DOI: 10.11817/j.issn.1672-7207.2019.04.002
辛基羟肟酸在氟碳铈矿表面的吸附机理
王介良1,2,3,4,曹钊1,2,3,王建英4,张雪峰4,雷霄4,冯宏杰1
(1. 内蒙古科技大学 矿业研究院,内蒙古 包头,014010;
2. 广东省资源综合利用研究所,广东 广州,510650;
3. 稀有金属分离与综合利用国家重点实验室,广东 广州,510650;
4. 内蒙古科技大学 内蒙古自治区白云鄂博矿多金属资源综合利用重点实验室,内蒙古 包头,014010)
摘要:通过吸附量测试、纯矿物浮选、Zeta 电位和XPS测试,研究辛基羟肟酸在氟碳铈矿表面的吸附行为和吸附机理。研究结果表明:辛基羟肟酸在氟碳铈矿表面吸附符合二级动力学模型,等温吸附过程符合Freundlich吸附模型,pH=9.5时辛基羟肟酸在氟碳铈矿表面的吸附速度和吸附量比pH=6.5时的大;辛基羟肟酸在氟碳铈矿表面的吸附为多层、不均匀吸附,是物理吸附和化学吸附共同作用的结果,但以化学吸附为主。吸附机理为:羟肟酸根阴离子OHA-与氟碳铈矿表面暴露的Ce3+发生螯合反应生成OHA-Ce沉淀,形成化学吸附;羟肟酸分子OHA与氟碳铈矿表面的氧以氢键形式形成物理吸附,同时,溶液中游离的羟肟酸分子OHA与氟碳铈矿表面已吸附上的OHA分子形成氢键和烃链间疏水缔合作用,产生不均匀的物理吸附。
关键词:氟碳铈矿;辛基羟肟酸;吸附;热力学;吸附机理
中图分类号:TD923 文献标志码:A 文章编号:1672-7207(2019)04-0762-09
Adsorption mechanism of octyl hydroxamic acid on bastnaesite surface
WANG Jieliang1,2,3,4, CAO Zhao1,2,3, WANG Jianying4, ZHANG Xuefeng4, LEI Xiao4, FENG Hongjie1
(1. Institute of Mining Engineering, Inner Mongolia University of Science and Technology, Baotou 014010, China;
2. Guangdong Institute of Resources Comprehensive Utilization, Guangzhou 510650, China;
3. State Key Laboratory of Rare Metals Separation and Comprehensive Utilization, Guangzhou 510650, China;
4. Key Laboratory of Integrated Exploitation of Bayan Obo Multi-Metal Resources,
Inner Mongolia University of Science and Technology, Baotou 014010, China)
Abstract: The adsorption behavior and mechanism of octyl hydroxamic acid (OHA) on bastnaesite surface was investigated through adsorption test, single mineral flotation, Zeta potential and XPS measurements. The results show that the adsorption of OHA on bastnaesite surface agrees with the second order kinetic model, and the isothermal adsorption process correlates well with the Freundlich isotherm model. The adsorption speed and adsorption amount of OHA on bastnaesite surface at pH=9.5 are greater than that at pH=6.5. The adsorption of OHA on bastnaesite surface is multilayered and heterogeneous, which is a combined result of physical and chemical adsorption, and the later is the dominating form. The adsorption mechanism is that hydroxamate anions OHA- can chelate react with the exposed Ce3+ on bastnaesite surface and form the OHA-Ce precipitate, resulting in chemical adsorption. Hydroxamic acid molecule OHA can form hydrogen bond with oxygen atom on bastnaesite surface and form physical adsorption, and meanwhile, the dissociated OHA molecule can form hydrogen bond and hydrophobic association of hydrocarbon chains with the adsorbed OHA on bastnaesite surface, resulting in heterogeneous physical adsorption.
