DOI: 10.11817/j.ysxb.1004.0609.2021-40060
独居石电子结构和辛基羟肟酸在其(100)面的吸附机理
史新章1,王介良1,曹 钊1, 2, 3, 4
(1. 内蒙古科技大学 矿业与煤炭学院,包头 014010;
2. 矿物加工科学与技术国家重点实验室,北京 100160;
3. 内蒙古自治区矿业工程重点实验室,包头 014010;
4. 中南大学 资源与生物工程学院,长沙 410083)
摘 要:通过密度泛函理论(DFT)从原子层面计算研究了独居石的表面性质、电子结构性质和辛基羟肟酸(OHA)在其(100)面的吸附机理。结果表明:独居石的(100)面在破碎和研磨中易于解理并稳定存在,其禁带宽度为3.87 eV,属非导电性矿物;Ce原子为电子供体,O原子为电子受体,P—O键的共价性较强,Ce—O键的离子性较强;OHA可取代水分子在独居石(100)面上以单核双配位构型形成五元环的稳定吸附;吸附后O与Ce原子间生成了化学键,此化学键的生成主要源于O原子的2p轨道电子以及Ce原子的6s、5d轨道电子的贡献。
关键词:独居石;电子结构;辛基羟肟酸;吸附;密度泛函理论
文章编号:1004-0609(2021)-08-2238-09 中图分类号:TD955 文献标志码:A
引文格式:史新章, 王介良, 曹 钊. 独居石电子结构和辛基羟肟酸在其(100)面的吸附机理[J]. 中国有色金属学报, 2021, 31(8): 2238-2246. DOI: 10.11817/j.ysxb.1004.0609.2021-40060
SHI Xin-zhang, WANG Jie-liang, CAO Zhao. Electronic structure of monazite and adsorption mechanism of octyl hydroxamic acid on its (100) surface[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(8): 2238-2246. DOI: 10.11817/j.ysxb.1004.0609.2021-40060
独居石属于磷酸盐稀土矿物,是稀土元素铈和镧的主要来源矿物之一。独居石的化学通式为(Ce,La,Nd,Th)PO4,由稀土元素含量不同可划分为铈独居石(独居石)、镧独居石、钕独居石和富钍独居石[1-2]。独居石的共伴生矿物丰富,其中以磷钇矿、氟碳铈矿、萤石、磷灰石、白云石和磁铁矿等矿物为主。开采出的独居石矿稀土氧化物(REO)含量较低,需通过选矿工艺将其与脉石矿物分离,从而提高REO含量以满足清洁冶炼的需求[3]。独居石与脉石矿物联系紧密,常充填于其颗粒间或孔洞中,复杂的嵌布关系使得浮选分离变得十分困难,选择普通的脂肪酸类捕收剂难以奏效。当下独居石的浮选通常采用羟肟酸类捕收剂,其中异羟肟酸同时具有“酰胺”和“肟”的性质,肟基可与金属阳离子发生络合吸附作用形成络合物[4-7]。ZHANG等[8-9]研究以异羟肟酸为独居石捕收剂,EDTA和硅酸钠为方解石抑制剂,可实现独居石和方解石浮选分离。ZHANG等[10]结合吸附动力学、微量热测定和红外光谱研究了辛基羟肟酸在独居石表面的吸附机理,结果表明低捕收剂浓度下羟肟酸主要在独居石表面活性位点形成化学吸附,在pH为9时饱和吸附量最高;高捕收剂浓度下羟肟酸则在独居石表面形成稀土羟肟酸盐沉淀。SARVARAMINI等[11]采用密度泛函理论模拟研究了羟肟酸捕收剂与溶剂化铈羟基络合物的作用机理,结果表明羟肟酸极性基团中的两个O原子与Ce发生共价键,生成羟基羟肟酸铈配合物。ABAKA-WOOD等[12]采用羟肟酸捕收剂浮选分离独居石、赤铁矿和石英,结果表明羟肟酸可有效捕收独居石和赤铁矿,对石英的捕收能力较弱。羟肟酸对独居石等稀土矿物具有较好的捕收能力和选择性,在实际稀土矿浮选中也获得较为广泛的应用,目前已通过纯矿物浮选和表面测试手段对羟肟酸在独居石表面的作用机理进行了较为深入的研究[13-14]。也有学者通过密度泛函理论计算了羟肟酸和油酸与Ce3+的相互作用,结果表明OHA阴离子有较强的反应活性,与Ce3+结合能最大[15]。然而在实际浮选中,游离的稀土离子和表面稀土离子有着很大的区别[16],羟肟酸在独居石表面吸附作用的量子化学计算研究鲜有报道。
