攀枝花钛铁矿固态还原行为
郭宇峰,游高,姜涛,邱冠周
(中南大学 资源加工与生物工程学院,湖南 长沙,410083)
摘 要:以我国攀枝花钛铁矿为研究对象,研究钛铁矿固态碳热还原反应的宏观动力学、钛铁矿还原产物的物相转变及显微结构的变化规律,探讨攀枝花钛铁矿固态碳热还原行为。研究结果表明:钛铁矿精矿还原速率较小,在温度为1 000~1 150 ℃时,攀枝花钛铁矿精矿的还原过程受界面化学反应控制,反应的表观活化能为125.92 kJ/mol;在钛铁矿还原过程中,将会形成M3O5型(M为Fe,Ti,Mg和Mn等)固溶体和TinO2n-1等导致铁离子活度降低的物相,使钛铁矿的还原难度加大,而且还原产物的结构和Mg在未反应内核处的富集都阻碍了攀枝花钛铁矿精矿的进一步还原。
关键词:钛铁矿;固态还原;动力学;微观机理
中图分类号:TF533 文献标志码:A 文章编号:1672-7207(2010)05-1639-06
Solid-state reduction behavior of Panzhihua ilmenite
GUO Yu-feng, YOU Gao, JIANG Tao, QIU Guan-zhou
(School of Minerals Processing and Bioengineering , Central South University, Changsha 410083, China)
Abstract: The macrodynamics of solid-state carbonthermic reduction of Panzhihua ilmenite and the mineral phases transformation and microstructure change of reduction products were researched, and the solid-state carbonthermic reduction behavior of Panzhihua ilmenite was also researched. The results show that the reduction rate of ilmenite is low, and the reduction reaction is controlled by interfacial chemical reaction and apparent activated energy of reaction is 125.92 kJ/mol at 1 000-1 150 ℃. The M3O5 (M is Fe, Ti, Mg, Mn, etc) solid solution and TinO2n-1 are found during the reduction processes, in which the activity of iron ion decreases and the reduction difficulty increases. Besides, the structure of reduction products and enrichment of Mg in the unreacted core restrict the further reduction of ilmenite.
Key words: ilmenite; solid-state reduction; kinetics; microscopic mechanism
