文章编号:1004-0609(2013)11-3248-06
攀枝花预氧化钛精矿的H2还原行为
危雪梅,鲁雄刚,肖 玮
(上海大学 上海市现代冶金与材料制备重点实验室,上海 200072)
摘 要:采用热重分析、X射线衍射、扫描电镜和光学显微镜等技术对攀枝花预氧化钛精矿的H2还原行为进行研究,包括H2还原反应的动力学、还原产物的物相转变及显微结构的变化规律。结果表明:预氧化钛精矿在1 100 ℃还原80 min后还原度达到96.04%;在温度为1 000 ~1 150 ℃范围内,还原过程受界面化学反应控制,表观活化能为95.25 kJ/mol。在还原过程中将会形成M3O5型(M=Ti, Fe, Mg等)固溶体,导致还原难度加大。显微结构表明,还原反应由颗粒边缘向内部进行,而Mg元素在未反应内核的富集阻碍了预氧化钛精矿的进一步还原。
关键词:预氧化钛精矿;H2;还原;动力学;物相;显微结构
中图分类号:TF 533 文献标志码:A
Reduction behavior of Panzhihua pre-oxidized ilmenite by hydrogen
WEI Xue-mei, LU Xiong-gang, XIAO Wei
(Shanghai Key Laboratory of Modern Metallurgy and Materials Processing, Shanghai University, Shanghai 200072, China)
Abstract: Reduction behavior of Panzhihua pre-oxidized ilmenite in hydrogen atmospheres, such as reduction kinetic, phase transformation and microstructures of reduced products was investigated by thermo-gravimetric,X-ray diffractometry (XRD), scanning electron microscopy (SEM), and optical microscopy. The results show that the reduction degree reaches 96.04% when the pre-oxidized ilmenite concentrates are reduced at 1 100 ℃ for 80 min and the reduction reaction is controlled by interfacial chemical reaction with the activation energy of 95.25 kJ/mol at 1 000-1 150 ℃. The M3O5 (M is Fe, Ti, Mg, etc) solid solution forms in the reduction process, in which the reduction difficulty increases. Microstructure analysis shows that the reaction takes place from the exterior of the grain to its interior and the enrichment of Mg in the unreacted area restricts the further reduction of pre-oxidized ilmenite.
Key words: pre-oxidized ilmenite; hydrogen; reduction; kinetics; phase; microstructure