采用分子模拟技术可以计算分析捕收剂与矿物表面相互作用时的能量、电荷变化情况以及捕收剂在矿物表面的吸附行为[17-19]。本文以独居石为研究对象,通过密度泛函理论计算研究了独居石晶体的电子结构性质以及OHA在独居石(100)面不同的吸附形态,最终确定了OHA在独居石(100)面的吸附构型并分析了成键机理,以期为独居石浮选实践和新型捕收剂设计提供理论依据。
1 实验
1.1 计算模型
根据美国矿物学家晶体结构数据库(AMCSD)中NI等[20]提供的晶胞参数构建了独居石单胞结构模型,如图1所示。
独居石晶体(CePO4)属单斜晶系,空间群为P21/n,晶胞参数为:a=0.679 nm,b=0.702 nm,c= 0.647 nm,α=γ=90°,β=103.38°,Z=4。独居石晶体结构由[PO4]四面体包围Ce构成,Ce的配位数为9,Ce—O键的平均键长为0.256 nm,属离子键;P—O键的平均键长为0.153 nm,属共价键[21]。
1.2 计算参数设置
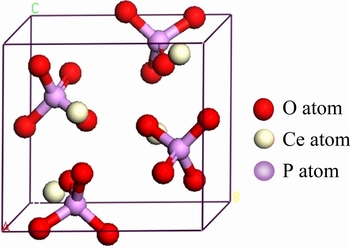
图1 独居石单胞结构模型[20]
Fig. 1 Cell structure model of monazite[20]
本文所有模拟工作均通过Material Studio 2017软件的CASTEP模块进行。交换关联函数、平面波截断能和布里渊区K点取样等参数对独居石体相结构优化影响较大[22]。本文对上述参数进行了收敛性测试,以优化计算后的总能量为主要参考依据,总能量越小独居石晶体结构越稳定,计算的精度越高得到的独居石结构越接近真实情况,但相应的计算时间也会增加。经过收敛性测试后的参数设置如下:交换关联函数选择GGA-PW91,截断能设置为450 eV,K点取样设置为3×3×3;原子最大位移误差不超过1.0×10-4 nm;原子间作用力误差不超过3.0×10-3 eV/nm;原子间内应力误差不超过5.0×10-2 GPa;体系总能量变化误差不超过1.0×10-6 eV/atom;SCF自洽场收敛误差不超过1.0×10-6 eV/atom,所有计算均在倒易空间中进行。
1.3 独居石吸附晶面的选择
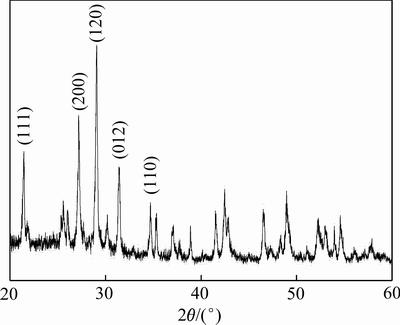
图2 独居石单矿物XRD谱
Fig. 2 XRD pattern of single monazite sample
独居石单矿物的X射线衍射分析结果如图2所示。由图2可知,衍射峰对应的(111)、(200)、(120)、 (012)和(110)面更容易通过机械粉碎和研磨获得,其中(200)面与(100)面为平行表面。
采用MS中的Build Surface模块分别构建(111)、(100)、(120)、(012)和(110)面并进行优化,同时对原子层数和真空层厚度进行了收敛性测试以确定最稳定的表面模型[23-24],随后采用式(1)和式(2)计算了独居石不同晶面的断裂键密度和表面能[25]:
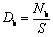 (1)
(1)
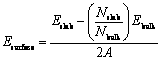 (2)
(2)
式中:Db和Nb分别为晶面断裂键密度和单位晶面断裂键个数;S为该晶面的面积;Eslab和Ebulk分别为表面结构和体相单胞的总能量;Nslab和Nbulk分别为表面结构和单胞总原子数;A是表面结构沿Z轴方向的面积;2表示表面结构沿Z轴方向有上下2个表面。独居石不同晶面的断裂键密度和表面能如表1和表2所示。