随着天然金红石资源的逐渐枯竭和价格上涨,储量丰富的钛铁矿已成为钛工业的主要生产原料,但存在流程长、设备产能低、“三废”量大等问题[1-2],因此,一般需预先将其富集成高品位的富钛料,再进行后续加工。已用于工业应用或正在研究的富集钛铁矿的方法[3-4]绝大多数是以还原法如两段还原熔炼法、弱还原酸浸法、还原锈蚀法和还原磨选法等作为主要技术手段,因此,研究钛铁矿的固态还原行为对于革新以还原法为主要技术手段的钛精矿制取富钛料工艺、开发高效率的钛铁矿富集方法具有重要的指导意义。我国攀枝花—西昌地区(即攀西地区)蕴藏着丰富的钛铁矿资源,占世界钛资源探明储量的35.17%。目前,攀西地区钛资源经选矿法回收的钛铁矿精矿,每年达到30万t,但由于攀西地区钛铁矿精矿属岩矿型钛铁矿,结构致密,氧化镁含量高,品位低,到目前为止,仍未开发出一条适合该资源特点的、高品位富钛料制备的有效途径。为此,本文作者以我国攀枝花钛铁矿为研究对象,系统研究钛铁矿固态碳热还原反应的宏观动力学,并采用X线衍射、光学显微镜和扫描电镜等微观测试手段,研究钛铁矿还原产物的物相变化及显微结构的变化,探寻攀枝花钛铁矿固态碳热还原行为,以期为采用还原法等对攀枝花钛铁矿的富钛料进行高效制备提供理论指导。
1 原料性能及研究方法
1.1 原料性能
研究所用钛铁矿为攀枝花钢铁公司提供的细粒浮选钛铁矿,其主要化学成分(质量分数)见表1。钛铁矿粒径较小,其中粒径小于0.076 mm的含量达到99.5%。还原剂为河南义马煤,其工业分析及灰渣熔点见表2。造球黏结剂为中南大学研制的专利产品即多功能复合黏结 剂F[5]。
表1 钛铁矿的主要化学成分(质量分数)
Table 1 Main composition of ilmenite concentrate %
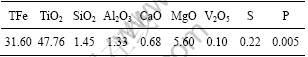
表2 还原煤工业分析含量(质量分数)及灰渣熔点
Table 2 Industrial analysis and ash melt point of reduced coal
1.2 试验研究方法
试验时,先将钛铁矿精矿配加1%的复合黏结剂F,在直径为100 cm的圆盘造球机上制成直径为10 mm左右的球团,湿球团在干燥箱内于(105±5) ℃干燥4 h,然后,将试样与还原煤置于耐热钢反应罐中,采用炉膛直径为60 mm、配有自动控温系统的底部密封的小型竖式电阻炉作为还原设备。为尽可能消除试样升温滞后现象和防止试样氧化,每次取直径为10 mm的干燥球10 g左右,还原煤质量为试样量的2倍,并将试样埋于还原煤中。在试验过程中,向炉内通入氮气保护。当达到指定还原时间后,快速取出反应罐并将其中的试样迅速倒入通有氮气的容器中快速冷却至室温,取样分析其全铁、金属铁和氧化亚铁的含量,计算还原产品的还原度。根据不同温度和时间下还原度的变化规律,对动力学进行分析和计算。
对钛铁矿还原产物的物相转变规律、钛铁矿还原产物的显微结构、形貌及镁锰等元素面分布进行研究。研究钛铁矿还原产物相变化规律的试验方法为:将反应罐放入炉内,以300 ℃/min的升温速度升温到设定温度后,快速取出反应罐,并将其中的试样迅速倒入通有氮气的容器中快速冷却至室温。取样后,采用日本理学D/max-rA型X线衍射仪(Cu靶,λ=1.540 56×10-10 m)对其物相转变规律进行分析。在对钛铁矿还原产物的显微结构、形貌及元素的面分布进行研究时,试验方法与还原动力学试验方法相同,根据研究要求,采用光学显微镜、JSM-56600LV型扫描电镜对所得产品显微结构、形貌及元素的面分布进行分析。
2 结果与分析
2.1 钛铁矿的等温还原试验
以煤为还原剂的攀枝花钛铁矿精矿等温还原试验结果见图1(其中,R为还原度)。
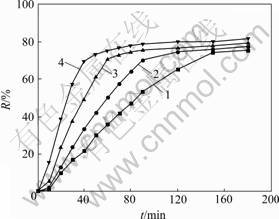
温度/℃: 1—1 000;2—1 050;3—1 100;4—1 150
图1 攀枝花钛铁矿精矿等温还原曲线
Fig.1 Isothermal curves of Panzhihua ilmenite reduction