Key words: bastnaesite; octyl hydroxamic acid; adsorption; adsorption thermodynamics; adsorption mechanism
稀土被誉为“工业味精”、新世纪高科技及功能材料的“宝库”,是发展高新技术的战略性元素[1],广泛应用于电子、石油化工、冶金、机械、能源、轻工、环境保护、农业及国防工业等领域[2-3]。氟碳铈矿是轻稀土的主要来源[4],其为半可溶性盐类矿物,主要包括铈和镧等稀土元素,分子式为 RECO3F,稀土氧化物的质量分数约为75%。浮选是氟碳铈矿富集回收的主要方法[5],针对氟碳铈矿捕收剂的研究主要集中在脂肪酸[6]、烷基羟肟酸[2]、萘系羟肟酸[7-9]、磷(膦)酸类[10-11]等。其中,脂肪酸类捕收剂选择性差,浮选温度高,添加大量抑制剂[12],磷(膦)酸盐类捕收剂在酸性条件下才能取得较好的浮选效果[13],均未被广泛应用于氟碳铈矿的工业生产。羟肟酸类捕收剂对稀土浮选的选择性强,在我国包头、冕宁、微山湖稀土矿[14]以及美国Mountain pass[15]、加拿大 Nechalacho[16]、澳大利亚 Mt Weld[17]等稀土矿得到成功应用。捕收剂在氟碳铈矿表面的作用机制已有相关研究。王成行等[18-19]通过结合红外光谱和 XPS 测试研究认为水杨羟肟酸、辛基羟肟酸在氟碳铈矿表面的吸附为化学吸附,生成稳定的—C=O—RE—O—N—五元环螯合物;任俊等[20]研究认为1-羟基-2-萘羟肟酸与氟碳铈矿表面Ce3+生成螯合物的化学吸附的同时,存在不均匀的物理吸附。吸附动力学和热力学计算广泛应用于环境科学技术[21]和选矿技术研究,曹永丹等[22]通过吸附动力学和热动力学计算,表明 Cu2+ 和 Ni2+ 在蛇纹石表面的吸附机理是单层吸附;朱玉霜等[23]在 12~55 ℃的条件下测试辛基羟肟酸在锡石表面的吸附量,并通过计算出的吸附热判断出辛基羟肟酸在锡石表面的吸附过程为化学吸附;徐龙华等[24]通过吸附等温线测量、荧光探针技术以及沉降实验等研究,从微观上描述了季铵盐在高岭石表面的吸附特性和吸附层结构;张国范等[25]通过吸附量测试及溶液化学计算等研究手段发现:当pH 为7左右时,油酸钠在闪锌矿表面主要发生化学吸附,矿物表面成分为(C17H33COO)2Zn,其表面还有可能有物理吸附;当pH为10时,吸附则主要以物理吸附为主;而通过吸附的动力学和热力学行为来分析羟肟酸捕收剂在氟碳铈矿表面的作用机理的研究少有报道。因此,本文作者通过辛基羟肟酸OHA在氟碳铈矿表面的吸附动力学和热力学研究阐明辛基羟肟酸OHA在氟碳铈矿表面的吸附行为,并结合纯矿物浮选实验、Zeta电位测试及XPS分析辛基羟肟酸OHA在矿物表面的吸附机理。
1 实验
1.1 试样制备
氟碳铈矿取自山东微山湖稀土矿,经手选除杂、破碎、磨矿及重选除杂和弱磁选除磁铁矿后,得到38~75 μm粒级的氟碳铈矿单矿物作为单矿物实验用纯矿物,其REO品位为71.21%,按氟碳铈矿理论REO品位74.88%计算,其纯度为95%左右。
1.2 主要试剂
实验所用辛基羟肟酸(OHA)、NaOH和HCl等试剂均为分析纯,实验用水为去离子水。
1.3 实验方法
1.3.1 氟碳铈矿吸附OHA动力学和热力学
1) 吸附量测定。使用UV-L5S紫外可见分光光度计进行吸附量的测定,根据辛基羟肟酸OHA与Fe3+络合显色的原理,在25 mL容量瓶中加入10 mL待测 OHA溶液,再加入5 mL无水乙醇,1 mL质量分数为1%的FeCl3溶液,加水定容,摇匀静置10 min,以空白试剂作参比,在500 nm处测定辛基羟肟酸的吸光度A。配置不同质量浓度OHA溶液,绘制OHA的标准曲线[26]如图1所示。通过标准曲线计算过滤液中的OHA质量浓度,再根据吸附量公式(1)计算OHA在矿物表面药剂的吸附量。
2) 吸附动力学。将0.5 g 氟碳铈矿加入30 mL、159.23 mg/L的 OHA溶液中,调至一定pH,吸附反应温度为25 ℃,在磁力转子搅拌器上搅拌,吸附 t 时刻后过滤,用UV-L5S紫外可见分光光度计测量滤液OHA质量浓度ρt,按照式(1)计算出氟碳铈矿 t 时刻的OHA吸附量qt。将氟碳铈矿对 OHA的吸附过程进行一级和二级动力学拟合,拟合方程分别如式(2)和式(3)所示,动力学拟合参数见表1。
