC曲线
Fig. 1 TG-DSC curves of decomposition of PbSO4
ZnSO4·7H2O的TG-DSC分析曲线如图2所示。由图2可知,TG曲线在362.4~576.5 K内有3个质量损失阶段,且DSC曲线在362.4、453.2、550.3和576.5 K附近分别有一个吸热峰,主要是ZnSO4·7H2O吸热脱水所致。TG曲线在1027.2 K附近质量损失不明显且DSC曲线出现明显吸热峰,与表2中(a)段(1000 K)的分析结果相吻合;TG曲线在1194.2 K处出现明显的质量损失阶段,且DSC曲线出现吸热峰,与表2中的(b)段(1027 K)的分析结果相吻合。热分析的温度比热力学计算在相应段(表2中的(a)和(b)段)的温度分别高出27.2和167.2 K。TG-DSC热分析结果与TAGAWA[13-14]等和KOLTA等[15]的研究结果一致,动力学分析及热力学分析表明ZnSO4的分解、ZnO·ZnSO4中间相的形成以及ZnO的升华都是产生质量损失阶段及吸热峰的原因。
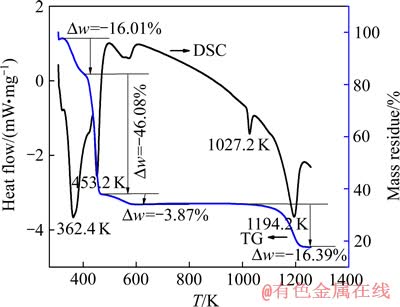
图2 ZnSO4·7H2O分解的TG-DSC曲线
Fig. 2 TG-DSC curves of decomposition of ZnSO4·7H2O
In2(SO4)3的TG-DSC分析曲线如图3所示。由图3可知,TG曲线在423.0 K后有明显质量损失阶段,且DSC曲线在518.8 K附近有明显的吸热峰,这是由于In2(SO4)3是吸潮性粉末,吸热脱水产生质量损失。TG曲线在1003.0 K之后有明显的质量损失阶段,且DSC曲线在1103.7 K附近有明显的吸热峰,与表3中900 K时的分析结果相吻合。因此,热分析的分解温度比热力学计算的温度高203.7 K。
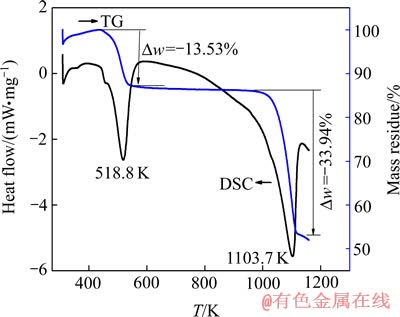
图3 In2(SO4)3分解的TG-DSC曲线
Fig. 3 TG-DSC curves of decomposition of In2(SO4)3
FeSO4·7H2O的TG-DSC分析曲线如图4所示。由图4可知,TG曲线在365.0 K之后存在明显的质量损失阶段,且DSC曲线在425.4 K附近有吸热峰出现,这是由于部分FeSO4分解为铁的氧化物[14]。TG曲线在973.0 K之后存在明显的质量损失阶段,DSC曲线在983.1 K附近有明显的吸热峰,与表4中700 K时的分析结果相吻合,主要是由于FeSO4完全分解成铁的氧化物[16]。热分析的分解温度比热力学计算的温度高283.1 K。
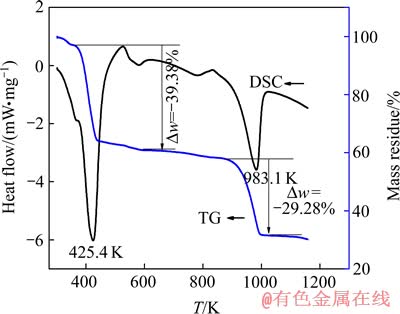
图4 FeSO4·7H2O分解的TG-DSC曲线
Fig. 4 TG-DSC curves of decomposition of FeSO4·7H2O
Fe2(SO4)3·xH2O的TG-DSC分析曲线如图5所示。由图5可知,TG曲线在373.0 K后存在明显的质量损失阶段,且DSC曲线在376.2和467.2 K附近出现吸热峰,主要是吸热脱水产生的质量损失。TG曲线在923.0 K之后有两个质量损失阶段,且DSC曲线在976.7 K附近有明显吸热峰,与表5中的(b)段(953 K)的分析结果相吻合;DSC曲线在1148.2 K附近有吸热峰,与表5中的(d)段(1053 K)的分析结果相吻合。热分析温度比热力学计算温度在相应段(表5中的(b)和(d)段)分别高23.7和95.2 K。SIRIWARDANE等[16]和PERLMUTTER等[17]的研究表明,Fe2(SO4)3·xH2O先脱水,然后分解为Fe2O3,温度高于923.0 K时完全分解为Fe2O3。
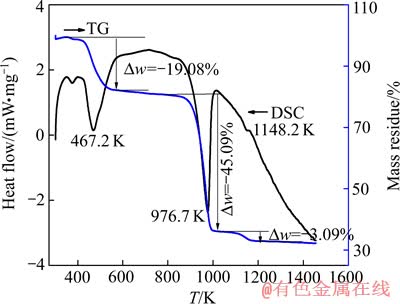
图5 Fe2(SO4)3·xH2O分解的TG-DSC曲线
Fig. 5 TG-DSC curves of decomposition of Fe2(SO4)3·xH2O
结合图1~5可知,PbSO4、ZnSO4、In2(SO4)3、FeSO4和Fe2(SO4)3发生分解反应的温度分别为1316.1、1194.2、1103.7、983.1和1148.2 K。因此,这5种金属硫酸盐发生分解反应由易到难的顺序为FeSO4、In2(SO4)3、Fe2(SO4)3、ZnSO4、PbSO4,这与热力学数据表1~5得出的结果相吻合。TG-DSC结果表明,硫酸盐的分解主要是以氧化物硫酸盐复盐为中间相逐渐分解为相应氧化物的过程。