由图1可见:在还原过程中,铁氧化物的还原度R随着还原温度的升高和还原时间的延长而不断增加;但是,当还原度接近70%左右时,继续提高还原温度和延长还原时间,钛铁矿中铁氧化物的还原度增加极其缓慢,即使还原温度达到1 150 ℃,还原时间延长到180 min,还原产物中铁氧化物的还原度也仅为80%左右。这与国内外的相关研究结果相一致[6-9]。攀枝花钛铁矿精矿MgO含量较高,达到5.6%。其原因一般认为是钛铁矿在还原过程中形成了难于还原的亚铁板钛矿(FeTi2O5),以及MgO和MnO易在还原过程中富集于反应界面形成的屏障效应[10-14],阻止了钛铁矿还原反应的进一步进行。
为查明煤还原钛铁矿的反应控制环节,应用收缩未反应核动力学模型对煤还原钛铁矿的等温还原试验结果进行分析。结果表明:由收缩未反应核模型中的界面化学反应控制模型所得结果与等温还原实验结果较符合。这表明在试验温度范围内,该反应过程受界面化学反应控制。按收缩未反应核模型界面化学反应控制分析,不同温度下的还原试验结果见图2。
图3所示为根据图2中各直线斜率求出的各温度下的速率常数k与温度的Arrhenius关系曲线,该曲线斜率为-E/R。图3中,曲线斜率为-1.514 6,由此可求出煤还原钛铁矿中铁氧化物的表观反应活化能为
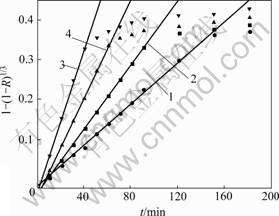
温度/℃: 1—1 000;2—1 050;3—1 100;4—1 150
图2 1 000~1 150 ℃还原时1-(1-R)1/3与t的关系曲线
Fig.2 Relationship between 1-(1-R)1/3 and t at 1 000-1 150 ℃
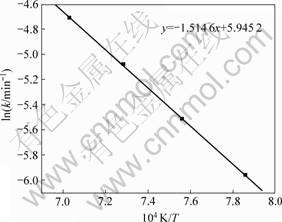
图3 化学反应速率常数k与温度的Arrhenius关系曲线
Fig.3 Arrhenius curve of reaction rate constant k and temperature
125.92 kJ/mol。普通铁矿石还原的表观反应活化能一般为50~80 kJ/mol。由此可见:在相同还原温度下,钛铁矿的还原速率比普通铁矿的还原速率慢得多。
2.2 钛铁矿还原过程的物相变化
为了解钛铁矿还原过程中产物的物相变化规律,揭示钛铁矿还原反应历程,采用XRD测定攀枝花钛铁矿精矿还原前后的物相组成。图4所示为攀枝花钛铁矿精矿和用煤还原攀枝花钛铁矿精矿以300 ℃/h的速度升温到不同温度后所得的还原产物的X线衍 射图。
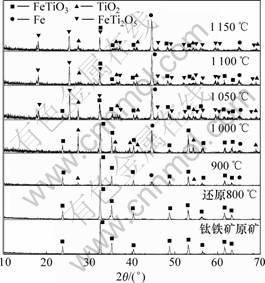
图4 攀枝花钛铁矿还原产物物相变化
Fig.4 Product phases transformation of Panzhihua ilmenite reduction
由图4可见:攀枝花钛铁矿精矿的主要物相是钛铁矿(FeTiO3),没有检测到其他物相。用煤还原攀枝花钛铁矿,当温度为800 ℃时,还原产物中的主要物相仍然为FeTiO3相,但代表FeTiO3的特征衍射峰强度有所减弱;当温度为900 ℃时,还原产物中的物相除了FeTiO3相外,还有金属铁和金红石相,这表明此时已开始发生如下反应:
FeTiO3 + C = Fe + TiO2 + CO (1)
当还原温度为1 000 ℃时,还原产物中存在的主要物相为钛铁矿、金属铁和金红石相,同时存在少量的新生物相亚铁板钛矿(FeTi2O5)。但与900 ℃时相比,1 000 ℃时钛铁矿的衍射峰强度明显减弱,金属铁和金红石相的衍射峰强度明显增强,这表明反应(1)在持续进行,并已开始发生如下反应:
2FeTiO3 + C = Fe + FeTi2O5 + CO (2)
当还原温度达到1 050 ℃时,还原产物的物相种类没有发生变化,但金属铁和亚铁板钛矿(FeTi2O5)的特征衍射峰明显比1 000 ℃时的要强,而钛铁矿的衍射峰续减弱,同时,TiO2的衍射峰也开始减弱。
当还原温度达到1 100 ℃时,还原产物的物相种类没有发生变化,但金属铁和亚铁板钛矿(FeTi2O5)的特征衍射峰继续增强,而钛铁矿和TiO2的特征衍射继续减弱。
当还原温度升到1 150 ℃时,与1 100 ℃时相比,钛铁矿和TiO2的特征衍射峰变化不大,产物中依然存在钛铁矿相。