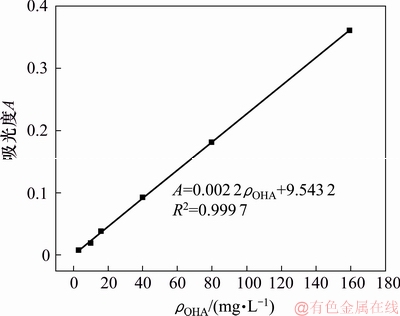
图1 辛基羟肟酸溶液的吸光度标准曲线
Fig. 1 Standard adsorption curve of OHA solution
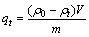 (1)
(1)
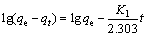 (2)
(2)
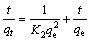 (3)
(3)
式中:ρ0和ρt分别为OHA初始质量浓度和吸附t时刻后过滤液残余质量浓度,mg/L;V为矿浆体积,L;m为氟碳铈矿质量,g;qe和 qt分别为平衡吸附量和 t 时刻吸附量,mg/g;K1为一级吸附速率常数,min-1;K2为二级吸附速率常数,g/(mg·min)。
3) 吸附热力学。配制质量浓度分别为15.923,39.808,79.615,119.423和159.230 mg/L的OHA溶液,每种各取30 mL置于100 mL 烧杯中,加入0.5 g的氟碳铈矿后调整到一定pH,吸附反应温度为25 ℃,达到吸附平衡后采用中速滤纸进行过滤,用UV-L5S紫外可见分光光度计测试滤液中残余OHA的质量浓度,按照式(4)计算出平衡吸附量qe,对氟碳铈矿吸附OHA过程分别进行Langmuir等温吸附方程拟合和Freundlich等温吸附方程拟合,Langmuir 等温吸附方程和 Freundlich 等温吸附方程公式分别如式(5)和式(6)所示。
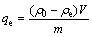 (4)
(4)
 (5)
(5)
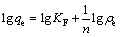 (6)
(6)
式中:ρe为OHA吸平衡后过滤液残余质量浓度,mg/L; qm为饱和吸附量,mg/g;KL为 Langmuir吸附常数,L/mg;KF为 Freundlich 吸附常数;1/n为吸附强度。
1.3.2 纯矿物浮选实验
氟碳铈矿浮选在XFGCII-35型试验室用充气挂槽浮选机中进行,叶轮转速1 992 r/min,浮选温度为室温,每次称取2.00 g实验矿样加入到40 mL浮选槽中,加30 mL去离子水,采用HCl或NaOH调整到指定pH,加入一定质量浓度的OHA,每次加药后调浆2 min,浮选前将pH再调整到指定pH,然后浮选刮泡4 min,对泡沫产品和槽内产品分别过滤、干燥称质量,计算浮选回收率。
1.3.3 Zeta电位测试
采用Brookhaven ZetaPlus Analyzer分析仪测试Zeta电位。将纯矿物用玛瑙研钵研磨至粒径小于5 μm,每次精确称取10 mg置于100 mL 1 mmol/L的KCl溶液中,用HCl或NaOH调节pH,依次加入一定量的EDTA或OHA,每次加药后磁力搅拌5 min,取上述样品加入样品池,在Zetaplus Zeta电位测定仪上测量,每个样品重复测量3次,取平均值作为相应pH条件下的Zeta电位。
1.3.4 XPS测试
采用Thermo Scientific ESCALAB 250Xi 型X线光电子能谱仪进行XPS测试。称取1 g氟碳铈样品置于小烧杯中,用HCl或NaOH调节pH至9.5左右,按照浮选条件加入一定浓度的浮选药剂,每次加药后磁力搅拌5 min,过滤并用相同pH去离子水洗涤矿物2次,将过滤产物50 ℃下真空干燥后进行XPS测试。XPS测试条件为:单色化Al Kα 射线光源,射线能量hν=1 486.6 eV,光斑直径为500 μm,真空度为5×10-8 Pa,C 1s矫正值为284.8 eV;全谱扫描结合能范围为0~1 300 eV,步长为1.0 eV,通过能为100 eV;N 1S高分辨谱通过能为30 eV,步长为0.05 eV。采用CasaXPS进行谱峰分析和分峰拟合。
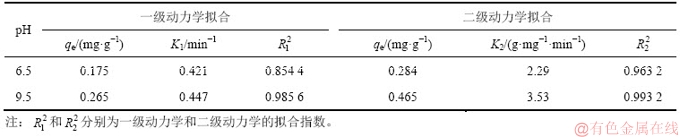
表1 氟碳铈矿吸附辛基羟肟酸的动力学拟合参数
Table 1 Kinetics fitting parameters of OHA adsorption on bastnaesite
2 结果与讨论
2.1 氟碳铈矿吸附OHA动力学和热力学
2.1.1 吸附动力学