随着钛工业的发展,高品位的天然金红石资源日趋枯竭,利用钛铁矿制取富钛料的工艺越来越受到人们的关注[1-2] 。目前,大规模应用的钛铁矿处理方法主要有电炉熔炉法、硫酸法和盐酸浸出法[3-4]。其中盐酸浸出法因为快速浸出、有效去除杂质、酸循环利用等优点逐渐成为攀枝花钛铁矿处理的主要工艺[5]。而钛铁矿的氧化-还原是盐酸浸出工艺中的关键步骤。因此,研究钛铁矿的还原行为对于钛铁矿富集方法具有重要的意义。
由于固体碳还原需要高温和较长时间,相比较而言,氢气还原具有速率更快、还原更彻底并且产物不污染空气的优势。考虑到低碳经济、节能减排的发展要求,氢气或者富氢气体还原越来越受到人们的重视[2, 6]。煤还原攀枝花钛铁矿过程中形成M3O5(M=Ti, Fe, Mg等)和TinO2n-1等导致铁离子活度降低的物相,同时杂质在未反应区域富集,阻碍了钛铁矿的进一步还原。因此,物相转变和杂质的迁移过程是研究氢气还原的重点[7]。
预氧化处理能提高还原的速率和产物的金属化 率[8-9]。因此,本文作者以攀枝花预氧化钛精矿为研究对象,通过热重恒温实验,采用X射线衍射、光学显微镜及和扫描电子显微镜等微观测试手段,研究预氧化钛精矿在H2还原反应中的宏观动力学以及还原过程中的物相转变及显微结构变化规律,探讨预氧化钛精矿的H2还原行为。
1 实验
1.1 原料性能
实验用预氧化钛精矿为攀枝花钢铁公司的细粒浮选钛精矿在850 ℃氧化30 min后的产物,其平均粒度为29 μm,主要化学成分及XRD谱分别见表1及图1。 可以判断,预氧化钛精矿中Fe2+全部氧化成Fe3+,主要化学成分为三价铁板钛矿(Fe2TiO5),同时还含有少量的赤铁矿(Fe2O3)和金红石(TiO2)相。还原气体为99.99%的H2,保护气体为99.99%的Ar。
表1 氧化钛精矿的主要化学成分
Table 1 Main chemical components of oxidized ilmenite (mass fraction, %)
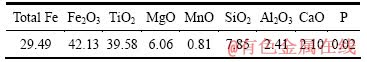
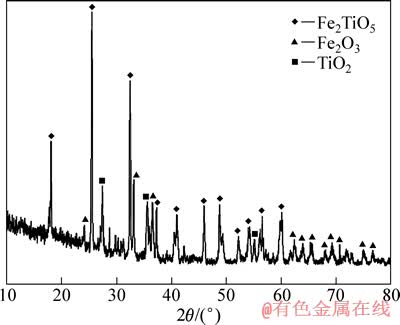
图1 预氧化钛精矿的XRD谱
Fig. 1 XRD pattern of pre-oxidized ilmenite
1.2 研究方法
每次称取1 000 mg样品,置于Cahn Thermax700型大载荷热重分析仪中,在Ar气氛下,以10 ℃/min升温速率快速升温,达到设定温度后切换成H2,恒温还原至设定时间后,通Ar保护冷却,气体流量均为100 mL/min,反应结束后取出样品。根据热重数据,计算还原过程的质量损失率和还原度,根据不同温度和时间下还原度的变化规律,对宏观动力学机理进行分析。 使用日产D/Max-2550型X射线衍射仪对还原产物的物相变化规律进行分析。 采用光学显微镜、HITACHI SU-1500型扫描电镜对所得产品的显微结构、形貌及元素迁移进行分析。
样品质量损失率(Rm)主要是由于氧的质量损失,计算公式为
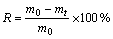 (1)
(1)
式中:m0为样品还原前的质量,g;mt为样品还原到t时刻的质量,g。
还原度(R)的计算公式为[6,10]
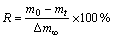 (2)
(2)
式中:m0为样品还原前的质量,g;mt为样品还原到t时刻的质量,g;Δm∞为还原过程中铁氧化物全部被还原为金属铁的最大质量损失量,g。
2 结果与分析
2.1 等温还原实验