从上述XRD测定结果可知:当还原温度升到 1 000 ℃时,亚铁板钛矿(FeTi2O5)就开始形成。
在还原温度为1 150 ℃时,在XRD测定结果中检测到TiO2相中含有TinO2n-1相,即此时发生如下反应:
nTiO2 + C = TinO2n-1 + CO, 4<n<9 (3)
因此,攀枝花钛铁矿精矿还原产物中(特别是在反应后期)将会有M3O5型(M为Fe,Ti,Mg和Mn等)固溶体和TinO2n-1相存在。
有文献报道[8, 15-17]:M3O5型固溶体的形成会降低铁离子的活度;此外,还原过程中形成的亚铁板钛矿(FeTi2O5)、低价钛氧化物在高温下易与未反应的钛铁矿互溶,其结果会导致铁离子活度降低。这可能是由于在还原过程中,亚铁板钛矿(FeTi2O5)和M3O5型固溶体和TinO2n-1等导致铁离子活度降低的物相形成,进一步加大了钛铁矿的还原难度,使钛铁矿的还原过程更加复杂,不利于钛铁矿的还原。
2.3 钛铁矿还原产物的形态以及Mg和Mn的分布
从攀枝花钛铁矿精矿等温还原曲线(见图1)可以看出:当还原度接近70%时,继续提高还原温度和延长还原时间,钛铁矿中铁氧化物的还原度极其缓慢增加。以往的研究表明[10-11, 14, 18-19]:钛铁矿在还原反应过程中,还原产物的形态和镁、锰等杂质的迁移行为对反应速率及进程有重要影响。风化程度较低的钛铁矿在还原反应后期,还原生成的金属铁易偏析在颗粒周围,使之烧结在一起;而锰和镁等杂质易在反应界面处聚集,这些都会妨碍反应物扩散接触,降低还原反应速率及进程。为查明攀枝花钛铁矿精矿还原行为是否与还原产物中金属铁的形态和还原过程中镁、锰等杂质的迁移行为有关,从而揭示其还原的微观机理,对攀枝花钛铁矿精矿还原产物的形态及Mg和Mn的分布情况进行分析。
图5所示为攀枝花钛铁矿精矿在1 000 ℃还原 60 min后还原产物的显微结构照片。从图5可见:还原产物的颗粒几乎都存在未反应的内核,生成的金属铁绝大部分呈粒状、条状附着在颗粒的边界处或裂缝处,粒度较大,但相互之间未形成连接。较少部分呈点状分散在颗粒的边界与未反应的内核之间,金属铁粒子粒径较小。
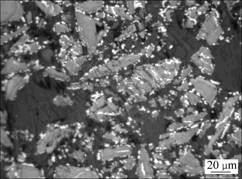
图5 于1 000 ℃还原60 min后攀枝花钛铁矿精矿还原产物显微组织
Fig.5 Microstructure of Panzhihua ilmenite reduced 60 min at 1 000 ℃
采用扫描电镜对还原产物中的典型颗粒进行分析,结果见图6。由图6可以看出:攀枝花钛铁矿精矿在1 000 ℃还原60 min后,还原产物中的颗粒结构致密,几乎没有孔洞。由从图6中Mg和Mn的面分布情况可以看出:Mg在未反应的内核处出现了富集现象,Mn则没有出现明显的富集现象。这可能与本研究采用的攀枝花钛铁矿精矿中Mg的含量较高、Mn的含量较低有关。攀枝花钛铁矿精矿在还原过程 中,Mg的这种富集现象与前人研究结果[10-11, 14, 18]有所不同。
图7所示为攀枝花钛铁矿精矿在1 100 ℃还原 60 min后还原产物的显微结构照片。可见:在此条件下,还原产物中仍有部分颗粒存在未反应的内核,生成的金属铁除部分呈粒状、条状附着在颗粒的边界处或裂缝处外,其余部分则呈点状、脉状向颗粒内部发展。与1 000 ℃时还原60 min相比,生成的金属铁粒子总体上有所增大,但附着在颗粒边界处或裂缝处的金属铁粒子相互之间仍未形成连接。
采用扫描电镜对还原产物中存在未反应内核的颗粒进行分析,结果见图8。从图8可以看出:攀枝花钛铁矿精矿在1 100 ℃还原60 min后,还原产物中的颗粒内部出现了因金属铁的大量生成而形成的孔洞,但颗粒边缘仍然较致密;Mg在未反应的内核处也出现了富集现象,而且这种富集与1 000 ℃时还原60 min
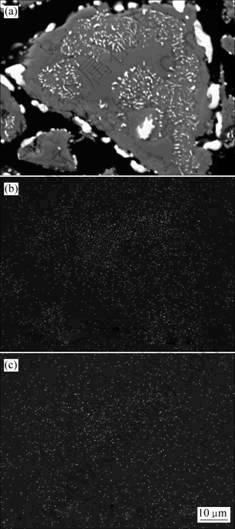
(a) SEM像; (b) Mg的面分布; (c) Mn的面分布
