TiO2光催化降解水中喹诺酮类抗生素
刘利伟,吴小莲,莫测辉,李彦文,高鹏,黄显东,邹星,黄献培
(暨南大学 环境工程系,广东省高校水土环境毒害性污染物防治与生物修复重点实验室,
广东 广州,510632)
摘要:研究TiO2光催化降解水中3种喹诺酮类抗生素的动力学特征,探讨TiO2用量、初始浓度、pH和水质对3种喹诺酮类抗生素光催化降解的影响。研究结果表明:当优化参数为TiO2用量1.0 g/L、抗生素初始浓度10 mg/L,pH=7时,反应80 min,3种喹诺酮类抗生素的降解率均在95%以上;光催化降解反应符合假一级反应动力学方程,反应速率常数为0.044 5~0.071 0,半衰期为9.76~15.57 min,降解难易程度从高到低的顺序为诺氟沙星、环丙沙星、洛美沙星;不同水质中诺氟沙星、环丙沙星的降解率从高到低的顺序为高纯水、河水、自来水,而洛美沙星的降解率从高到低的顺序为河水、自来水、高纯水。
关键词:抗生素;水污染;催化;降解;动力学;TiO2
中图分类号:X52 文献标志码:A 文章编号:1672-7207(2012)08-3300-08
Photocatalytic degradation of quinolone antibiotics in water using TiO2
LIU Li-wei, WU Xiao-lian, MO Ce-hui, LI Yan-wen, GAO Peng,
HUANG Xian-dong, ZOU Xing, HUANG Xian-pei
(Key Laboratory of Water/Soil Toxic Pollutants Control and Bioremediation,
Department of Education of Guangdong Province, Department of Environment Engineering,
Jinan University, Guangzhou 510632, China)
Abstract: The efficiency of photodegradation of three quinolones in water by TiO2-catalysed irradiation was investigated through analyzing TiO2 dosage, initial concentration, the pH value and water quality on the photodegradation of quinolones. The degradation efficiency of quinolones is above 95% under the optimum conditions of TiO2 dosage 1.0 g/L, initial concentration 10 mg/L, and pH value 7.0 after 80 min of photodegradation. TiO2-catalysed UV photodegradation reaction of three quinolone compounds follows the pseudo-first-order kinetic formula, with reaction rate constants from being 0.044 5 to 0.071 0. The half-life periods are 9.76-15.57 min for lomefloxacin, ciprofloxacin and norfloxacin, which indicates that the photodegradation efficiency order are norfloxacin, ciprofloxacin, lomefloxacin. The photodegradation efficiencies of different quinolone compounds vary in various sources of water, with the descending order of ultra pure water, river water, tap water for both norfloxacin and ciprofloxacin, while the descending order of river water, tap water, ultra pure water for lomefloxacin.