通过比较独居石不同晶面的断裂键密度和表面能可知,断裂键密度与表面能呈线性关系,与高志勇教授提出的断裂键理论相吻合[26]。值得注意的是,(100)面的表面能最小,但是(120)面的断裂键密度最低,这是由于P—O键参与了(120)面的解理,该键共价性较大,键能较高。由此可知,(100)面在破碎和研磨中很容易解理并稳定存在。因此将(100)面作为辛基羟肟酸的吸附面来阐释吸附机理。(100)面结构中的原子层厚度为0.697 nm,真空层厚度为2.00 nm。K点取样设置为Gamma点,其余参数与独居石体相优化参数保持一致,优化后的独居石(100)面如图3所示。
2 结果与讨论
2.1 独居石能带结构和态密度分析
独居石体相的能带结构如图4所示,其中能量为0处称为费米能级(EF)。计算给出的独居石禁带宽度为3.87 eV,由此可知独居石晶体为非导电性矿物。
独居石体相的总态密度图和局域分波态密度图如图5所示。由图5可知,独居石的态密度峰主要分布在-40~20eV之间,独居石的导带能级分布在5~20 eV范围内,价带能级分布在-40~0 eV范围内。最高占据的价带主要是由O 2p轨道贡献,费米能级之上的窄空带主要由Ce 4f轨道贡献。由固体能带理论可知,当物体的费米能级与一个或多个能带相交且交错的能带较宽时,其才能成为优良导体[27]。结合独居石体相的能带结构图与态密度图分析可知,独居石的导电性较弱。
同时,在费米能级附近观察到O 2p轨道的态密度峰最强,Ce 4f和Ce 5d轨道次之。研究表明费米能级附近的原子电子性质活泼,易与其他原子发生化学反应[28],因此可得知独居石中O原子的活性较强,其次是Ce原子。
2.2 独居石Mulliken电荷和键布居分析
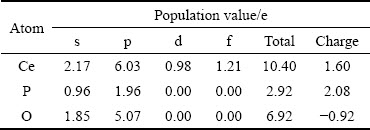
独居石优化前各原子的价电子构型为Ce 4f15s25p65d16s2、P 3s23p3、O 2s22p4,价电子数分别为12 e、5 e、6 e。独居石优化后Ce原子、P原子和O原子的Mulliken电荷布居值如表3所示。
表1 独居石不同晶面的断裂键密度
Table 1 Broken bond density of monazite crystal with different surfaces
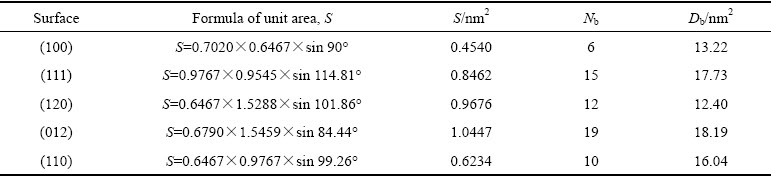
表2 独居石不同晶面的表面能
Table 2 Surface energy of monazite crystal with different surfaces
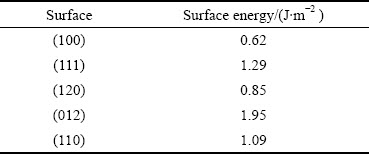
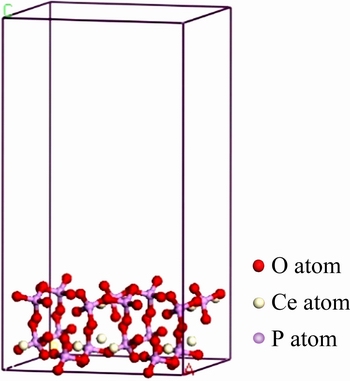
图3 独居石(100)面结构模型
Fig. 3 Structure model of monazite (100) surface
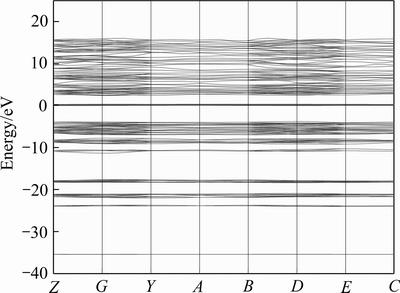
图4 独居石的能带结构
Fig.4 Energy band structure of monazite
由表3可知,独居石优化后各原子的价电子构型分别为Ce 4f1.215s2.175p6.035d0.98、P 3s0.963p1.96、O 2s1.852p5.07,价电子数分别为10.40 e、2.92 e、6.92 e。其中Ce原子的6s、5d轨道共失去1.60 e,P原子的3s、3p轨道共失去2.08 e,为电子供体;O原子的2p轨道得到0.92 e,为电子受体。
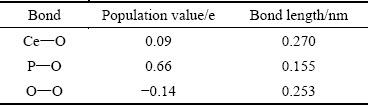
原子间成键的离子性与共价性强弱可通过Mulliken键布居值来体现[29]。布居值大于0,则原子间成键,布居值越大键的共价性越强;布居值小于0,则原子间为反键,布居值越小键的离子性越强;布居值接近0,则原子间为非键形态[30]。独居石的Mulliken键布居值如表4所示。
