J. Cent. South Univ. Technol. (2011) 18: 1389-1394
DOI: 10.1007/s11771-011-0851-y
Electrochemical mechanism of rusticyanin (Rus.) isolated from A. ferrooxidans measured by Rus.-ZnS-QDs/L-Cys/Au electrode
SUN Jing(孙静), YU Run-lan(余润兰), MIAO Lei(苗雷), ZHONG Dai-li(钟代立),
LIU Jie(刘杰), GU Guo-hua(顾帼华)
Key Laboratory of Biometallurgy of Ministry of Education, School of Minerals Processing and Bioengineering,
Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract: Electrochimcal behaviors of rusticyanin (Rus.) isolated from Acidithiobacillus ferrooxidans were investigated through Rus.-ZnS-QDs/L-Cys/Au electrode. The cyclic voltammetric results indicate that rusticyanin immobilized on the surface of Rus.-ZnS-QDs/L-Cys/Au electrode can undergo a direct quasi-reversible electrochemical reaction. The immobilized rusticyanin is not denatured and still retains its activity in the temperature range of 19-43 °C. The reduction ability of the protein increases and its oxidation ability becomes weak with the increase of pH from 6.0 to 7.8. Fe2+ ions in the solution can promote the electron transfer kinetics of the immobilized rusticyanin and make its peak potentials (φp) markedly move negatively.
Key words: rusticyanin; acidithiobacillus ferrooxidans; cyclic voltammetry; electrochemistry
1 Introduction
Rusticyanin is a small (Mr 16,500) periplasmic protein isolated from Acidithiobacillus ferrooxidans (A. ferrooxidans) (previously called Thiobacillus ferrooxidans). It is generally considered to be an important component in iron oxidation since the protein is presented with high concentration (up to 5% of total soluble proteins) when the bacterium is grown on iron [1-3]. Though several different models for the oxidative iron respiratory chain in A. ferrooxidans have been proposed, all include rusticyanin as a common component [4]. Rusticyanin is a member of the family of blue copper-containing proteins called cupredoxins, and shows homology to other members in its C-terminus [5]. However, unlike other cupredoxins, the protein has a Cu(I)-Cu(II) redox couple of 680 mV, whereas typical redox potentials for the type I copper proteins are around 300 mV [6]. In addition to this remarkable property, rusticyanin has an extremely wide pH range (1.5-9.0) and exhibits some activity even at pH 0.5 [7-8].
Quantum dots (QDs) are semiconductor nanocrystals which have distinctive photoluminescence properties and possess unique optical properties including remarkable photostability, large absorption cross sections and tunable emission peaks [9-12]. Equally important, QDs also serve as versatile nanoscale objects of precisely tunable size and morphology, having exceptionally narrow size distributions. These advances have resulted in the large-scale preparation of relatively monodisperse QDs [13].
A class of wide-gap II–VI semiconductor nanomaterials such as X(IIB) and Y(VIA) (where X=Zn, Cd and Y=S, Se, Te) have unique optical and electrical properties [14]. ZnS is a semiconductor in terms of relatively large band-gap energy of 3.66 eV and good conductivity. It is a well-known luminescence material having prominent application in displays, sensors and lasers [15-17]. Therefore, more attention has been focused on the synthesis of ZnS semiconductor materials because of their potential applications [18]. ZHANG et al [19] demonstrated that a kind of ZnS QDs with free carboxyl groups, which is available for covalent coupling to protein by cross-linking to reactive amine groups on its surface, can be synthesized in aqueous solution. These nanometer-sized conjugates are water-soluble and biocompatible which are used to link bio-molecules such as peptides, proteins and DNA [20]. They can also enhance the direct electron transfer between the active sites of proteins and the electrode, and keep the activity of proteins [21].
In this context, it is the first time to investigate the electrochemical behaviors of rusticyanin isolated from A. ferrooxidans by Rus.-ZnS-QDs/L-Cys/Au electrode, and the effect of some factors such as environmental temperature, pH, concentration of Fe2+ ions on electron transfer of rusticyanin was also investigated.