Key words: antibiotics; water pollution; catalysis; degradation; kinetics; TiO2
喹诺酮类抗生素广泛用于人类医疗和动物养殖,在体内代谢通常低于25%,大部分以药物原形随粪尿排出体外[1]。大量喹诺酮类抗生素随着生活污水[2-4]、养殖污水[5]和制药工业废水[6-7]等不断进入环境,造成水环境污染,各种水体包括河水、海水、地下水等普遍检出喹诺酮类抗生素[8-11]。不同水环境介质中喹诺酮类抗生素的浓度虽然比较低,但可能诱导病原菌产生耐药性,对生态系统和人类健康产生严重威胁[12-13],因此,有必要对水环境中喹诺酮类抗生素污染的治理技术进行研究。近年来,关于深度氧化法利用半导体材料如TiO2作为光催化剂降解有机污染物的报道逐渐增多[14-16]。目前,国外对常见种类抗生素,如喹诺酮类抗生素[17-18]、磺胺类抗生素[19]、四环素 类[20]等中的少数化合物进行光催化降解研究,主要集中在反应动力学、降解产物鉴定及其微生物毒性方面。例如,Knapp等[21]发现恩诺沙星在日光照射下的模拟生态池中快速光解,生成环丙沙星, 其光解符合一级动力学。Araki 等[18]发现西他沙星的光解也符合一级动力学, 并且光解速率受溶液pH和Cl-的影响。在国内,对于抗生素光降解研究主要基于药物稳定性方面进行,如葛林科等[22]考察模拟日光照射对水中加替沙星的光降解动力学、影响因素与机理,加替沙星光降解生成具有较高风险的中间产物;但对于环境中抗生素光催化降解的研究很少,肖健等[23]研究大环内酯类抗生素红霉素和罗红霉素的光催化降解动力学及其影响因素。目前,国内外基于水体中抗生素污染治理而进行光催化降解参数优化和水质影响等方面的研究还鲜见报道。为此,本文作者针对环境中普遍检出的喹诺酮类抗生素进行此方面的研究,旨在为抗生素污染水体治理提供科学依据和实用技术。
1 材料与方法
1.1 材料与仪器
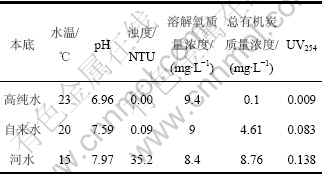
(1) 材料:3种喹诺酮类抗生素诺氟沙星(Norfloxacin,NOR)、环丙沙星(Ciprofloxacin,CIP)和洛美沙星(Lomefloxacin,LOM),购自中国药品生物制品检定所,纯度为95.0%;喹诺酮标准品,产自德国Dr. Ehrenstorfer公司,纯度均>98.0%;甲醇、乙腈,均为色谱纯(Sigma公司);其他化学试剂均为分析纯;实验用水均为高纯水。自来水和河水中喹诺酮类抗生素光催化降解的对比实验中,供试自来水为水龙头开启5 min 后采集样品,并在20 ℃下放置24 h后进行实验;供试河水采自流溪河广州段表层水。高纯水、自来水和河水的水质参数见表1。
(2) 仪器为:Shimadzu LC-20AT高效液相色谱仪(High performance liquid chromatography, HPLC),配有荧光检测器、Shimadzu SIL-20A自动进样器、柱温控制器和LC Solution工作站;色谱柱:waters (250 mm×4.6 mmI.D.,5 μm);高压汞灯(功率125 W,主要发射波长250 nm);UVA + UVB 紫外辐射计(YK234UV, 金坛市泰纳仪器厂);Shimadzu TOC-VCSH检测器;雷磁PHS-3C精密pH计。
表1 试验用水水质参数
Table 1 Water quality parameters of experiments
1.2 实验方法
配制一定浓度的喹诺酮类抗生素水溶液,倒入耐热玻璃槽(有效体积500 mL),用硫酸或NaOH调节pH。加入一定量TiO2后,避光静置30 min,使TiO2吸附喹诺酮类抗生素达到饱和。溶液以磁力搅拌器进行搅拌,并利用空气泵持续通气以使溶液充分反应。以高压汞灯作为光源悬于液面上方,在室温下进行光催化反应,间隔一定时间取样。每次采样2 mL,取3份平行样,样品经过孔径为0.22 μm的滤膜过滤,稀释后待测。实验过程中设置黑暗处理作为对照。