以H2为还原剂的等温还原实验中,不同还原温度下质量损失率和还原时间的关系如图2所示。 由图2可以看出,当还原温度从800 ℃提高到1 050 ℃时,质量损失率有大幅度增加。 当温度由1 050 ℃提高到1 150 ℃时,开始阶段质量损失率大幅提升,还原50 min后,温度对质量损失率的影响减小;当还原温度为1 000~1 150 ℃时,在还原反应的初期,质量损失率增加很快,还原50 min后,质量损失率随着还原时间的延长增加缓慢,最终反应趋于平衡。当预氧化钛精矿在1 100℃还原80 min后,质量损失率趋于稳定,达到12.06%。
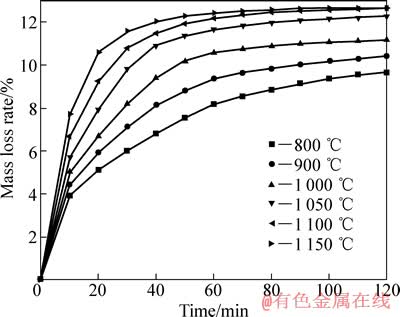
图2 不同还原温度下质量损失率与还原时间的关系
Fig. 2 Relationship between mass loss rate and reduction time at different temperatures
通过式(2)计算出还原度R,结果如图3所示。当还原温度为1 000~1 150℃时,在还原反应的初期,还原度增加很快,还原50 min后,还原度随着还原时间的延长增加缓慢。根据相关研究结果,预氧化钛精矿的还原反应分两步进行:第一步是Fe3+→Fe2+反应,第二步是Fe2+→Fe反应。在反应开始阶段,第一步反应和第二步反应同时进行,当还原进行到一定时间后只有第二步反应进行[10]。反应进行到后期,还原度增加缓慢,这与矿物中含有氧化镁、氧化锰等杂质阻碍反应进一步还原有关[1]。当预氧化钛精矿在1 100 ℃还原80 min后,还原度趋于稳定,达到96.04%。
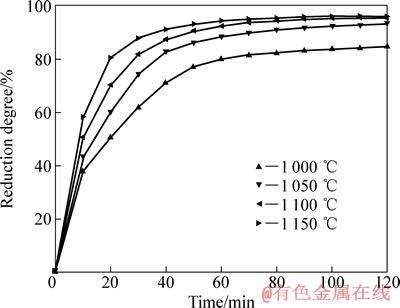
图3 不同还原温度下还原度与还原时间的关系
Fig. 3 Relationship between reduction degree and reduction time at different temperatures
2.2 反应动力学
为查明H2还原氧化钛精矿的反应控制环节,先将如图3 所示的还原度R转换成还原分数x(x=R/100%),然后用x与时间t的关系,分别以1-(1-x)1/3对时间t、1-2x/3-(1-x)2/3对时间t以及1-2(1-x)2/3+(1-x)1/3对t/[1-(1-x)1/3]进行线性回归。计算结果表明,在1 000~ 1 150 ℃,1-(1-x)1/3与t最符合线性关系,这表明在试验温度范围内还原反应为界面化学反应控制。
根据Arrhenius方程:
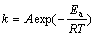 (3)
(3)
式中:Ea为化学反应的活化能,J/mol;A为指数前系数;k为速率常数,min-1;R为摩尔气体常数,其值为8.314 J/(mol·K);T为热力学温度,K。作关于ln k和1/T的线性回归曲线,其线性拟合结果如图4所示。所以Ea=11 456.7×8.314=95.25 kJ/mol。
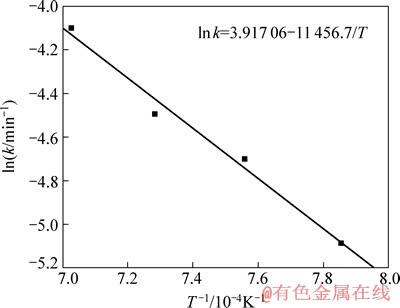
图4 ln k和1/T的关系
Fig. 4 Relationship between ln k and 1/T
2.3 热力学计算
采用HSC软件计算Fe2TiO5、Fe2O3和H2间发生反应的标准吉布斯自由能变化(ΔGΘ),结果如图5所示。图中的序号对应于下列反应方程式:
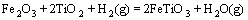 (4)
(4)
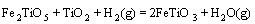 (5)
(5)
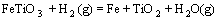 (6)
(6)
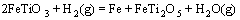 (7)