2 Experimental
2.1 Materials
Rusticyanin was obtained from BL21(DE3) Escherichia coli cells with pLM1::RUS plasmid (the cells were got from ZENG et al [3]). A Hi-trap chelating metal affinity column was purchased from GE healthcare LTD. All other chemicals were of analytical grade. All the solutions were prepared with doubly distilled water. The 0.02 mol/L phosphate buffer solutions (PBS), which were made up from Na2HPO4 and NaH2PO4, were always employed as supporting electrolyte except that Fe2+ concentrations experiments were carried out in HAc-NaAc buffer (pH4.0).
2.2 Purification of A. ferrooxidans rusticyanin
The method as ZENG et al [3] referred to in previous work was employed. Firstly, the E. coli strain BL21 (DE3) cells with pLM1::RUS plasmid were cultured in LB medium; secondly, the cells were harvested by centrifugation; thirdly, the cells were lysed using lysozyme and sonicator, and then the A. ferrooxidans rusticyanin was purified with Hi-trap column; at last, the Cu2+ ions and the dialysis of the purified protein were combined. The method of Bradford was used to determine the protein content with bovine serum albumin as the standard. The eluted fractions were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) with the discontinuous buffer of LAEMMLI [3].
2.3 Preparation of ZnS quantum dots
Zinc sulfide quantum dots with free carboxyl groups on their surface were prepared according to Ref.[19]. Briefly, 19.2 μL mercaptoacetic acid was added to a 25 mL solution of Zn(NO3)2 (5 mmol/L) under vigorous stirring and adjusted to pH 8.0 with NaOH (0.5 mol/L), then thoroughly deoxygenated with pure nitrogen for about 30 min. A solution of Na2S (6.7 mmol/L) in 25 mL doubly distilled water was slowly added dropwise to the solution above and the mixture was further stirred for 24 h under nitrogen environment. Thus, the colloidal solution of ZnS quantum dots was obtained and stored at room temperature for several weeks [22].
2.4 Pretreatment of gold electrode
The bare gold electrode (2 mm in diameter) was polished to a mirror-like surface with 1.0, 0.3 and 0.05 μm alumina slurry respectively, and rinsed ultrasonically with doubly distilled water and absolute ethanol for 5 min. The electrode was voltammetrically cycled in 0.5 mol/L H2SO4 until a steady cyclic voltammogram was obtained.
2.5 Preparation of Rus.-ZnS-QDs/L-Cys/Au electrode
The pretreated gold electrode was immersed in an aqueous solution of L-cysteine (0.02 mol/L) for approximately 6 h. Then it was rinsed by doubly distilled water to remove physically absorbed L-cysteine. Then the electrode was put into ZnS quantum dots solution with 1-ethyl-3(3-di-methylaminopropyl carbodiimide) hydrochloride (EDC) for 24 h at 4 °C. Finally, the prepared electrode was immersed into rusticyanin solution for 24 h and the Rus.-ZnS-QDs/L-Cys/Au electrode was obtained.
2.6 Measurement
Electrochemical measurements were performed with CHI 660B electrochemical workstation (CH Instruments, Shanghai, China). A three-electrode system was used in measurements. It consisted of a Rus.-ZnS-QDs/L-Cys/Au electrode (diameter: 2 mm) as the working electrode, Pt as the counter electrode, and an Ag/AgCl electrode as the reference electrode. All potentials were given with respect to the Ag/AgCl electrode. All the solutions were purged with highly purified nitrogen for 30 min before the experiments and a nitrogen environment was kept over the solutions during the electrochemical measurements. All electrochemical experiments were carried out at 37 °C except that the temperature experiments were carried out with various temperature values. The UV–vis absorption spectroscopic measurements were performed using a UV–vis 8453 spectrophotometer (Agilent, USA) and Techcomp UV-2300 spectrophotometer was used for recording absorption. Scanning electron microscopy (SEM) images of the samples were taken with a JSM-6360 instrument (JEOL, Japan).
3 Results and discussion
3.1 Purification of A. ferrooxidans rusticyanin
After purification of A. ferrooxidans rusticyanin, the purity of the protein was examined with SDS-PAGE. Figure 1 shows a clear single band between 18.4 ku and 14.4 ku, which is about 17 ku. The relative molecular mass of rusticyanin is about 16.5 ku [1], which means that the protein purified is the right one. The concentration of the protein is 8 mg/mL, which is determined by Bradford method.