从图1~5与表1~5的对比分析说明,热力学计算得出的数据与TG曲线质量损失阶段、DSC曲线吸热峰和放热峰位置吻合较好;热分析相变温度、分解温度比热力学计算在相应段的温度高,但两者差距在7.5~283.1 K范围内。由浸锌渣和沉铁渣涉及的主要金属硫酸盐的分解温度可知,熔池熔炼炉内的温度高于1316.1 K可以使冶炼废渣较好地脱硫分解,便于对分解产物(金属氧化物)进行还原处理,有利于废渣冶炼新工艺的连续进行。
TG-DSC热分析实验验证了上述热力学计算结果的准确性,同时证实了热力学计算方法的可靠性。通过化学反应的标准平衡常数计算的分解压确定金属硫酸盐的分解温度,这种方法可以为冶炼工艺提供有效的数据参数,避免由偶然因素造成的实验测定方法的不准确性。
4 结论
1) 从硫酸盐分解过程的热力学计算结果可知:通过化学反应标准平衡常数计算硫酸盐分解反应的吉布斯自由能变化量和分解压时,取△cp=0进行计算是可行的;硫酸盐分解反应的平衡常数随温度的升高而增大,一定氧分压下分解气氛中的SO2平衡分压随温度的升高而增加;5种金属硫酸盐发生分解反应由易到难的顺序为FeSO4、In2(SO4)3、Fe2(SO4)3、ZnSO4、PbSO4。
2) 5种金属硫酸盐的TG-DSC分析结果表明:通过化学反应平衡常数的热力学计算得出的数据与TG曲线质量损失阶段、DSC曲线吸热峰和放热峰位置吻合较好;热力学计算得到的相变温度和分解温度比热分析在相应段的温度低,但两者差距在7.5~283.1 K范围内。
3) 熔池熔炼炉内的温度达到1316.1 K以上可使浸锌渣、沉铁渣中涉及的主要金属硫酸盐较好地分解脱硫,以利于废渣处理新工艺的连续进行。
4) TG-DSC热分析实验证实了热力学计算方法的可靠性。通过化学反应的标准平衡常数计算金属硫酸盐的分解压并确定分解温度,这种方法可以为冶炼工艺提供有效的参考数据。
REFERENCES
[1] 蒋继穆. 我国铅锌冶炼现状与持续发展[J]. 中国有色金属学报, 2004, 14(S1): s52-s62.
JIANG Ji mu. Status and sustainable development of lead and zinc smelting industry in China[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(S1): s52-s62.
[2] 彭容秋. 铅锌冶金学[M]. 北京: 科学出版社, 2003: 19-25.
PENG Rong-qiu. Metallurgy of lead-zinc[M]. Beijing: Science Press, 2003: 19-25.
[3] 郭朝晖, 程 义, 邱冠周, 刘学端, 潘凤开. Pb/Zn冶炼废渣 中有价金属生物浸出条件优化[J]. 中国有色金属学报, 2008, 18(5): 923-928.
GUO Zhao-hui, CHENG Yi, QIU Guan-zhou, LIU Xue-duan, PAN Feng-kai. Optimization on bioleaching of metal values from Pb/Zn smelting slag[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(5): 923-928.
[4] 丁倩倩, 吴克明, 袁芳沁, 王 磊, 柯 兴. 高温挥发法从沉铁渣中回收锌的试验研究[J]. 湿法冶金, 2011, 30(3): 234-236.
DING Qian-qian, WU Ke-ming, YUAN Fang-qin, WANG Lei, KE Xing. Study on recovery of zinc from iron slag by high-temperature volatilization[J]. Hydrometallurgy, 2011, 30(3): 234-236.
[5] 黄柱成, 杨永斌, 蔡江松, 郭宇峰, 李光辉, 姜 涛, 邱冠周. 浸锌渣综合利用新工艺及镓的富集行为[J]. 中南工业大学学报: 自然科学版, 2002, 33(2): 133-136.