图2所示为辛基羟肟酸OHA质量浓度为159.23 mg/L、温度为25 ℃、吸附时间为15 min时,pH对氟碳铈矿吸附辛基羟肟酸OHA的影响。由图2可知:当pH小于9.5时,随pH升高,辛基羟肟酸OHA在氟碳铈矿表面吸附量增大;当pH大于9.5时,随pH升高,辛基羟肟酸OHA在氟碳铈矿表面吸附量减小。当pH=9.5时,辛基羟肟酸在氟碳铈矿表面吸附量最大。分析认为,随pH增大,辛基羟肟酸解离程度大,溶液中辛基羟肟酸根离子含量增加,从而增大其在氟碳铈矿表面的化学吸附;而pH过高(pH>9.5)时,矿物表面电负性增强,由于静电斥力而阻碍辛基羟肟酸阴离子在矿物表面的吸附,导致辛基羟肟酸在氟碳铈矿表面的吸附量减小。
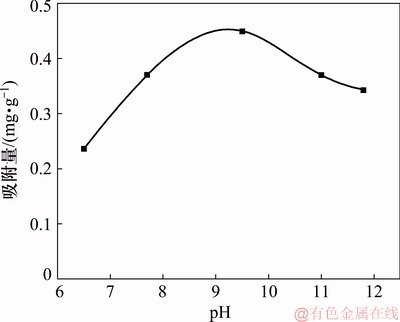
图2 pH对氟碳铈矿吸附辛基羟肟酸的影响
Fig. 2 Effect of pH on adsorption amount of OHA on bastnaesite
图3所示为辛基羟肟酸OHA质量浓度为159.23 mg/L、温度为25 ℃时,在pH为6.5和9.5条件下,氟碳铈矿对辛基羟肟酸OHA吸附量随时间的变化曲线。由图3可知:随吸附时间增加,辛基羟肟酸吸附量逐渐增加,吸附时间为10 min时,氟碳铈矿吸附辛基羟肟酸OHA趋于平衡。
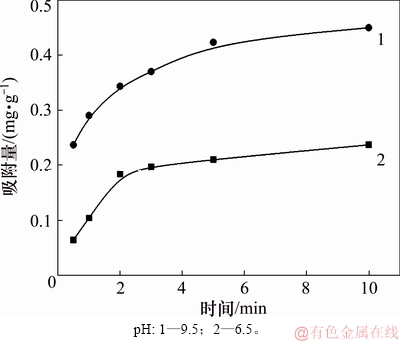
图3 吸附时间对氟碳铈矿吸附辛基羟肟酸的影响
Fig. 3 Effect of time on adsorption amount of OHA on bastnaesite
图4和图5所示分别为辛基羟肟酸OHA质量浓度为159.23 mg/L、温度为25 ℃时,在pH为6.5和9.5条件下,氟碳铈矿吸附辛基羟肟酸的一级动力学拟合和二级动力学拟合结果,拟合参数见表1。由表1可知:拟合指数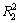 >
>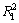 ,说明氟碳铈矿吸附辛基羟肟酸OHA更符合二级动力学模型;pH=9.5时氟碳铈矿吸附辛基羟肟酸的二级动力学拟合吸附速率常数(3.53)大于pH=6.5时吸附速率常数(2.29),说明pH=9.5时,辛基羟肟酸OHA在氟碳铈矿吸附更快。
,说明氟碳铈矿吸附辛基羟肟酸OHA更符合二级动力学模型;pH=9.5时氟碳铈矿吸附辛基羟肟酸的二级动力学拟合吸附速率常数(3.53)大于pH=6.5时吸附速率常数(2.29),说明pH=9.5时,辛基羟肟酸OHA在氟碳铈矿吸附更快。

图4 氟碳铈矿吸附辛基羟肟酸的一级动力学拟合
Fig. 4 First-order kinetics fitting of OHA adsorption on bastnaesite
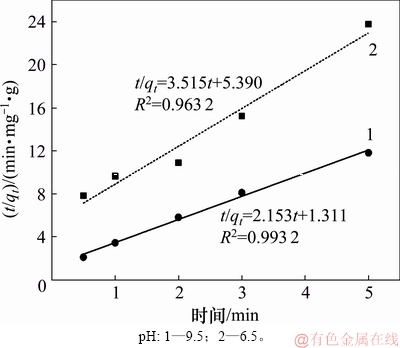
图5 氟碳铈矿吸附辛基羟肟酸的二级动力学拟合
Fig. 5 Second-order kinetics fitting of OHA adsorption on bastnaesite
2.1.2 吸附热力学
图6所示为pH分别为6.5和9.5、温度为25℃时,氟碳铈矿对辛基羟肟酸OHA吸附量随浓度的变化曲线。由图6可知:随辛基羟肟酸OHA质量浓度的增加,氟碳铈矿对辛基羟肟酸OHA吸附量随之增大;pH=9.5时,辛基羟肟酸OHA在氟碳铈矿表面吸附量随辛基羟肟酸OHA质量浓度的增加而增大的趋势更明显,说明溶液pH为9.5时较pH为6.5时更有利于辛基羟肟酸OHA在氟碳铈矿表面的吸附。
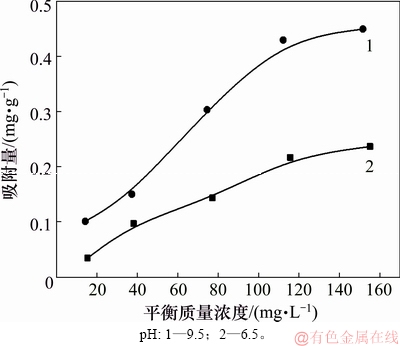
图6 辛基羟肟酸浓度对吸附量的影响
Fig. 6 Effect of OHA mass concentration on adsorption mount of OHA on bastnaesite
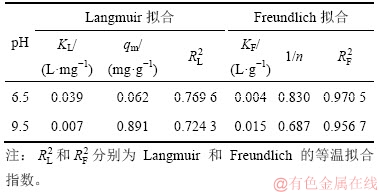
图7和图8所示分别为pH为6.5和9.5、温度为25 ℃时,氟碳铈矿吸附辛基羟肟酸的Langmuir和Freundlich拟合,拟合参数见表2。由表2可知:等温拟合指数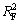 >