图6 于1 000 ℃还原60 min后攀枝花钛铁矿精矿还原产物的SEM像及Mg和Mn的面分布
Fig.6 SEM of Panzhihua ilmenite reduced at 1 000 ℃ for 60 min and surface distribution of Mg and Mn
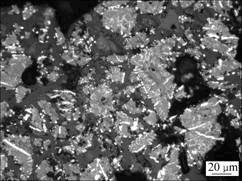
图7 于1 100 ℃还原60 min后攀枝花钛铁矿精矿还原产物显微照片
Fig.7 Microstructure of Panzhihua ilmenite reduced at 1 100 ℃ for 60 min
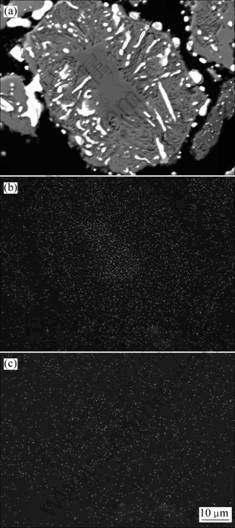
(a) SEM像; (b) Mg的面分布; (c) Mn的面分布
图8 于1 100 ℃还原时间60 min后攀枝花钛铁矿精矿还原产物的SEM像及Mg和Mn的面分布
Fig.8 SEM of Panzhihua ilmenite reduced at 1 100 ℃ for 60 min and surface distribution of Mg and Mn
相比要明显得多。这与提高温度后Mg的迁移速度加快有关。Mn则同样没有出现明显的富集现象。可见:生成的金属铁的形态不会影响攀枝花钛铁矿精矿的还原,还原产物的结构和Mg在未反应的内核处的富集可能是影响攀枝花钛铁矿精矿还原的主要因素。因为在颗粒边界处或裂缝处生成的金属铁粒子相互之间未形成连接,因而也就不会妨碍气体反应物的扩散。在较低温度(如1 000 ℃)时还原产物结构较致密,此时会妨碍气体反应物的扩散,虽然提高还原温度(如1 100 ℃)后,还原产物中的颗粒内部出现了因金属铁的大量生成而形成的孔洞,但颗粒边缘仍然较致密。而Mg在未反应内核处的富集在攀枝花钛铁矿精矿的还原过程中始终存在,并且随着温度提高明显加强。根据Grey等的研究结果[18],少量的锰和镁等杂质离子能使M3O5型连续固溶体稳定。攀枝花钛铁矿精矿还原过程中Mg的这种富集现象,必将会在未反应内核处形成大量稳定的M3O5型固溶体,从而使原钛铁矿中铁氧化物的活度降低,导致钛铁矿还原反应速率降低。
3 结论
(1) 钛铁矿精矿还原速率较小,产物很难达到较高的还原度。在1 000~1 150 ℃,攀枝花钛铁矿精矿的还原过程受界面化学反应控制,反应的表观活化能为 125.92 kJ/mol。
(2) 当还原温度升高到1 000 ℃时,攀枝花钛铁矿精矿在还原过程中将会形成亚铁板钛矿(FeTi2O5)和M3O5型(M为Fe,Ti,Mg和Mn等)固溶体和TinO2n-1等导致铁离子活度降低的物相,使钛铁矿的还原难度加大,不利于钛铁矿的还原。
(3) 攀枝花钛铁矿精矿的还原产物中存在未反应的内核,还原产物结构较致密;生成的金属铁绝大部分呈粒状、条状分散附着在颗粒的边界处或裂缝处,粒度较大,较少部分呈点状分散在颗粒的边界与未反应的内核之间,粒度较小;Mg在未反应的内核处出现了富集现象。还原产物的致密结构和Mg在未反应内核处的富集阻碍了攀枝花钛铁矿精矿的进一步 还原。
参考文献:
[1] 莫畏, 邓国珠, 罗方. 钛冶金[M]. 2版. 北京: 冶金工业出版社, 1998: 118-198.
MO Wei, DENG Guo-zhu, LUO Fang. Titanium metallurgy[M]. 2nd ed. Beijing: Metallurgical Industry Press, 1998: 118-198.
[2] 孙康. 钛提取冶金物理化学[M]. 北京: 冶金工业出版社, 2001: 24-64.
SUN Kang. Extractive metallurgical physical chemistry of titanium[M]. Beijing: Metallurgical Industry Press, 2001: 24-64.