HUANG Zhu-cheng, YANG Yong-bin, CAI Jiang-song, GUO Yu-feng, LI Guang-hui, JIANG Tao, QIU Guan-zhou. Comprehensive utilization of Zinc-leaching residue and concentration mechanism of gallium[J]. Journal of Central South University of Technology: Science and Technology, 2002, 33(2): 133-136.
[6] 任鸿九. 有色金属熔池熔炼[M]. 北京: 冶金工业出版社, 2001: 2-11.
REN Hong-jiu. Non-ferrous metal bath smelting[M]. Beijing: Metallurgical Industry Press, 2001: 2-11.
[7] MACKEY P J, CAMPOS R. Modern continuous smelting and concerting by bath smelting technology[J]. Canadian Metallurgical Quarterly, 2001, 40(3): 355-376.
[8] 彭容秋. 铅冶金[M]. 长沙: 中南大学出版社, 2004: 71-86.
PENG Rong-qiu. Metallurgy of lead[M]. Changsha: Central South University Press, 2004: 71-86.
[9] 张乐如. 铅锌冶炼新技术[M]. 长沙: 湖南科学技术出版社, 2006: 49-64.
ZHANG Le-ru. Advanced technology for lead and zinc metallurgy[M]. Changsha: Hunan Science and Technology Press, 2006: 49-64.
[10] STREET S, BROOKS G, REILLY L, WORNER H K. Environment and other bath smelting processes for treating organic and ferrous wastes[J]. JOM, 1998, 50(4): 43-47.
[11] LI Mi, PENG Bing, CHAI Li-yuan, PENG Ning, YAN Huan, HOU Dong-ke. Recovery of iron from zinc leaching residue by selective reduction roasting with carbon[J]. Journal of Hazardous Materials, 2012, 237/238: 323-330.
[12] TOMASZEWICA E, KOTFICA M. Mechanism and kinetics of thermal decomposition of nickel(Ⅱ) sulfate(Ⅵ) hexahydrate[J]. Journal of Thermal Analysis and Calorimetry, 2004, 77(1): 25-31.
[13] TAGAWA H, SAIJO H. Kinetics of the thermal decomposition of some transition metal sulfates[J]. Thermochimica Acta, 1985, 91: 67-77.
[14] TAGAWA H. Thermal decomposition temperatures of metal sulfates[J]. Thermochimica Acta, 1984, 80: 23-33.
[15] KOLTA G A, ASKE M H. Thermal decomposition of some metal sulphates[J]. Thermochimica Acta, 1975, 11(1): 65-72.
[16] SIRIWARDANE R V, POSTON JR J A, FISHER E P, SHEN M S, MILTZ A L. Decomposition of the sulfates of copper, iron(Ⅱ), iron(Ⅲ), nickel, and zinc: XPS, SEM, DRIFTS, XRD, and TGA study[J]. Applied Surface Science, 1999, 152(3): 219-236.
[17] MU J, PERLMUTTER D D. Thermal decomposition of inorganic sulfates and their hydrates[J]. Industrial & Engineering Chemistry Process Design and Development, 1981, 20(4): 640-646.
[18] PETKOVA V, PELOVSKI Y. Comparative DSC study on thermal decomposition of iron sulphates[J]. Journal of Thermal Analysis and Calorimetry, 2008, 93(3): 847-852.
[19] 姚腾猛, 谭 军, 刘常青, 李 洁, 陈启元, 王 辉, 廖 舟, 张平民. 基于拓扑规则Pb-S-O体系优势区图的绘制与应用[J]. 中国有色金属学报, 2013, 23(3): 835-842.
YAO Teng-meng, TAN Jun, LIU Chang-qing, LI Jie, CHEN Qi-yuan, WANG Hui, LIAO Zhou, ZHANG Ping-min. Plotting and application of predominance area diagram of Pb-S-O system based on topological rules[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(3): 835-842.
[20] 叶大伦, 胡建华. 实用无机物热力学数据手册[M]. 北京: 冶金工业出版社, 2002: 366-1169.
YE Da-lun, HU Jian-hua. Practical inorganic thermodynamic datamanual[M]. Beijing: Metallurgical Industry Press, 2002: 366-1169.
[21] 陈定盛, 石 林. 钾长石-硫酸钙-碳酸钙热分解体系产物形成过程的热力学分析[J]. 非金属矿, 2006, 29(4): 5-8.
CHEN Ding-sheng, SHI Lin. Thermodynamic study on forming process of calcined products in orthoclase-CaSO4-CaCO3 system[J]. Non-Metallic Mines, 2006, 29(4): 5-8.
(编辑 陈卫萍)
基金项目:国家高技术研究发展计划资助项目(2011AA061003)
收稿日期:2013-05-08;修订日期:2013-09-13
通信作者:谭 军,副教授,博士;电话:0731-88877364;E-mail: yytanjun@163.com