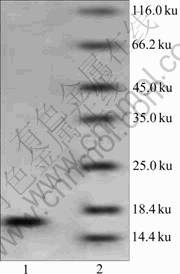
Fig.1 Coomassie blue-stained SDS-PAGE of purified rusticyanin (wild type): Lane 1—Purified rusticyanin; Lane 2— Molecular mass standards
The UV-visible spectrum of the recombinant A. ferrooxidans rusticyanin is shown in Fig.2. The rusticyanin with copper ions shows a maximum absorbance at 600 nm and a second band at 450 nm. The absorption band at 600 nm is the characteristic one of type 1 copper sites corresponding to a charge transfer from Cu(II) to S-Cys. The band at 450 nm is believed to be associated with a separate cysteine-copper charge transfer transition [3, 23]. On the other hand, the protein without combining copper ions has no absorbance at 600 nm and 450 nm, which suggests that copper is correctly incorporated into the active site of the wild-type rusticyanin.
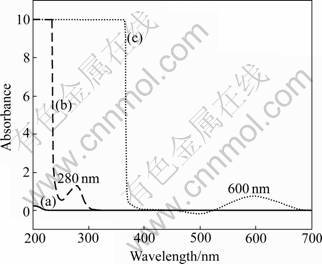
Fig.2 UV-vis scanning of blank (a); rusticyanin without combining copper ions (b) and rusticyanin with copper ions (c)
3.2 Preparation of ZnS quantum dots
50 mL colloidal solution of ZnS quantum dots was centrifuged and washed by ethanol twice, then dried by vacuum drier. The deposition of ZnS quantum dots was measured by SEM. The average diameter of ZnS quantum dots is about 10 nm, as shown in Fig.3, which is an appropriate size for protein incorporating [19].
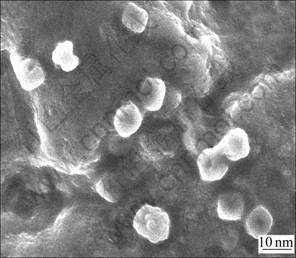
Fig.3 SEM image of ZnS quantum dots
3.3 Electrochemical characterization of Rus.-ZnS- QDs/L-Cys/Au electrode
Figure 4(a) shows the typical cyclic voltammograms (CVs) of bare gold electrode, ZnS-QDs/L-Cys/Au electrode and Rus.-ZnS-QDs/L-Cys/Au electrode in 0.20 mol/L phosphate buffer solution of pH 6.86 at scan rate of 10 mV/s, respectively. As shown in CVs (Fig.4(a)), there is only one cathodic peak in both bare gold electrode and ZnS-QDs/L-Cys/Au electrode, while a couple of stable and well-defined redox peaks are observed at the Rus.-ZnS-QDs/L-Cys/Au electrode between -0.3 V and 0.9 V. The anodic peak potential and cathodic peak potential of Rus.-ZnS-QDs/L-Cys/Au electrode are located at 0.815 V and 0.532 V, respectively, which correspond to Cu(II)/Cu(I) center in the rusticyanin molecules entrapped in the ZnS-QDs/L-Cys film.
To further investigate the characteristics of Rus.- ZnS-QDs/L-Cys/Au electrode, the dependence of the peak currents on the scan rate was systematically studied. Figure 4(b) shows the cyclic voltammograms of the Rus.-ZnS-QDs/L-Cys/Au electrode in 0.2 mol/L PBS (pH 6.86) at different scan rates and Fig.4(c) shows the linear regression curves of cathodic and anodic current. As shown in Fig.4(c), the anodic and cathodic peak currents change linearly with the scan rate in the examined range of 10-40 mV/s. The linear regression equation is Ipc=0.071 5υ+1.143, with a correlation coefficient of R2= 0.988 1 for cathodic current; Ipa=-1.094 1υ-1.229 5, with a correlation coefficient of R2=0.999 8 for anodic current, which is the characteristic peak of thin-layer electro- chemical behavior. All these results demonstrate a quasi- reversible surface-confined feature of the electron transfer between rusticyanin and electrode [24-25].