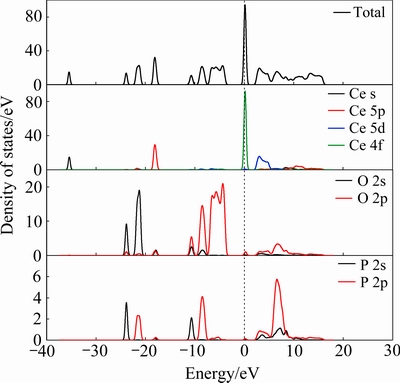
图5 独居石的态密度图
Fig. 5 State density of monazite
表3 独居石中不同原子的Mulliken电荷布居分析
Table 3 Mulliken charge population analysis of different atoms in monazite
由表4可知,独居石中P—O键的布居值最大,为0.66,具有较强的共价性。O—O的布居值为-0.14,呈现出较弱的反键作用。Ce—O键的布居值最小,为0.09,具有较强的离子性。
表4 独居石中不同化学键的Mulliken键布居分析
Table 4 Mulliken bond population analysis of different chemical bonds in monazite
2.3 辛基羟肟酸在独居石(100)面的吸附机理
2.3.1 辛基羟肟酸在独居石(100)面的吸附形态分析
为研究辛基羟肟酸(OHA)在独居石(100)面的吸附机理,将OHA以不同吸附形态摆放在独居石(100)面进行优化计算,OHA在独居石(100)面吸附前后的吸附构型如图6所示。
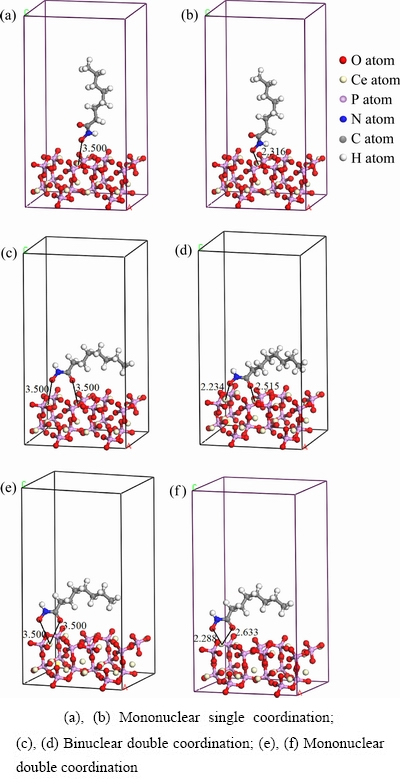
图6 OHA在独居石(100)面的不同吸附形态
Fig.6 Different adsorption forms of OHA on monazite (100) surface
不同分子或离子与相同矿物表面作用时的吸附能有差异,由此可凭借吸附能来判断药剂与矿物表面结合作用的强弱。药剂在矿物表面的吸附能计算公式如下:
△E=Ecom-Esurf -Erea
式中:△E为吸附能;Ecom、Esurf和Erea分别为优化后的吸附络合物、表面结构和吸附质的总能量。吸附能为负值时说明吸附可以自发进行,吸附能越小代表着吸附越稳定;吸附能为零或正值时表明吸附不能自发进行[31]。不同吸附形态的OHA以及H2O在独居石(100)面的吸附能计算结果如表5所示。
表5 OHA和H2O在独居石(100)面的吸附能
Table 5 Adsorption energy of OHA and H2O on monazite (100) surface

由表5可知,水分子在(100)面上的吸附能为-76.154 kJ/mol,表明浮选体系中水分子可以自发地吸附在独居石表面使之亲水。图6所示的双核双配位和单核双配位模型的吸附能分别是-431.760 kJ/mol和-466.863 kJ/mol,因此可以推断,OHA在独居石(100)面上最终的稳定吸附形态是形成五元环的单核双配位构型。该计算结果与徐彩丽等[13]对OHA浮选行为的研究结果相吻合。
2.3.2 辛基羟肟酸在独居石(100)面的吸附机理分析
通过分析OHA肟基上的O原子和独居石表面Ce原子反应前后的Mulliken电荷布居和态密度变化,讨论OHA在独居石(100)面上的吸附机理。O1和Ce1原子吸附前后的Mulliken电荷布居值如表6所示。
由表6可知,OHA阴离子在独居石(100)面吸附后,O1原子的2s轨道和Ce1原子的6s、4f和5d轨道均失去电子,而O1原子的2p轨道和Ce1原子的5p轨道均得到电子;呈现出O1原子的电子增多,Ce原子的电子减少,导致O1原子的电荷负值更大,Ce1原子的电荷正值增加。上诉变化的发生可能是由于成键过程中O1原子核靠近Ce原子核,2个原子核间的相互作用导致2个原子的部分内层s轨道和f轨道电子被激发跃迁到p轨道;因为O1原子的电负性更大,所以O1原子和C e1原子间电子云的重叠部分更偏向于O1原子,从而总体上O1原子表现出得到电子且Ce原子失去电子。
表6 吸附前后O和Ce原子的Mulliken电荷布居值
Table 6 Mulliken charge population value of O and Ce atoms before and after adsorption