1.3 分析方法
采用HPLC检测波长。激发波长为280 nm,发射波长为450 nm;柱温为25 ℃;进样量为20 μL;流动相为乙腈-0.05 mol/L 磷酸溶液(用三乙胺调节pH=2.5,V(水):V(乙腈)=15:85);流速为1.0 mL/min。采用环丙沙星、诺氟沙星和洛美沙星化合物标样,质量浓度设为0,0.01,0.02,0.05,0.10,0.20,0.50 mg/L作工作曲线,外标法定量,3种化合物的加标回收率为95%~105%。在空白实验中未检出目标化合物,说明实验操作过程中无人为污染。HPLC测定相对标准误差<5%,TOC测定相对标准误差<2%。
采用草酸铁钾露光计测定紫外灯光子辐射流 率[24]。采用UVA + UVB 紫外辐射计测定紫外辐射灯的辐射强度为600 μW/cm2。
2 结果与讨论
2.1 TiO2对紫外光降解喹诺酮类抗生素的催化效能
在3种喹诺酮初始质量浓度均为10 mg/L,pH=3和TiO2用量为1.0 g/L条件下,TiO2对紫外降解喹诺酮类抗生素的催化效能见图1。
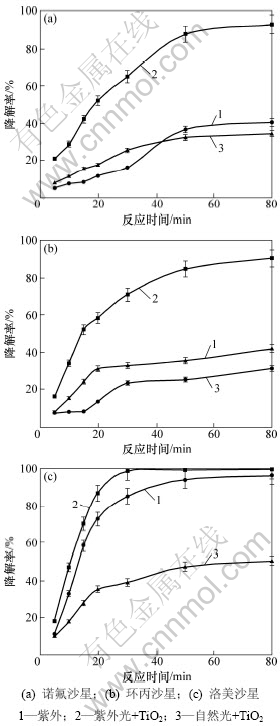
图1 TiO2对紫外光降解喹诺酮类抗生素的催化效能
Fig.1 Effect of TiO2 on ultraviolet photodegradation of quinolone antibiotics in water
从图1可见:仅进行紫外光辐射或在自然光下添加TiO2催化剂可以有效降解水体中的喹诺酮,随着反应时间的增加,3种喹诺酮浓度逐渐降低。当溶液只用紫外灯照射时,反应80 min,诺氟沙星降解率为39.81%,盐酸环丙沙星降解率为27.39%,盐酸洛美沙星降解率为96.28%;而在自然光下只添加TiO2,反应80 min时,诺氟沙星降解率为34.61%,盐酸环丙沙星降解率为42.01%,盐酸洛美沙星降解率为50.66%。但是,同时用紫外照射并添加TiO2时喹诺酮降解率大大增加,诺氟沙星、环丙沙星和洛美沙星降解率分别为92.51%,90.33%和99.44%。这说明溶液中加入催化剂TiO2会明显促进光降解反应的进行。
2.2 喹诺酮类抗生素的光催化降解动力学特征
3种喹诺酮类抗生素的TiO2光催化降解反应过程均符合假一级反应动力学方程(表2),其反应速率常数(k)在0.044 5~0.071 0 之间,半衰期(t1/2)在9.76~15.57 min之间,与文献[25]对环丙沙星的光催化降解研究结果相近。因此,3种喹诺酮类化合物之间,洛美沙星较易被降解,其次是环丙沙星,而诺氟沙星相对较难降解。各化合物均是在光催化反应前30 min降解速率较快,之后降解速率趋缓。光催化速率随着反应时间而减小可能与降解所形成的中间产物竞争溶液中的氧化剂有关[26]。
2.3 TiO2用量对光催化降解的影响
在抗生素初始质量浓度为10 mg/L、pH为3条件下,TiO2用量对喹诺酮类抗生素光催化降解的影响见图2。从图2可见:TiO2用量为1.0和1.5 g/L时,对3种喹诺酮类抗生素的光催化降解效果相当,在50 min反应时间内,3种喹诺酮类抗生素的降解率均达到92%以上,均明显高于TiO2质量浓度分别为0.5 g/L和3.0 g/L时的降解效果。溶液中投加TiO2提高了紫外光的吸收和增加了可利用光敏位点,促进更多氧化物质形成,如羟基自由基的数量增加,从而加快光催化氧化的反应速度[22]。但当TiO2用量超过一定值时,其粒子产生一定的遮蔽作用, 并导致光散射现象[27],从而使喹诺酮类抗生素的降解率下降。关于有机污染物的光催化降解,通常存在催化剂用量的最优值,与催化剂和污染物的性质以及光化学反应器的性能有 关[28]。本实验确定TiO2催化剂的最佳用量为1.0 g/L。
2.4 抗生素初始质量浓度对光催化降解的影响