(7)
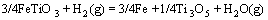 (8)
(8)
由标准吉布斯自由能变化曲线可以看出,Fe2TiO5和Fe2O3很容易被H2还原为FeTiO3;随着温度升高,FeTiO3还原反应的ΔGΘ降低,生成金属铁、FeTi2O5和低价的钛氧化物。
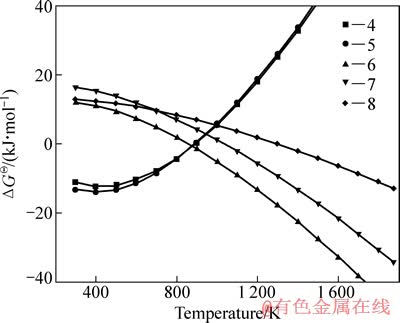
图5 反应式(4) ~ (8)的ΔGΘ和T的关系
Fig. 5 Relationship between ΔGΘ and T for reactions (4)-(8)
2.4 预氧化钛精矿还原过程的物相变化
为了解攀枝花预氧化钛精矿还原过程中产物的物相变化规律,揭示预氧化钛精矿还原反应历程,采用 XRD 测定预氧化钛精矿还原前后的物相组成。 图6所示为预氧化钛精矿利用H2在不同温度下恒温还原80 min所得还原产物的XRD谱。
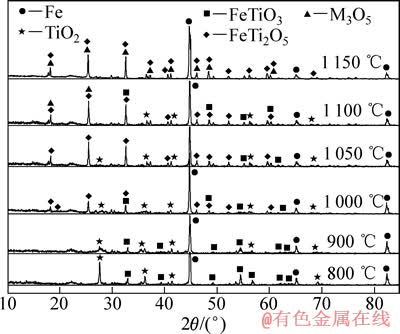
图6 不同温度下预氧化钛精矿还原80 min产物的XRD谱
Fig. 6 XRD patterns of products reduced for 80 min by hydrogen at different temperatures
由图1可知,预氧化钛精矿的主要化学成为三价铁板钛矿(Fe2TiO5),同时还含有少量的赤铁矿(Fe2O3) 和金红石(TiO2)相。由图6可以看出,在 800 ℃时,还原产物中Fe2TiO5和Fe2O3消失,出现了钛铁矿 (FeTiO3)和金属铁相,由此可以推测预氧化钛精矿还原过程中Fe2TiO5和Fe2O3全部转化为FeTiO3,即反应(4)和(5)反应完全,同时部分FeTiO3还原成金属铁和TiO2,即发生了反应(6)。
当还原温度为900 ℃时,金属铁的衍射峰增强,钛铁矿的衍射峰减弱,这是反应(6)进一步进行的结果。当温度升高到1 000 ℃时,预氧化钛精矿还原产物开始出现亚铁板钛矿(FeTi2O5),钛铁矿的衍射峰减弱,这是发生反应(7)的结果。当温度继续升高时,金属铁和亚铁板钛矿(FeTi2O5)的特征衍射峰继续增强,而TiO2的特征衍射开始减弱。
当还原温度为1 100 ℃时,在XRD 测定结果中检测到FeTi2O5相中含有M3O5(M=Ti,Fe,Mg等)相;当温度升高到1 150 ℃时,M3O5衍射峰增强。这是因为在反应(7)和(8)进行过程中,矿石中的MgO等杂质会固溶于FeTi2O5中,增加了FeTi2O5的稳定性,最终将形成固溶有FeO、MgO、TiO2、Ti3O5等的 M3O5(M=Ti,Fe,Mg等)型固溶体,尤其是在出现液相之后, M3O5 型固溶体导致氧化物的活度变得越来越小,使得铁氧化物的还原变得困难[3-4, 7-8, 11]。预氧化钛精矿在1 100 ℃还原80 min后的产物主要为金属铁、TiO2和M3O5型固溶体。
2.5 预氧化钛精矿还原产物的形态以及Mg的分布
以往的研究表明[7-8, 12-15]:钛精矿在固体碳还原反应过程中,MgO、MnO2等杂质的迁移行为对反应速率及进程有重要影响。试验所用预氧化钛精矿中Mg含量高达6.06%,而Mn含量较低。为了揭示攀枝花预氧化钛精矿氢气还原的微观机理,对预氧化钛精矿还原产物的显微结构及Mg、Fe和Ti的分布情况进行了分析。图7(a)和(b)所示分别为攀枝花预氧化钛精矿在1 100 ℃还原40 min和80 min后还原产物的金相显微结构。
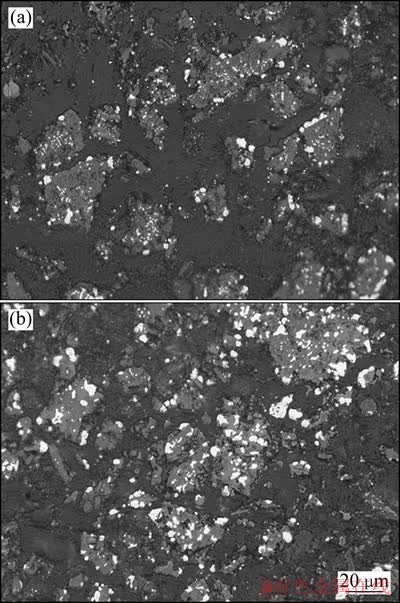
图7 在1 100 ℃还原攀枝花氧化钛精矿还原产物金相显微结构