>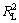 ,说明氟碳铈矿吸附辛基羟肟酸符合Freundlich等温吸附方程,辛基羟肟酸OHA在氟碳铈矿表面的吸附是多层、不均匀吸附,即同时存在物理吸附和化学吸附。Freundlich方程中非线性指数1/n反映吸附质吸附位点能量分布特征,吸附常数KF反映吸附能力的强弱,KF越大,吸附能力越大;1/n越小,吸附强度越大[27]。由表2可知:氟碳铈矿在pH=9.5时吸附辛基羟肟酸OHA能力优于pH=6.5时的吸附能力。
,说明氟碳铈矿吸附辛基羟肟酸符合Freundlich等温吸附方程,辛基羟肟酸OHA在氟碳铈矿表面的吸附是多层、不均匀吸附,即同时存在物理吸附和化学吸附。Freundlich方程中非线性指数1/n反映吸附质吸附位点能量分布特征,吸附常数KF反映吸附能力的强弱,KF越大,吸附能力越大;1/n越小,吸附强度越大[27]。由表2可知:氟碳铈矿在pH=9.5时吸附辛基羟肟酸OHA能力优于pH=6.5时的吸附能力。
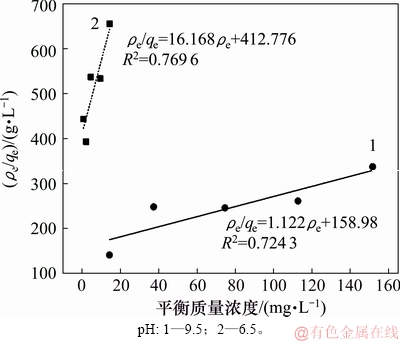
图7 氟碳铈矿吸附辛基羟肟酸的Langmuir拟合
Fig. 7 Langmuir fitting of OHA adsorption on bastnaesite

图8 氟碳铈矿吸附辛基羟肟酸的Freundlich拟合
Fig. 8 Freundlich fitting of OHA adsorption on bastnaesite
表2 氟碳铈矿吸附辛基羟肟酸的等温拟合参数
Table 2 Isothermal adsorption fitting parameters of OHA on bastnaesite
2.2 纯矿物浮选
固定辛基羟肟酸OHA用量为40 mg/L、浮选温度为25 ℃,考察pH对氟碳铈矿可浮性的影响,结果如图9所示。由图9可知:当矿浆pH小于9.5时,随着pH升高,氟碳铈矿的浮选回收率增大,在pH=9.5时,氟碳铈矿达到最大回收率86.73%;当矿浆pH大于9.5时,随着pH升高,氟碳铈矿的浮选回收率减小,氟碳铈矿浮选的最佳pH为9.5,结合pH对氟碳铈矿吸附辛基羟肟酸的影响结果(图2)可知,辛基羟肟酸在氟碳铈矿表面吸附量越大,其浮选回收率越高。
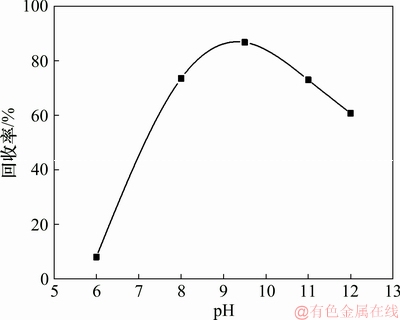
图9 pH对氟碳铈矿可浮性的影响
Fig. 9 Effect of pH on floatability of bastnaesite
固定矿浆pH=9.5、浮选温度为25 ℃,考察辛基羟肟酸OHA用量对氟碳铈矿可浮性的影响,结果如图10所示。由图10可知:随着辛基羟肟酸OHA用量的增加,氟碳铈矿的浮选回收率增大,当辛基羟肟酸OHA用量大于40 mg/L时,继续增加辛基羟肟酸OHA用量,氟碳铈矿回收率基本不再增加,因此,氟碳铈矿浮选的最佳辛基羟肟酸OHA用量为40 mg/L。
2.3 辛基羟肟酸与氟碳铈矿作用的Zeta电位测试
图11所示为辛基羟肟酸OHA质量浓度对氟碳铈矿Zeta电位的影响。由图11可知:无OHA时,氟碳铈矿等电点为 pH=6.8 左右;与OHA作用后的氟碳铈矿Zeta电位负移,主要为带负电的羟肟酸根离子和中性羟肟酸分子在氟碳铈矿表面吸附后,降低或屏蔽了氟碳铈矿表面的正电性所致;且随着辛基羟肟酸OHA质量浓度的大,氟碳铈矿Zeta电位负移程度增大,说明随辛基羟肟酸质量浓度增大,其在氟碳铈矿表面吸附量增加,这与辛基羟肟酸OHA浓度和氟碳铈矿吸附量的关系研究结果一致。
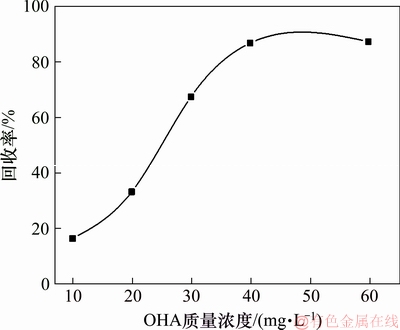
图10 辛基羟肟酸质量浓度对氟碳铈矿可浮性的影响
Fig. 10 Effect of OHA mass concentration on floatability of bastnaesite
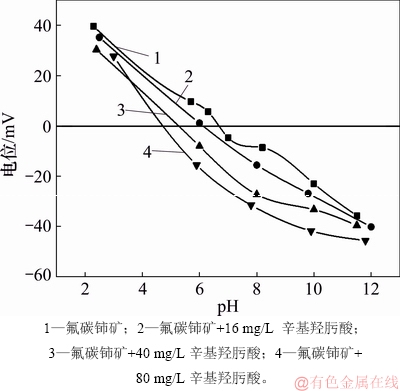
图11 辛基羟肟酸质量浓度对氟碳铈矿Zeta电位的影响
Fig. 11 Zeta potentials of bastnaesite as function of OHA mass concentration
2.4 辛基羟肟酸与氟碳铈矿作用的 XPS分析