在TiO2质量浓度为1.0 g/L和pH=3条件下,喹诺酮类抗生素初始质量浓度对其光催化降解的影响如图3所示。从图3可见:3种喹诺酮类抗生素均是在较低初始质量浓度时具有较高的降解率;当初始质量浓度低于10 mg/L时,喹诺酮类抗生素的降解率与初始质量浓度成正相关;而当初始质量浓度高于20 mg/L时,喹诺酮类抗生素的降解率与初始质量浓度成负相关。当光催化降解反应30 min后,初始质量浓度低于10 mg/L时的降解率均达到80%以上,特别是洛美沙星,其降解率达到95%以上;而初始质量浓度高于20 mg/L时,诺氟沙星和环丙沙星的降解率均低于48%;当光催化降解反应80 min后,初始质量浓度低于10 mg/L时的降解率均达到97%以上;而初始质量浓度高于20 mg/L时,诺氟沙星和环丙沙星的降解率均低于73%。但初始质量浓度为50 mg/L时洛美沙星的降解率也达到90%以上。当初始质量浓度较低时,TiO2表面的吸附位点未达到充分饱和,初始质量浓度增大有利于TiO2与喹诺酮类化合物相互接触反应,从而提高其降解率。
表2 喹诺酮类抗生素的光催化降解动力学参数
Table 2 Photolysis kinetic parameters of quinolone anbiotics in water
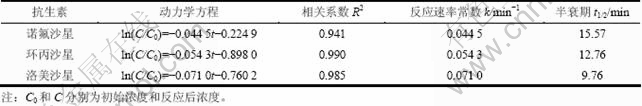
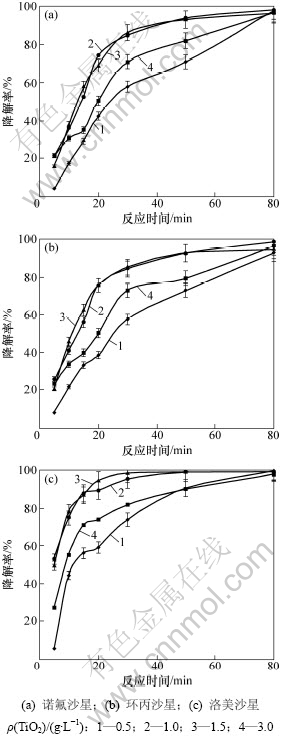
图2 TiO2用量对喹诺酮类抗生素光催化降解的影响
Fig.2 Effect of TiO2 dosage on photodegradation of quinolone antibiotics in water
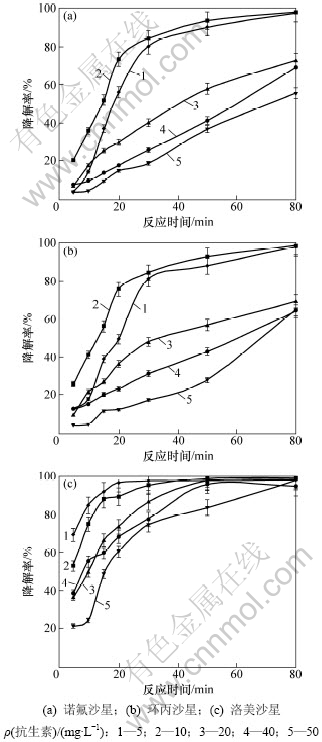
图3 喹诺酮类抗生素初始质量浓度对其光催化降解的影响
Fig.3 Effect of initial concentration on photodegradation of quinolone antibiotics in water
2.5 pH对光催化降解的影响
在TiO2用量为1.0 g/L,抗生素初始质量浓度为10 mg/L时,pH对喹诺酮类抗生素光催化降解的影响见图4。从图4可见:在中性条件下喹诺酮类抗生素的光催化降解效果最好,越偏离中性条件降解效果越差,但在偏酸性条件下的降解效果优于偏碱性条件;当pH为7时,光催化反应30 min后3种喹诺酮类抗生素的降解率均达90%以上;当pH小于5或大于9时,光催化反应30 min后3种喹诺酮类抗生素的降解率均在85%以下。Torniainen等[29]在pH为7、抗生素初始浓度为5×10-3 mol/L,反应90 min后获得环丙沙星的光催化降解率为98.0%。pH对光催化降解效果的影响主要是改变TiO2表面电荷及有机物的质子化状态[30]。TiO2的零电荷位点约为6.5,在碱性条件下呈负价,使正价态有机物更易进行光催化降解。但在酸性条件下,正价的TiO2使负价态有机物更易进行光催化降解。喹诺酮类化合物分子是中性基团,在中性条件下更易发生质子化反应,从而具有较高的光活性,更易进行光催化降解。另外,光催化降解反应会产生羟基自由基作为主要活性物质,而pH会影响羟基自由基的生成,使光催化降解反应的效率发生改变[31]。
2.6 光催化降解喹诺酮类抗生素的矿化特征
有机物光催化降解过程中的矿化特征通常以总有机碳(TOC)质量浓度来表示。当TOC质量浓度越低,表明矿化程度越高,降解越彻底当。TiO2用量为1.0 g/L,抗生素初始质量浓度为10 mg/L,pH=7时,喹诺酮类抗生素混合溶液光催化降解过程中的矿化特征见图5。喹诺酮类抗生素光催化降解反应前10 min,TOC质量浓度迅速降低,并于20 min时降到最低值(154.86 mg/L)。但随着反应时间的延长,TOC质量浓度逐渐增大,并于30 min时达到最高值(164.94 mg/L),之后TOC质量浓度又迅速下降。30 min后,TOC质量浓度降低逐渐减缓,反应至80 min时,降到反应的最低值(120.06 mg/L)。这可能是由于喹诺酮类抗生素光催化降解产生的一系列中间产物之间相互发生反应,生成新的有机物,导致TOC质量浓度逐渐增大。研究表明:喹啉羧酸类喹诺酮抗菌药具有光降解和光聚合2种特性[32]。在光催化降解反应初期,有机物的降解物多为其衍生物且发生多次降解现象,在后期则形成聚合物,可能是光促反应的次级反应[33]。Paul 等[26]利用可见光进行环丙沙星的光催化降解实验,在3 h内TOC质量浓度基本不变,说明环丙沙星的降解不彻底。因此,紫外光更有利于光催化降解喹诺酮类抗生素的矿化过程。