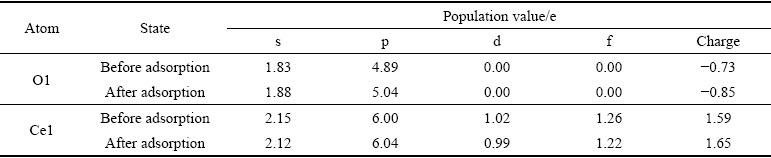
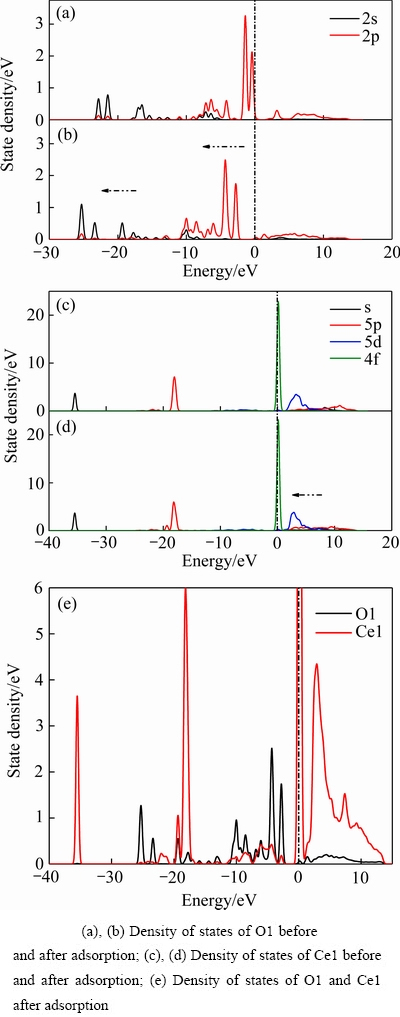
图7 吸附前后O1原子和Ce1原子的态密度变化
Fig.7 State density changes of O1 and Ce1 atoms before and after adsorption:
吸附前后O1原子和Ce1原子的态密度变化如图7所示。由图7可知,吸附前O1原子的2p轨道为费米能级附近的态密度峰提供了主要贡献,而且部分2p轨道的态密度峰越过费米能级进入了导带区,表明吸附前O1原子活性很强,其活性主要由2p轨道提供。吸附前Ce1原子的6s、5d和4f轨道为费米能级附近的态密度峰提供了主要贡献,部分5d和4f轨道越过了费米能级,表明吸附前Ce1原子的反应活性由6s、5d和4f轨道提供。
吸附后形成O1—Ce1键,O1原子的态密度峰整体向能量较低的方向移动,费米能级之上的态密度峰基本消失,并且态密度在-12~0 eV能量范围内的非局域性明显增加。吸附后的Ce1原子在费米能级之上的态密度峰也呈现出向低能量的方向移动的趋势,4f轨道的态密度峰变化不大,可能是由于5s和5p轨道电子层的包裹屏蔽作用导致。O1原子和Ce1原子吸附后的态密度峰在-10~0 eV能量范围内出现了强烈“共振”,表明O1原子和Ce1原子间有化学键生成。
结合以上Mulliken电荷布居和态密度分析可知,吸附后O1和Ce1原子变得更为稳定,二者之间生成O—Ce配位键,该化学键的生成主要由O1原子的2p轨道电子以及Ce1原子的6s,5d轨道电子参与反应。
3 结论
1) 交换关联函数选择GGA-PW91,截断能设置为450 eV,K点取样设置为3×3×3时,对独居石晶胞模型进行优化,其晶胞参数与实验值的误差不超过2%。能带结构和态密度分析表明,独居石晶体最高占据的价带主要是由O 2p轨道贡献,费米能级之上的窄空带主要由Ce 4f轨道贡献,其禁带宽度为3.87 eV,为非导电性矿物。
2) 独居石的Mulliken电荷布居分析表明,Ce原子6s、5d轨道以及P原子3s、3p轨道均失去了一定电子,为电子供体;O原子的2p轨道获得了大量电子,为电子受体。独居石中P—O键的布居值最大,原子间作用力最强,呈现出较强的共价性;Ce—O键的布居值最小,原子间作用力较弱,呈现出较强的离子性。
3) OHA可取代H2O在独居石(100)表面上以单核双配位构型形成五元环的稳定吸附形态,其吸附能最大,为-466.863 kJ/mol。吸附前后的Mulliken电荷布居和态密度分析比较表明,O1和Ce1原子之间产生了化学键,O1原子的2p轨道和Ce1原子的6s、5d轨道杂化对该化学键的生成贡献较大。
REFERENCES
[1] 张福良, 李政林. 我国独居石资源开发利用现状及政策建议[J]. 现代矿业, 2015(11): 1-4.
ZHANG Fu-liang, LI Zheng-lin. Present situation and policy suggestions of development and utilization of monazite resources in China[J]. Modern Mining, 2015(11): 1-4.
[2] 肖 勇, 陈月华. 独居石与独居石渣利用研究进展[J]. 稀土, 2016, 37(4): 129-135.
XIAO Yong, CHEN Yue-hua. Research progress on the utilization of monazite and monazite slag[J]. Chinese Rare Earths, 2016, 37(4): 129-135.