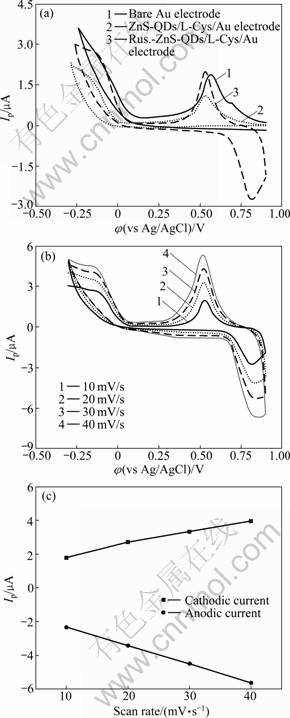
Fig.4 Cyclic voltammograms in pH 6.86 PBS buffer solution: (a) With different electrodes at scan rate of 10 mV/s; (b) At different scan rates with Rus.-ZnS-QDs/L-Cys/Au electrode; (c) linear regression cuves
3.4 Effect of temperature and pH on electrochemical behaviors of Rus. protein
The influence of environmental temperature between 19 °C and 45 °C on the CVs of the Rus.-ZnS- QDs/L-Cys/Au electrode is shown in Fig.5. The cathodic peak current continues to increase with the increase of temperatures, which reaches a maximum value at 43 °C. Probably, this is due to the activity enhancement of rusticyanin with the increase of temperature. However, when the temperature increases above 45 °C, cathodic peak current decreases slightly, which probably is due to the partial denaturation of rusticyanin. As shown in Fig.5(b), the anodic peak currents increase slightly and linearly with the scan rate in the examined range of 19-43 °C. The linear regression equation is Ipa=0.134 7T- 1.150 4, with a correlation coefficient of R2=0.994 2.
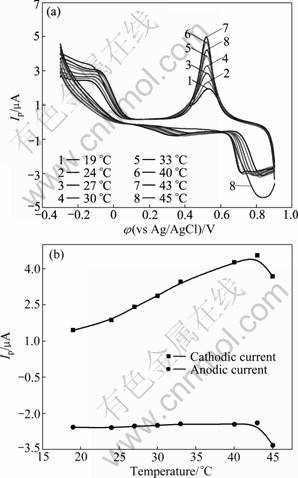
Fig.5 Cyclic voltammograms of Rus.-ZnS-QDs/L-Cys/Au electrode at different temperature in pH 6.86 PBS buffer solution at the scan rate of 10 mV/s (a) and linear regression curves (b)
Solution pH value has an obvious influence on the electrochemical behaviors of rusticyanin, as shown in Fig.6. The potential of reduction peak moves negatively and the peak current rises with the increase of pH. The potential of oxidation peak also moves negatively but the peak current declines with the increase of pH. This result indicates that the protons in rusticyanin affect chelate Cu2+ ions and take part in redox reactions. Its reduction ability of the protein increases and its oxidation ability becomes weak with the increase of pH.
3.5 Effect of Fe2+ concentrations on electrochemical behaviors of rusticyanin
The electrochemical behavior of Rus./ZnS-QDs/L- Cys/Au electrode in the solution containing ferrous ions is quite different from that without ferrous ions. Peak potential (φp) markedly moves negatively and peak current (Ip) obviously amplifies, as shown in Fig.7(a). Anodic peak at about 0.82 V changes into about 0.23 V and cathodic peak at about 0.53 V changes into about -0.04 V. But the φp values almost do not change and the Ip values only increase a little with increasing the concentration of Fe2+ ions, as shown in Fig.7(b). As rusticyanin in Rus.-ZnS-QDs/L-Cys/Au electrode is activated for 5 min by 10 mmol/L Cu2+ ions and then Cu2+ ions were dialyzed and separated by pH 4.0 NaAc for a night, the experimental results indicate that: 1) Fe2+ ions are catalysis reagents and can promote electron transfer kinetics; 2) if there are no metallic ions in the solution, the redox potential of rusticyanin will be very high and the kinetics of electron transfer will be weak when there are no coexisting ions which can transfer electrons in the solution.