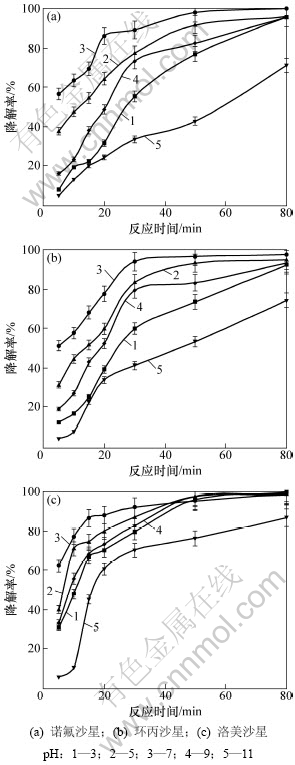
图4 pH对喹诺酮类抗生素光催化降解的影响
Fig.4 Effect of pH on photodegradation of quinolone antibiotics in water
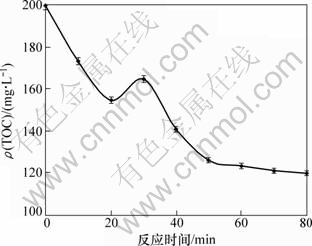
图5 喹诺酮类抗生素混合溶液光催化降解过程中的矿化特征
Fig.5 Mineralization characteristics of quinolone antibiotics in water in process of photodegradation
2.7 水质对喹诺酮类抗生素光催化降解的影响
当TiO2用量为1.0 g/L、抗生素初始质量浓度为10 mg/L,pH=7时,水质对喹诺酮类抗生素光催化降解的影响见图6。从图6可见:诺氟沙星和环丙沙星的降解率从高到低均为高纯水、河水、自来水,而洛美沙星的降解率从高到低则为河水、自来水、高纯水。河水的水质较差,一方面较高的浊度使紫外光发生散射、反射,不利于目标化合物的光催化降解[34];而另一方面,水溶性有机物吸收光子形成一系列较稳定的光氧化剂(如H2O2),腐殖质吸收光子产生激发态分子和活性氧等活性中间体,从而促进目标化合物的光催化降解[35]。自来水中含有Cl-等卤素离子,因其重原子效应而降低目标化合物的光降解效果[36]。但对于洛美沙星,Cl-参与并促进了其光降解反应过程,而对于诺氟沙星和环丙沙星则没有相应的作用[32]。
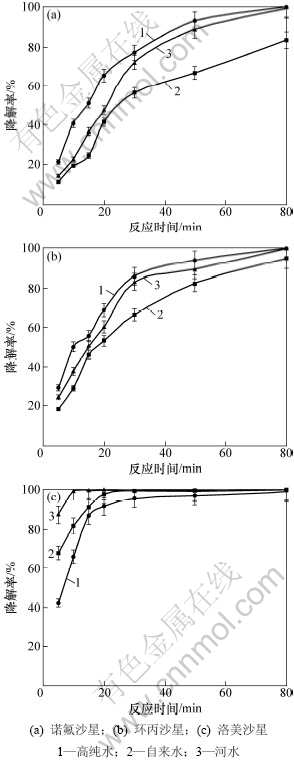
图6 水质对喹诺酮类抗生素光催化降解的影响
Fig.6 Effect of water quality on photodegradation of quinolone antibiotics in water
3 结论
(1) 光催化降解反应符合假一级反应动力学方程,反应速率常数在0.044 5~0.071 0 之间,半衰期在9.76~15.57 min之间,降解难易程度从高到低为诺氟沙星,环丙沙星,洛美沙星。
(2) 在中性条件下喹诺酮类抗生素的光催化降解效果最好,越偏离中性条件降解效果越差,但在偏酸性条件下的降解效果优于偏碱性条件下的降解效果。当优化参数为TiO2用量1.0 g/L,初始质量浓度10 mg/L,pH=7时,反应80 min,3种喹诺酮类抗生素的降解率均在95%以上。不同水质中诺氟沙星、环丙沙星的降解率从高到低的顺序为高纯水、河水、自来水,而洛美沙星的降解率从高到低的顺序为河水、自来水、高纯水。
参考文献:
[1] Haque M M, Muneer M. Photodegradation of norfloxacin in aqueous suspensions of titanium dioxide[J]. Journal of Hazardous Materials, 2007, 145: 51-57.
[2] Karthikeyan K G, Meyer Michael T. Occurrence of antibiotics in wastewater treatment facilities in Wisconsin, USA[J]. Science of the Total Environment, 2006, 361: 196-207.
[3] Lindberg R H, Wennberg P, JohanssonM I, et al. Screening of human antibiotic substances and determination of weekly mass flows in five sewage treatment plants in Sweden[J]. Environmental Science and Technology, 2005, 39: 3421-3429.
[4] Nakata H, Kanaan K, Jones P D, et al. Determination of fluoroquinolone antibiotics in wastewater effluents by liquid chromatography-mass spectroscopy and fluorescence detection[J]. Chemosphere, 2005, 58: 759-766.