图12和图13所示分别为辛基羟肟酸OHA、辛基羟肟酸铈沉淀物及辛基羟肟酸作用前后氟碳铈矿的XPS全谱及N 1s XPS 窄谱。由图12(a)和(b)可知:OHA XPS全谱在400 eV左右处出现N峰,OHA与Ce反应生成络合沉淀XPS全谱在880~915 eV,100 eV和200 eV处出现Ce峰。从图13(a)和(b)可知:OHA在400.3 eV处为1对称峰,OHA与Ce反应后N峰分裂为400.63 eV 和398.38 eV 2处峰;由图12(c)和(d)可知:与辛基羟肟酸作用前,氟碳铈矿表面无N峰,与辛基羟肟酸作用后,氟碳铈矿表面400 eV处出现N峰。从图13(c)和(d)可知:OHA作用后氟碳铈矿表面 N 1s 峰拟合为400.71 eV和399.62 eV 2处峰,这与OHA与Ce反应生成络合沉淀物的N 1s 峰位相一致。Ni等[28-29]研究表明辛基羟肟酸 N 1s 峰仅在 400.3 eV 处有1对称峰,但在烧绿石等矿物表面吸附后,其 N 1s 峰分裂为 400.6 eV 和 399 eV 左右的2处峰,分别对应于中性羟肟酸分子R—CO—NH—OH 和去质子后的羟肟酸根 R—CO—NH—O—,即分别代表羟肟酸在矿物表面的物理吸附和化学吸附,表明辛基羟肟酸在氟碳铈矿表面的吸附是化学吸附和物理吸附共存,这与吸附热力学研究结果相符合,且辛基羟肟酸在氟碳铈矿表面吸附后N 1s 399.62 eV处化学吸附峰较400.71 eV处物理吸附峰更强,说明辛基羟肟酸在氟碳铈矿表面的吸附以化学吸附为主。
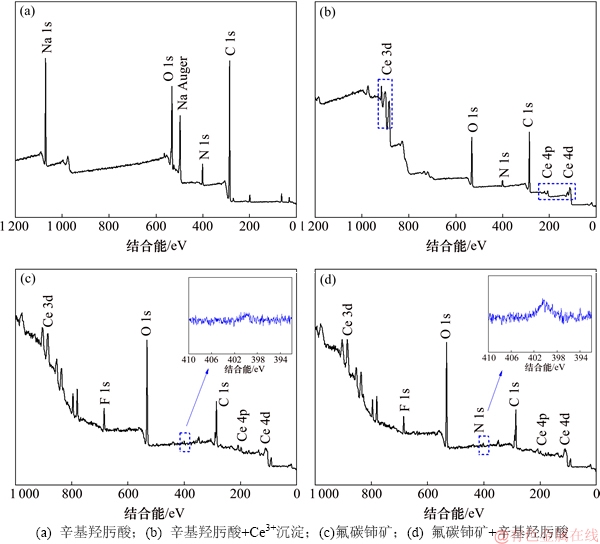
图12 辛基羟肟酸、辛基羟肟酸铈沉淀物及辛基羟肟酸作用前后氟碳铈矿XPS全谱
Fig. 12 XPS survey scan of OHA, OHA-Ce, bastnaesite before and after conditioned with OHA
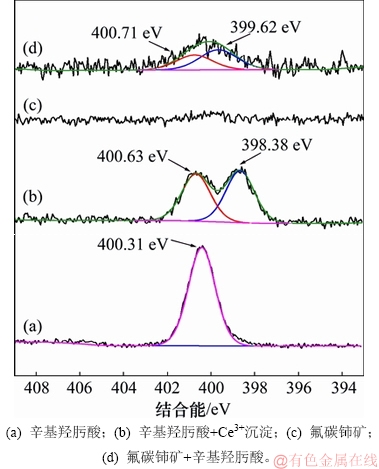
图13 辛基羟肟酸、辛基羟肟酸铈沉淀物及辛基羟肟酸作用前后氟碳铈矿N 1s XPS 窄谱
Fig. 13 N1s XPS narrow scan of OHA, OHA-Ce, bastnaesite before and after conditioned with OHA
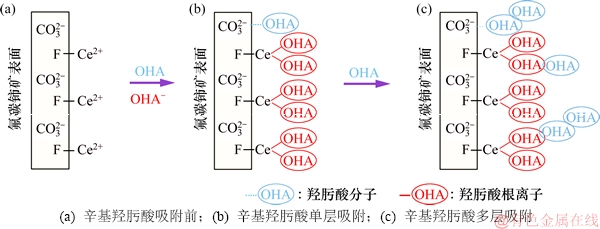
图14 辛基羟肟酸在氟碳铈矿表面的吸附过程模型
Fig. 14 Possible adoption process of OHA on bastnaesite surface
根据以上实验及测试分析,可推测辛基羟肟酸OHA在氟碳铈矿表面的吸附过程如图14所示。由图14可知:辛基羟肟酸在氟碳铈矿表面同时发生化学吸附和物理吸附,辛基羟肟酸对氟碳铈矿的吸附作用机制:1) 羟肟酸根阴离子OHA-与氟碳铈矿表面暴露的Ce3+发生螯合反应生成OHA-Ce沉淀,形成化学吸附;2) 羟肟酸OHA分子中的氢与氟碳铈矿表面 中的氧形成氢键,发生物理吸附;3) 溶液中游离的羟肟酸分子OHA与在氟碳铈矿表面已吸附的OHA分子生成氢键和烃链间疏水缔合作用,形成不均匀的物理吸附层。
中的氧形成氢键,发生物理吸附;3) 溶液中游离的羟肟酸分子OHA与在氟碳铈矿表面已吸附的OHA分子生成氢键和烃链间疏水缔合作用,形成不均匀的物理吸附层。
3 结论
1) 辛基羟肟酸在氟碳铈矿表面吸附符合二级动力学模型,等温吸附过程符合Freundlich吸附模型;弱碱性条件下(pH=9.5)辛基羟肟酸在氟碳铈矿表面的吸附速度更快、吸附量更大,该条件下氟碳铈矿的浮选回收率最高。
2) 辛基羟肟酸在氟碳铈矿表面的吸附为多层、不均匀吸附,是物理吸附和化学吸附共同作用的结果,但以化学吸附为主。吸附机理为:羟肟酸根阴离子OHA-与氟碳铈矿表面暴露的Ce3+发生螯合反应生成OHA-Ce沉淀,形成化学吸附;羟肟酸分子OHA与氟碳铈矿表面的氧以氢键形式形成物理吸附,同时,溶液中游离的羟肟酸分子OHA与氟碳铈矿表面已吸附上的OHA分子形成氢键和烃链间疏水缔合作用,产生不均匀的物理吸附。
参考文献:
[1] 程建忠, 车丽萍. 中国稀土资源开采现状及发展趋势[J]. 稀土, 2010, 31(2): 65-69.