[3] CHELGANI S C, RUDOLPH M, LEISTNER T, et al. A review of rare earth minerals flotation: Monazite and xenotime[J]. International Journal of Mining Science and Technology, 2015, 25(6): 877-883.
[4] 车丽萍, 余永富, 庞金兴, 等. 羟肟酸类捕收剂在稀土矿物浮选中的应用及发展[J]. 稀土, 2004(3): 49-54.
CHE Li-ping, YU Yong-fu, PANG Jin-xing, et al. Application and development of hydroxamic acid collectors in flotation of rare earth minerals[J]. Chinese Rare Earths, 2004(3): 49-54.
[5] 黄林旋, 吴祥林. 异羟肟酸类型捕收剂的研制与浮选稀土矿物试验[J]. 稀土, 1985, 6(1): 1-7.
HUANG Lin-xuan, WU Xiang-lin. Preparation of hydroxamic acid type collector and experiment of flotation of rare earth minerals[J]. Chinese Rare Earths, 1985, 6(1): 1-7.
[6] 刘明宝, 裴 丹, 李 航, 等. 蓖麻油酸钠协同体系中独居石的浮选行为及机制研究[J]. 中国稀土学报, 2020, 38(5): 696-705.
LIU Ming-bao, PEI Dan, LI Hang, et al. Flotation characteristics and mechanisms of monazite in synergistic systems containing sodium ricinoleate[J]. Journal of the Chinese Society of Rare Earths, 2020, 38(5): 696-705.
[7] 刘明宝, 李 亮, 裴 丹, 等. N-亚硝基苯胲铵在独居石表面的吸附特性研究[J]. 稀土, 2019, 40(6): 74-80.
LIU Ming-bao, LI Liang, PEI Dan, et al. Adsorption characteristics of cupferron on monazite[J]. Chinese Rare Earths, 2019, 40(6): 74-80.
[8] ZHANG W, HONAKER R Q, GROPPO J G. Flotation of monazite in the presence of calcite part I: Calcium ion effects on the adsorption of hydroxamic acid[J]. Minerals Engineering, 2017, 100: 40-48.
[9] ZHANG W, HONAKER R Q. Flotation of monazite in the presence of calcite part II: Enhanced separation performance using sodium silicate and EDTA[J]. Minerals Engineering, 2018, 127: 318-328.
[10] ZHANG W, HONAKER R Q. A fundamental study of octanohydroxamic acid adsorption on monazite surfaces[J]. International Journal of Mineral Processing, 2017, 164: 26-36.