[5] Rooklidge S J. Environmental antimicrobial contamination from terraccumulation and diffuse pollution pathways[J]. Science of the Total Environment, 2004, 325: 1-13.
[6] Akmehmet B I, Otker M. Treatment of pharmaceutical wastewater containing antibiotics by O3 and O3 /H2O2 processes[J]. Chemosphere, 2003, 50: 85-95.
[7] Ma W, Yang M, Wang J, et al. Treatment of antibiotics wastewater utilizing successive hydrolysis, denitrification and nitrification[J]. Environmental Technology, 2002, 23: 685-941.
[8] Thomas H. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review in recent research data[J]. Toxicology Letters, 2002, 131: 5-17.
[9] Campagnolo E R, Johnson K R, Karpati A, et al. Antimicrobial residues in animal waste and water resources proximal to large scale swine and poultry feeding operations[J]. Science of the Total Environment, 2002, 299: 89-95.
[10] Koplin D W. Pharmaceuticals, hormones, and other organic wastewater contaminates in U. S. streams, 1999-2000: A National Reconnaissance[J]. Environmental Science and Technology, 2002, 36(6): 1202-1211.
[11] Frank S, Frank T L Heinz J B, et al. Pharmaceuticals in groundwater analytical methods and results of a monitoring program in baden wurttemberg, Germany[J]. Journal of Chromatography A, 2001, 938: 199-210.
[12] Volmer D A, Mansoori B, Locke S J. Study of 4-quinoloneantibiotics in biological samples by short column liquid chromatography coupled with electro spray ionization tandem mass spectrometry[J]. Analytical Chemistry, 1997, 69(9): 4143-4155.
[13] Hartmann A, Alder A C, Koller T, et al. Identification of fluoroquinolone antibiotics as the main source of genotoxicity in native hospital wastewater[J]. Environmental Toxicology and Chemistry, 1998, 17: 377-382.