CHENG Jianzhong, CHE Liping. Current mining situation and potential development of rare earth in China[J]. Chinese Rare Earths, 2010, 31(2): 65-69.
[2] ZHANG Xia, DU Hao, WANG Xuming, et al. Surface chemistry aspects of bastnaesite flotation with octyl hydroxamate[J]. International Journal of Mineral Processing, 2014, 133: 29-38.
[3] 程建忠, 侯运炳, 车丽萍. 白云鄂博矿床稀土资源的合理开发及综合利用[J]. 稀土, 2007, 28(1): 70-74.
CHENG Jianzhong, HOU Yunbing, CHE Liping. Making rational multipurpose use of resources of RE in Bayan Obo deposit[J]. Chinese Rare Earths, 2007, 28(1): 70-74.
[4] GUPTA C K, KRISHNAMURTHY N. Extractive metallurgy of rare earths[J]. International Materials Reviews, 2013, 37(1): 197-248.
[5] JORDENS A, CHENG Y P, WATERS K E. A review of the beneficiation of rare earth element bearing minerals[J]. Minerals Engineering, 2013, 41(1): 97-114.
[6] PRADIP P. The surface properties and flotation of rare-earth minerals[D]. Berkeley: University of California. Department of Materials Science and Mineral Engineering, 1981: 222.
[7] 徐金球, 徐晓军, 王景伟. 1-羟基2-萘甲羟肟酸的合成及对稀土矿物的捕收性能[J]. 有色金属, 2002, 54(3): 72-73.
XU Jinqiu, XU Xiaojun, WANG Jingwei. Synthesis of 1-hydroxy-2-naphthyl hydroximic acid and application to collecting rare earth minerals[J]. Nonferrous Metals, 2002, 54(3): 72-73.
[8] REN J, LU S, SONG S, et al. A new collector for rare earth mineral flotation[J]. Minerals Engineering, 1997, 10(12): 1395-1404.
[9] CUI J L, HOPE G A, BUCKLEY A N. Spectroscopic investigation of the interaction of hydroxamate with bastnaesite (cerium) and rare earth oxides[J]. Minerals Engineering, 2012, 36/37/38: 91-99.
[10] FERRON C J, BULATOVIC S M, SALTER R S. Beneficiation of rare earth oxide minerals[J]. Materials Science Forum, 1991, 70/71/72: 251-270.
[11] REN Jun, WANG Wenmei, LUO Jiake, et al. Progress of flotation reagents of rare earth minerals in China[J]. Journal of Rare Earths, 2003, 21(1): 1-8.
[12] BULATOVIC S M. Handbook of flotation reagents: chemistry, theory and practice[M]. Oxford: Elsevier Science & Technology, 2010: 151-173.
[13] NI X, PARRENT M, CAO M, et al. Developing flotation reagents for niobium oxide recovery from carbonatite Nb ores[J]. Minerals Engineering, 2012, 36/37/38: 111-118.
[14] 罗家珂, 任俊, 唐芳琼, 等. 我国稀土浮选药剂研究进展[J]. 中国稀土学报, 2002, 20(5): 385-391.
LUO Jiake, REN Jun, TANG Fangqiong, et al. Development on flotation reagents of rare earth minerals in China[J]. Journal of the Chinese Society of Rare Earths, 2002, 20(5): 385-391.
[15] PRADIP P, FUERSTENAU D W. Design and development of novel flotation reagents for the beneficiation of Mountain Pass rare-earth ore[J]. Minerals & Metallurgical Processing, 2013, 30(1): 1-9.
[16] JORDENS A, MARION C, GRAMMATIKOPOULOS T, et al. Beneficiation of the Nechalacho rare earth deposit: flotation response using benzohydroxamic acid[J]. Minerals Engineering, 2016, 99: 158-169.
[17] HE X J, VAISEY M. Research on process development for Mt Weld rare earths resources of Australia[C]// Proceedings of XXVI International Mineral Processing Congress. New Delhi: New Concept Information Systems Pvt. Ltd., 2012: 140.