[11] SARVARAMINI A, AZIZI D, LARACHI F. Hydroxamic acid interactions with solvated cerium hydroxides in the flotation of monazite and bastnaesite—Experiments and DFT study[J]. Applied Surface Science, 2016, 387: 986-995.
[12] ABAKA-WOOD G B, ADDAI-MENSAH J, SKINNER W. A study of flotation characteristics of monazite, hematite, and quartz using anionic collectors[J]. International Journal of Mineral Processing, 2017, 158: 55-62.
[13] 徐彩丽, 池汝安, 吕仁亮, 等. 辛基羟肟酸浮选行为的研究进展[J]. 武汉工程大学学报,2019,41(6): 566-572.
XU Cai-li, CHI Ru-an, Lü Ren-liang, et al. Research progress in flotation behavior of octylhydroxamic acid[J]. Journal of Wuhan Institute of Technology, 2019, 41(06): 566-572.
[14] 王 振, 丁 威, 肖军辉, 等. 辛基异羟肟酸钠在独居石表面的吸附及浮选机理研究[J]. 矿冶工程, 2019, 39(01): 58-60.
WANG Zhen, DING Wei, XIAO Jun-hui, et al. Study on the adsorption and flotation mechanism of sodium octyl hydroxamate on the surface of monazite[J]. Mining and Metallurgical Engineering, 2019, 39(1): 58-60.
[15] 王 鹏, 曹 钊, 王介良, 等. 辛基羟肟酸与油酸的密度泛函理论计算及其对氟碳铈矿和萤石浮选效果的对比[J]. 中国有色金属学报, 2020, 30(8): 1974-1981.
WANG Peng, CAO Zhao, WANG Jie-liang, et al. DFT caculation of octyl hydroxamic acid and oleic acid and their flotation performance comparison on bastnasite and fluorite[J]. The Chinese Journal of Nonferrous Metals, 2020, 30(8): 1974-1981.
[16] GENEYTON A, FILIPPOV L O, RENARD A, et al. Advances in carboxylate collectors adsorption on monazite surface: Part 1—Assessment of the hydroxylation and carbonation of surface lanthanide ions[J]. Applied Surface Science, 2019, 485: 283-292.
[17] ZHU Y M, LUO B B, SUN C Y, et al. Density functional theory study of α-Bromolauric acid adsorption on the α-quartz(101) surface[J]. Minerals Engineering, 2016, 92: 72-77.
[18] ESPIRITU, EILEEN R L, SILVA G R, et al. The effect of dissolved mineral species on bastnasite, monazite and dolomite flotation using benzohydroxamate collector[J]. Colloids and Surfaces A, 2018, 539: 319-334.
[19] LIU W G, LIU W B, WANG X Y, et al. Effect of butanol on flotation separation of quartz from hematite with N-dodecyl ethylenediamine[J]. International Journal of Mining Science and Technology, 2016, 26(6): 1059-1063.
[20] NI Y, HUGHES J M, MARIANO A N. Crystal chemistry of the monazite and xenotime structures[J]. American Mineralogist, 1995, 80(1): 21-26.
[21] EILEEN R L, ESPIRITU, SHIVA N, et al. Surface chemistry and flotation behavior of dolomite, monazite and bastnasite in the presence of benzohydroxamate, sodium oleate and phosphoric acid ester collectors[J]. Colloids and Surfaces A, 2018, 546: 254-265.
[22] 宫贵臣, 刘 杰, 韩跃新, 等. 苯乙烯膦酸在锡石(100)表面吸附的密度泛函理论研究[J]. 中南大学学报(自然科学版), 2018, 49(12): 2901-2907.
GONG Gui-chen, LIU Jie, HAN Yue-xin, et al. Density functional theory calculations of adsorption of SPA on cassiterite (100) surface[J]. Journal of Central South University (Science and Technology), 2018, 49(12): 2901-2907.
[23] 史新章, 曹 钊, 张金山, 等. 萤石的晶体化学基因特性量化计算与分子动力学模拟[J]. 金属矿山, 2020(6): 48-55.
SHI Xin-zhang, CAO Zhao, ZHANG Jin-shan, et al. Quantitative calculation and molecular dynamics simulation of crystal chemistry gene characteristics of fluorite[J]. Metal Mine, 2020(6): 48-55.
[24] 宫贵臣, 刘 杰, 韩跃新, 等. 油酸在锡石(100)表面吸附机理的密度泛函理论研究[J]. 中国矿业大学学报, 2018, 47(3): 639-644.