Fig. 7 Microstructures of Panzhihua pre-oxidized ilmenite reduced at 1 100 ℃ for 40 min (a) and 80 min (b)
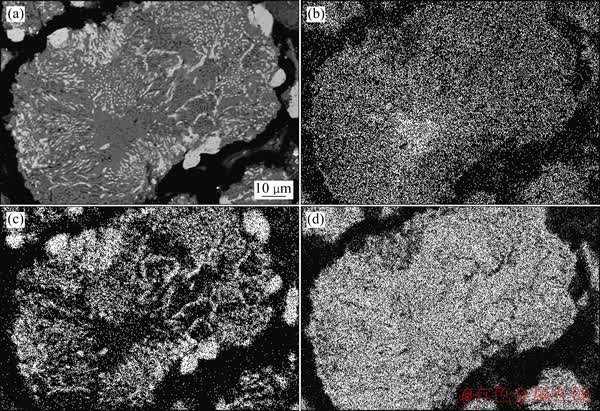
图8 在1 100 ℃还原80 min后攀枝花预氧化钛精矿还原产物的BSE像
Fig. 8 BSE image (a) of Panzhihua pre-oxidized ilmenite reduced at 1 100 ℃ for 80 min, and surface distributions of elements Mg (b), Fe (c) and Ti (d)
从图7(a)可见,还原产物颗粒几乎都存在未反应内核,大颗粒金属铁主要分布在颗粒边缘及孔隙处,微小蠕虫状金属铁镶嵌于颗粒内部和边缘。 从图7(b)可见,还原产物仍有部分颗粒存在未反应的内核,金属铁粒子明显增多,生成的金属铁粒子明显增大,微小蠕虫状金属铁粒子由颗粒边缘继续向颗粒内部发展。
通过扫描电镜对预氧化钛精矿在1 100 ℃还原80 min后还原产物的显微结构及主要元素的面分布进行了研究,结果如图8所示。从图8背散射成像可以看出,攀枝花预氧化钛精矿在1 100 ℃还原 80 min 后,还原产物中的颗粒内部出现了因金属铁的大量生成而形成的孔洞, Mg在未反应的内核出现富集现象。
由XRD谱可知,在1 100 ℃还原80 min后,攀枝花预氧化钛精矿还原产物主要由金属铁、M3O5 和TiO2组成。结合元素面分布(见图8(b)~(d))可知,图8(a)中颗粒亮色部分主要是金属铁相,灰色部分主要是M3O5和TiO2相。以往的研究表明[7],少量的Mn和Mg等杂质离子能使M3O5型连续固溶体稳定。攀枝花预氧化钛精矿还原过程中Mg的这种富集现象,必将会在未反应内核处形成大量稳定的M3O5型固溶体,从而使得铁氧化物的活度降低,还原反应速率降低。
3 结论
1) 攀枝花预氧化钛精矿在1 100 ℃还原80 min后还原度达到96.04%,在1 000~1 150 ℃温度范围内,预氧化钛精矿的还原过程受界面化学反应控制,反应的表观活化能为 95.25 kJ/mol。
2) 预氧化钛精矿在还原过程中形成亚铁板钛矿(FeTi2O5),FeO、MgO、TiO2、Ti3O5等固溶于其中形成M3O5型(M=Fe,Ti,Mg等)固溶体,使得铁离子活度降低,不利于预氧化钛精矿的继续还原。预氧化钛精矿在1 100 ℃还原80 min后还原产物主要由金属铁、TiO2和M3O5型固溶体构成。
3) 预氧化钛精矿还原产物颗粒金属铁粒子由颗粒边缘向颗粒内部发展,颗粒内部出现因金属铁的大量生成而形成的孔洞。Mg 在未反应内核出现富集现象,阻碍了预氧化钛精矿的进一步还原。
REFERENCES
[1] WANG Yu-ming, YUAN Zhang-fu, GUO Zhan-cheng, TAN Qiang-qiang, LI Zhao-yi, JIANG Wei-zhong. Reduction mechanism of natural ilmenite with graphite[J]. Transactions of Nonferrous Metals Society of China, 2008,18(4):962-968.
[2] WANG Yu-ming, YUAN Zhan-fu, MATSUURA H, TSUKIHASHI F. Reductive extraction kinetics of titania and iron and ilmenite by H2-CO gas mixtures[J]. ISIJ International, 2009, 49(2): 164-170.