[18] 王成行, 邱显扬, 胡真. 水杨羟肟酸对氟碳铈矿的捕收机制研究[J]. 中国稀土学报, 2014, 32(6): 727-735.
WANG Chenghang, QIU Xianyang, HU Zhen. Flotation mechanism of bastnaesite by salicylhydroxamic acid[J]. Journal of the Chinese Society of Rare Earths, 2014, 32(6): 727-735.
[19] 饶金山, 何晓娟, 罗传胜, 等. 辛基羟肟酸浮选氟碳铈矿机制研究[J]. 中国稀土学报, 2015, 33(3): 370-377.
RAO Jinshan, HE Xiaojuan, LUO Chuansheng, et al. Flotation mechanism of octyl hydroximic acid on bastnaesite[J]. Journal of the Chinese Society of Rare Earths, 2015, 33(3): 370-377.
[20] 任俊, 卢寿慈, 池汝安. 1-羟基-2-萘羟肟酸浮选氟碳铈矿作用机理[J]. 中国有色金属学报, 1996, 6(4): 24-28.
REN Jun, LU Shouci, CHI Ru’an. Flotation mechanism of bastnaesite with 1-hydroxy 2-naphthyl hydroxamic acid[J]. The Chinese Journal of Nonferrous Metals, 1996, 6(4): 24-28.
[21] 付宏渊, 邱祥, 王琼, 等. 铁盐改性柚子皮对含铬废水的吸附性能[J]. 中南大学学报(自然科学版), 2017, 48(9): 2271-2278.
FU Hongyuan, QIU Xiang, WANG Qiong, et al. Adsorption performance of Fe(Ⅲ)-modified pomelo peel on wastewater containing Cr(Ⅵ) [J]. Journal of Central South University (Science and Technology), 2017, 48(9): 2271-2278.
[22] 曹永丹, 曹钊, 张亚辉, 等. Cu(II)、Ni(II)离子在蛇纹石表面的吸附及其浮选的影响[J]. 工程科学学报, 2016, 38(4): 461-467.
CAO Yongdan, CAO Zhao, ZHANG Yahui, et al. Effect of Cu(II) and Ni(II) adsorption on serpentine flotation[J]. Chinese Journal of Engineering, 2016, 38(4): 461-467.
[23] 朱玉霜, 朱丹. 辛基羟肟酸在锡石表面吸附的热力学研究[J]. 有色金属, 1994, 46(1): 24-28.
ZHU Yushuang, ZHU Dan. Adsorption thermodynamics of octyl hydroxamic acid on cassiterite[J]. Nonferrous Metals, 1994, 46(1): 24-28.
[24] 徐龙华, 蒋昊, 巫侯琴, 等. 季铵盐在高岭石表面的吸附特性[J]. 中南大学学报(自然科学版), 2013, 44(11): 4379-4384.
XU Longhua, JIANG Hao, WU Houqin, et al. Absorption characteristics of quaternary ammonium on kaolinite[J]. Journal of Central South University (Science and Technology), 2013, 44(11): 4379-4384.
[25] 张国范, 张佰发, 石晴. 油酸钠在闪锌矿表面的吸附机理[J]. 中南大学学报(自然科学版), 2017, 48(1): 16-24.
ZHANG Guofan, ZHANG Baifa, SHI Qing. Adsorption mechanism of sphalerite by sodium oleate[J]. Journal of Central South University(Science and Technology), 2017, 48(1): 16-24.
[26] ZHANG W, HONAKER R Q, GROPPO J G. Flotation of monazite in the presence of calcite part I: calcium ion effects on the adsorption of hydroxamic acid[J]. Minerals Engineering, 2017, 100: 40-48.
[27] 印海, 刘伟, 王慧. 生物炭对水中五氯酚的吸附性能研究[J]. 中国环境科学, 2014, 34(8): 2017-2023.
YIN Hai, LIU Wei, WANG Hui. Adsorption efficiencies of pentachlorophenol from aqueous solution onto biochars[J]. China Environmental Science, 2014, 34(8): 2017-2023.
[28] NI Xiao, LIU Qi. Notes on the adsorption of octyl hydroxamic acid on pyrochlore and calcite[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2013, 430: 91-94.
[29] NI Xiao, LIU Qi. The adsorption and configuration of octyl hydroxamic acid on pyrochlore and calcite[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2012, 411: 80-86.
(编辑 杨幼平)
收稿日期:2018-04-27;修回日期:2018-07-21
基金项目(Foundation item):国家自然科学基金资助项目(51764045);内蒙古自治区科技计划项目(4050901051701);稀有金属分离与综合利用国家重点实验室开放基金资助项目(GK-201804);内蒙古高等学校“青年科技英才支持计划”(NJYT-18-B08))(Project(51764045) supported by the National Natural Science Foundation of China; Project(4050901051701) supported by Science and Technology Program of Inner Mongolia; Project (GK-201804) supported by the Research Fund Program of State Key Laboratory of Rare Metals Separation and Comprehensive Utilization; Project(NJYT-18-B08) supported by Inner Mongolia Young Science & Technology Talent Support Plan)
通信作者:曹钊,博士,副教授,博士生导师,从事复杂多金属矿选矿工艺和理论研究;E-mail:caozhao1217@163.com