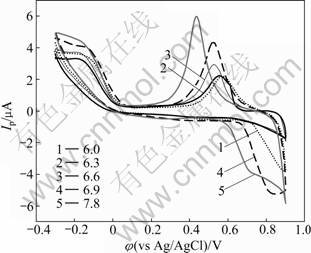
Fig.6 Cyclic voltammograms of Rus.-ZnS-QDs/L-Cys/Au electrode in PBS buffer solutions with different pH at scan rate of 10 mV/s
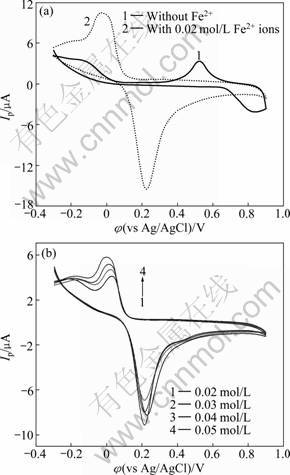
Fig.7 Effect of Fe2+ ions on Rus.-ZnS-QDs/L-Cys/Au electrode in pH4.0 HAc-NaAc buffer solution at scan rate of 20 mV/s
4 Conclusions
1) Rusticyanin immobilized on the surface of ZnS-QDs/L-Cys/Au electrode can undergo a direct quasi-reversible electrochemical reaction.
2) The immobilized rusticyanin is not denatured and still retains its activity in the temperature range of 19- 43 °C.
3) The reduction ability of the protein increases and the oxidation ability becomes weak with the increase of pH.
4) Fe2+ ions in the solution can promote the electron transfer kinetics of the immobilized rusticyanin and make its peak potentials (φp) markedly move negatively.
References
[1] YARZABAL A, DUQUESNE K, BONNEFOY V. Rusticyanin gene expression of Acidithiobacillus ferrooxidans ATCC 33020 in sulfur- and in ferrous iron media [J]. Hydrometallurgy, 2003, 71(1/2): 107-114.
[2] COMBA P, LLEDóS A, MASERAS F. Hybrid quantum mechanics/molecular mechanics studies of the active site of the blue copper proteins amicyanin and rusticyanin [J]. Inorganica Chimica Acta, 2001, 324(1/2): 21-26.
[3] ZENG Jia, GENG Mei-mei, LIU Yuan-dong. The sulfhydryl group of Cys138 of rusticyanin from Acidithiobacillus ferrooxidans is crucial for copper binding [J]. Biochimica et Biophysica Acta (BBA) - Proteins & Proteomics, 2007, 1774(4): 519-525.
[4] CHIGUSA I, KAZUHIRO S, AKIKAZU A. Kinetic rate constant for electron transfer between ferrous ions and novel Rusticyanin isoform in Acidithiobacillus ferrooxidans [J]. Journal of Bioscience and Bioengineering, 2003, 95(5): 534-537.
[5] YAMADAL T, HIRALKAL Y. Rusticyanin, a bacterial electron transfer protein, causes G1 arrest in J774 and apoptosis in human cancer cells[J]. Cell Cycle, 2004, 3(9): 1182-1187.
[6] OLSSON M H M, HONG Gong-yi, WARSHEL A. Frozen density functional free energy simulations of redox proteins: Computational studies of the reduction potential of plastocyanin and rusticyanin [J]. Journal of the American Chemical Society, 2003, 125(17): 5025-5039.
[7] BARRETT M L, HARVEY I, SUNDARARAJAN M. Atomic resolution crystal structures, EXAFS, and quantum chemical studies of rusticyanin and its two mutants provide insight into its unusual properties [J]. Biochemistry, 2006, 45(9): 2927-2939.
[8] HOLT S D, PIGGOTT B, INGLEDEW W J. EXAFS of the type-1 copper site of rusticyanin [J]. Febs Letters, 1990, 269(1): 117-121.
[9] VANNOY C H, XU Jian-min, LEBLANC R M. Bioimaging and self-assembly of lysozyme fibrils utilizing CdSe/ZnS quantum dots [J]. J Phys Chem, 2010, 114(2): 766-773.
[10] LI Hui, SHIH W Y, SHIH W H. Highly photoluminescent and stable aqueous ZnS quantum dots [J]. Ind Eng Chem Res, 2010, 49(2): 578-582.