[3] 孙康. 钛铁矿的富集方法[J]. 钒钛, 1995(5): 12-22.
SUN Kang. Beneficiation methods of titanium-containing iron ores[J]. Vanadium Titanium, 1995(5): 12-22.
[4] 邱冠周, 郭宇峰. 钛铁矿富集方法评述[J]. 矿产综合利用, 1998(5): 29-33.
QIU Guan-zhou, GUO Yu-feng. A review on beneficiation methods of titanium-containing iron ores[J]. Multipurpose Utilization of Mineral Resources, 1998(5): 29-33.
[5] 徐经沧, 杨有育, 刘仲辉, 等. 复合粘结剂冷固球团煤基直接还原新工艺. 中国专利, ZL92111782.5[P]. 1994-06-29.
XU Jing-cang, YANG You-yu, LIU Zhong-hui, et al. New technology of coal-based direct reduction of cold bond pellets with complex binders. China Patent, ZL92111782.5[P]. 1994-06-29.
[6] 范晓慧, 邱冠周, 姜涛, 等. 攀钢钛精矿制取富钛料新工艺的研究[J]. 金属矿山, 2002(6): 20-22.
FAN Xiao-hui, QIU Guan-zhou, JIANG Tao, et al. Study on the new technology of making titanium-rich materials from Panzhihua steel’s titanomagnetite concentrate[J]. Metal Mine, 2002(6): 20-22.
[7] 余伟. 钛精矿回转窑还原制取富钛料补充试验研究[J]. 稀有金属与硬质合金, 2003, 31(2): 26-30.
YU Wei. Supplementary test of enriched Ti material preparation with rotary kiln material from Ti concentrate[J]. Rare Metals and Cemented Carbide, 2003, 31(2): 26-30.
[8] Wouterlood H J. The reduction of ilmenite with carbon[J]. Chem Tech Biotechnol, 1979, 29(10): 603-618.
[9] WANG Yu-ming, YUAN Zhang-fu. Reductive kinetics of the reaction between a natural ilmenite and carbon[J]. Int J Miner Process, 2006, 81(3): 133-140.
[10] Merk R, Pickles C A. Reduction of ilmenite by carbon monoxide[J]. Can Metall Q, 1988, 27(3): 179-185.
[11] Suresh K G, Rajakumar V, Grieveson P. Kinetics of reduction of ilmenite with graphite at 1 000 to 1 100 ℃[J]. Metall Trans, 1987, 18B: 713-717.
[12] Grey I E, Jones D G, Reid A F. Reaction sequences in the reduction of ilmenite: 1-introduction[J]. Trans Instn Min Metall, 1973, 82: 151-152.
[13] Grey I E, Reid A F. Reaction sequences in the reduction of ilmenite: 3-reduction in a commercial rotary kiln: An X-ray diffraction study[J]. Trans Instn Min Metall, 1974, 83: 39-46.
[14] Jones D G. Optical microscopy and electronprobe microanalysis study of ilmenite reduction[J]. Trans Inst Min Metal, 1974(82): 1-9.
[15] Pesl J, Eric R H. High temperature carbothermic reduction of Fe2O3-TiO2-MxOy oxide mixtures[J]. Minerals Engineering, 2002, 15: 971-984.
[16] Welham N J, Williams J S. Carbothermic reduction of ilmenite (FeTiO3) and rutile (TiO2)[J]. Metallurgical and Materials Transactions B, 1999, 30B(12): 1075-1081.
[17] ELGuindy M I, Davenport W G. Kinetics and mechanism of ilmenite reduction with graphite[J]. Metall Trans, 1970(1): 1729-1734.
[18] Suresh K G, Rajakumar V, Grieveson P. The influence of weathering on the reduction of ilmenite with carbon[J]. Metall Trans B, 1989, 18B: 735-745.
(编辑 陈灿华)
收稿日期:2009-09-15;修回日期:2009-12-08
基金项目:国家自然科学基金资助项目(50504018);国家杰出青年科学基金资助项目(507254);国家高技术研究发展计划(“973”计划)项目(2007CB613606)
通信作者:郭宇峰(1970-),男,内蒙古阿荣旗人,副教授,从事复合矿综合利用研究;电话:13975894856;E-mail: guo.yf@126.com