GONG Gui-chen, LIU Jie, HAN Yue-xin, et al. Density functional theory study on the adsorption mechanism of oleate on cassiterite (100)[J]. Journal of China University of mining and technology, 2018, 47(3): 639-644.
[25] XY L, TIAN J, WU H, et al. New insights into the oleate flotation response of feldspar particles of different sizes: Anisotropic adsorption model[J]. Journal of colloid and interface science, 2017, 505: 500-508.
[26] 高跃升, 高志勇, 孙 伟. 萤石表面性质各向异性研究及进展[J]. 中国有色金属学报, 2016, 26(2): 415-422.
GAO Yue-sheng,GAO Zhi-yong,SUN Wei. Research and development of anisotropy of fluorite surface properties[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(2): 415-422.
[27] MONKHORST H J, PACK J D. Special point for brillouin zone integrations[J]. Physical Review B, 1976, 13: 5188-5196.
[28] 任尚元. 有限晶体中的电子态[J]. 物理, 2003, 32(10): 682-686.
REN Shang-yuan. Electronic states in finite crystals[J]. Physics, 2003, 32(10): 682-686.
[29] SEGALL M D, SHAH R, PICKARD C J, et al. Population analysis of plane wave electronic structure calculations of bulk materials[J]. Physical Review B, 1996, 54(23): 16317-16320.
[30] 陈建华, 钟建莲, 李玉琼, 等. 黄铁矿、白铁矿和磁黄铁矿的电子结构及可浮性[J]. 中国有色金属学报, 2011, 21(7): 1719-1727.
CHEN Jian-hua, ZHONG Jian-lian, LI Yu-qiong, et al. Electronic structure and floatability of pyrite, white iron and pyrrhotite[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(7): 1719-1727.
[31] 孙 伟, 柯丽芳, 孙 磊. 苯甲羟肟酸在锡石浮选中的应用及作用机理研究[J]. 中国矿业大学学报, 2013, 42(1): 62-68.
SUN Wei, KE Li-fang, SUN Lei. Study of the application and mechanism of benzohydroxamic acid in the floatation of cassiterite[J]. Journal of China University of Mining & Technology, 2013, 42(1): 62-68.
Electronic structure of monazite and adsorption mechanism of octyl hydroxamic acid on its (100) plane
SHI Xin-zhang1, WANG Jie-liang1, CAO Zhao1, 2, 3, 4
(1. School of Mining and Coal Engineering, Inner Mongolia University of Science and Technology, Baotou 014010, China;
2. State Key Laboratory of Mineral Processing, Beijing 100160, China;
3. Inner Mongolia Key Laboratory of Mining Engineering, Baotou 014010, China;
4. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: The surface and electronic structure properties of monazite and the adsorption mechanism of octyl hydroxamic acid (OHA) on the (100) plane of monazite were studied by density functional theory (DFT) calculations from the atomic level. The results show that the (100) plane of monazite is easy to cleave and exist stably during crushing and grinding. The band gap of monazite is 3.87 eV, which is a non-conductive mineral. Ce atom is an electron donor, O atom is an electron acceptor, P—O bond exhibits strong covalent properties, and Ce—O bond exhibits strong ionic properties. OHA can replace water molecules to form a stable five-membered ring adsorption on the (100) plane of monazite via bidentate configuration. A chemical bond is formed between O and Ce atoms after adsorption. Its formation is mainly due to the contribution of 2p orbital electrons of O atom and 6s, 5d orbital electrons of Ce atom.
Key words: monazite; electronic structure; OHA; adsorption; DFT
Foundation item: Project(51764045) supported by the National Natural Science Foundation of China; Project (BGRIMM-KJSKL-2020-23) supported by the Open Foundation of State Key Laboratory of Mineral Processing, China; Project(NJYT-18-B08) supported by the Inner Mongolia Young Science & Technology Talent Support Plan, China; Project(2017YQL05) supported by the Outstanding Youth Science Foundation of Inner Mongolia University of Science and Technology, China
Received date: 2020-12-07; Accepted date: 2021-04-25
Corresponding author: CAO Zhao; Tel: +86-18747235897; E-mail: caozhao1217@163.com
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51764045);矿物加工科学与技术国家重点实验室开放基金资助项目(BGRIMM-KJSKL- 2020-23);内蒙古高等学校“青年科技英才支持计划”资助项目(NJYT-18-B08);内蒙古科技大学优秀青年基金资助项目(2017YQL05)
收稿日期:2020-12-07;修订日期:2021-04-25
通信作者:曹 钊,教授,博士;电话:18747235897;E-mail:caozhao1217@163.com