[11] SU Yuan-yuan, HE Yao, LU Hao-ting. The cytotoxicity of cadmium based, aqueous phase—Synthesized, quantum dots and its modulation by surface coating[J]. Biomaterials, 2009, 30(1): 19-25.
[12] FU Xin, HUANG Ke-long, LIU Su-qin. Citrate-stabilized CdSe/CdS quantum dots as fluorescence probe for protein determination [J]. J Cent South Univ Technol, 2010, 17(4): 720-725
[13] LIU Wen-hao, CHOI H S, ZIMMER J P. Compact cysteine-coated CdSe(ZnCdS) quantum dots for in vivo applications [J]. J Am Chem SOC, 2007, 129(47): 14530-14531.
[14] YANG Jia-xiang, WANG Shao-min, ZHAO Xiao-lu. Preparation and characterization of ZnS nanocrystal from Zn(II) coordination polymer and ionic liquid [J]. Journal of Crystal Growth, 2008, 310(19): 4358-4361.
[15] YANG Jian, PENG Jun-jun, ZOU Ren-xian. Mesoporous zinc-blende ZnS nanoparticles: Synthesis, characterization and superior photocatalytic properties [J]. Nanotechnology, 2008, 19(25): 1-7.
[16] LUO Yuan-yuan, DUAN Guo-tao, YE Min. Poly(ethylene glycol)-mediated synthesis of hollow ZnS microspheres [J]. J Phys Chem C, 2008, 112(7): 2349-2352.
[17] LI Zhu-lai, WANG Jin, XU Xiu-zhi. The evolution of optical properties during hydrothermal coarsening of ZnS nanoparticles [J]. Materials Letters, 2008, 62(23): 3862-3864.
[18] LI Yao, HE Xiao-yan, CAO Min-hua. Micro-emulsion-assisted synthesis of ZnS nanospheres and their photocatalytic activity [J]. Materials Research Bulletin, 2008, 43(11): 3100-3110.
[19] ZHANG Fen-fen, LI Chen-xin, LI Xiao-hua. ZnS quantum dots derived a reagentless uric acid biosensor [J]. Talanta, 2006, 68(4): 1353-1358.
[20] CHAN W C W, MAXWELL D J, GAO Xiao-hu. Luminescent quantum dots for multiplexed biological detection and imaging [J]. Current Opinion in Biotechnology, 2002, 13(1): 40-46.
[21] ZHANG Fen-fen, WANG Xiao-li, AI Shi-yun. Immobilization of uricase on ZnO nanorods for a reagentless uric acid biosensor [J]. Analytica Chimica Acta, 2004, 519(2): 155-160.
[22] WANG Qing-jiang, DING Fei, ZHU Ning-ning, LI Hui, HE Pin-gang, FANG Yu-zhi. Determination of hydroxyl radical by capillary zone electrophoresis with amperometric detection [J]. Journal of Chromatography A, 2003. 1016(1): 123-128.
[23] BOTUYAN M V. NMR solution structure of Cu(I) rusticyanin from Thiobacillus ferrooxidans: Structural basis for the extreme acid stability and redox potential [J]. Journal of Molecular Biology, 1996, 263(5): 752-767.
[24] CHAN Yu, YANG Xiao-jing, GUO Li-rong. Direct electrochemistry and electrocatalysis of hemoglobin at three-dimensional gold film electrode modified with self-assembled monolayers of 3-mercaptopropylphosphonic acid [J]. Analytica Chimica Acta, 2009, 644(1/2): 83-89.
[25] SHAN Dan, CHENG Guo-xing, ZHU Dao-bin. Direct electrochemistry of hemoglobin in poly(acrylonitrile-co-acrylic acid) and its catalysis to H2O2 [J]. Sensors and Actuators B, 2009, 137(1): 259-265.
(Edited by HE Yun-bin)
Foundation item: Project(2010CB630903) supported by the National Basic Research Program of China; Project(50621063) supported by the National Natural Science Foundation of China
Received date: 2010-09-09; Accepted date: 2010-12-14
Corresponding author: YU Run-lan, Professor, PhD; Tel: +86-731-88877472; E-mail: YRL715@sina.com.cn