[3] 杨绍利, 盛继孚. 钛铁矿熔炼钛渣与生铁技术[M]. 北京: 冶金工业出版社, 2006: 42-47.
YANG Shao-li, JI Fu-sheng. Ilmenite smelting titanium slag and pig iron technology[M]. Beijing: Metallurgical Industry Press, 2006: 42-47.
[4] 邓国珠. 钛冶金[M]. 北京: 冶金工业出版社, 2010: 56-96.
DENG Guo-zhu. Titanium metallurgy[M]. Beijing: Metallurgical Industry Press, 2010: 56-96.
[5] TAN Ping, HU Hui-ping, ZHANG Li. Effects of mechanical activation and oxidation-reduction on hydrochloric acid leaching of Panxi ilmenite concentration[J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 1414-1421.
[6] SI Xing-guo, LU Xiong-gang, LI Chuang-wei, LI Chong-he, DING Wei-zhong. Phase transformation and reduction kinetics during the hydrogen reduction of ilmenite concentrate[J]. Journal of University of Science and Technology Beijing, 2012, 19(5): 384-390.
[7] 郭宇峰, 游 高, 姜 涛, 邱冠周. 攀枝花钛铁矿固态还原行为[J]. 中南大学学报: 自然科学版, 2010, 41(5): 1639-1644.
GUO Yu-feng, YOU Gao, JIANG Tao, QIU Guan-zhou. Solid-state reduction behavior of Panzhihua ilmenite[J]. Journal of Central South University: Science and Technology, 2010, 41(5): 1639-1644.
[8] 郭宇峰, 吕亚男, 姜 涛, 邱冠周. 预氧化在攀枝花钛铁矿固态还原过程中的作用[J]. 北京科技大学学报, 2010, 32(4): 413-419.
GUO Yu-feng,  Ya-nan, JIANG Tao, QIU Guan-zhou. Effect of pre-oxidation on Panzhihua ilmenite in solid state reduction process[J]. Journal of University of Science and Technology Beijing, 2010, 32(4): 413-419.
Ya-nan, JIANG Tao, QIU Guan-zhou. Effect of pre-oxidation on Panzhihua ilmenite in solid state reduction process[J]. Journal of University of Science and Technology Beijing, 2010, 32(4): 413-419.
[9] 肖 玮, 鲁雄刚, 危雪梅, 丁伟中. 钛铁矿预氧化的物相转变及对气基还原的影响[J]. 中国稀土学报, 2012, 30: 392-296.
XIAO Wei, LU Xiong-gang, WEI Xue-mei, DING Wei-zhong. Phase Transitions of ilmenite preoxidation and effect on gaseous reduction[J]. Journal of Chinese Society of Rare Earths, 2012, 30: 392-296.
[10] SUN Kang, TAKASHI R, YAGI J. Reduction kinetics of cement-bonded natural ilmenite pellets with hydrogen[J]. ISIJ International, 1992, 32(4): 496-504.
[11] PESL J, ERIC R H. High temperature carbothermic reduction of Fe2O3-TiO2-MxOy oxide mixtures[J]. Mineral Engineering, 2002, 15 (11): 971-984.
[12] WANG Yu-ming, YUAN Zhan-fu. Reductive kinetics of the reaction between a natural ilmenite and carbon[J]. International Journal of Minerals Processing, 2006, 81(3): 133-140.
[13] MERK R, PICKLES C A. Reduction of ilmenite by carbon monoxide[J]. Canadian Metallurgical Quarterly, 1988, 27(3): 179-185.
[14] SURESH K G, RAJAKUMAR V, GRIEVESON P. Kinetics of reduction of ilmenite with graphite at 1 000 to 1 100 ℃[J]. Metallurgical Transactions B, 1987, 18(4): 713-717.
[15] JONES D G. Optical microscopy and electron-probe microanalysis study of ilmenite reduction[J]. Transactions of the Institution of Mining and Metallurgy, 1974, 83: 1-9.
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51074105);国家杰出青年科学基金资助项目(51225401)
收稿日期:2012-12-03;修订日期:2013-04-03
通信作者:鲁雄刚,研究员,博士;电话: 021-66132237;E-mail: luxg@shu.edu.cn