[14] 李红, 胥学鹏, 王英臣. 二氧化钛纳米微粒的制备及对染料甲基紫的降解[J]. 环境保护科学, 2008, 34(4): 28-30.
LI Hong, Xu Xue-peng, Wang Ying-cheng. Preparation of TiO2 nano particles and the photocatalytic degradation on methyl violet[J]. Environmental Protection Science, 2008, 34(4): 28-30.
[15] Kim S, Choi W. Visible-light-induced photocatalytic degradation of 4-chlorophenol and phenolic compounds in aqueous suspension of pure titania: Demonstrating the existence of a surface-complex-mediated path[J]. Physical Chemistry B, 2005, 109: 5143-5149.
[16] Budaia M, Grofb P, Zimmerc A, et al. UV light induced photodegradation of liposome encapsulated fluoroquinolones: An MS study[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2008, 198: 268-273.
[17] Vasconcelos TG, Kummerer K, Henriques DM, et al. Ciprofloxacin in hospital effluent: Degradation by ozone and photoprocesses[J]. Journal of Hazardous Materials, 2010, 169(1/3): 1154-1158.
[18] Araki T, Kawai Y, Ohta I, et al. Photochemical behavior of sitafloxacin, fluoroquinolone antibiotic, in an aqueous solution[J]. Chemical and Pharmaceutical Bulletin, 2002, 50(2): 229-234.
[19] Ziemianska J, Adamek E, Sobczak A, et al. The study of photocatalytic degradation of sulfonamides applied to municipal wastewater[J]. Physicochemical Problems of Mineral Processing, 2010, 45: 127-140.
[20] Alatrache A, Laoufi N A, Pons M N, et al. Tylosin abatement in water by photocatalytic process[J]. Water science and technology, 2010, 62(2): 435-441.
[21] Knapp C W, Cardoza L A, Hawes J N. Fate and Effects of enrofloxacin in aquatic systems under different light conditions[J]. Environmental Science and Technology, 2005, 39: 9140-9146.
[22] 葛林科, 陈景文, 张思玉, 等. 水中氟喹诺酮类抗生素加替沙星的光降解[J]. 科学通报, 2010, 55(11): 996-1001.
GE Lin-ke, CHEN Jing-wen, ZHANG Si-yu et al. Photodegradation of fluoroquinolone antibiotic gatifloxacin in aqueous solutions[J]. Chinese Science Bulletin, 2010, 55(11): 996-1001.
[23] 肖健, 刘林梅, 邹世春. 水环境中红霉素和罗红霉素抗生素光降解的研究[J]. 广州化学, 2008, 33(2): 1-12.
XIAO Jian, LIU Lin-hai, ZOU Shi-chun. Photodegradation behavior of representative macrolide antibiotics in water environment [J]. Guangzhou Chemistry, 2008, 33(2): 1-12.
[24] 赵伟荣. 阳离子红X-GRL染料的UV、O3、O3/UV 氧化处理研究[D]. 杭州: 浙江大学材料与化学工程学院, 2004: 67-70.
ZHAO Wei-rong. UV, O3, O3/UV oxidation of Cationic Red X-GRL dyes[D]. Hangzhou: Zhejiang University. School of Materials and Chemical Engineering, 2004: 67-70.
[25] JIAO Shao-jun, ZHENG Shou-rong, YIN Da-qiang, et al. Aqueous oxytetracycline degradation and the toxicity change of degradation compounds in photo irradiation process[J]. Journal of Environmental Sciences, 2008, 20: 806-813.
[26] Paul T, Miller P L, Strathmann T J, et al. Visible-light-mediated TiO2 photocatalysis of fluoroquinolone antibacterial agents [J]. Environment Science and Technology, 2007, 41: 4720-4727.
[27] Parra S, Olivero J, Pulgarin C. Relationships between physicochemical properties and photoreactivity of four biorecalcitrant phenylurea herbicides in aqueous TiO2 suspension[J]. Applied Catalysis B: Environment, 2002, 36(1): 75-85.
[28] Curco D, Gimenez J, Addardak A, et al. Effects of radiation absorption and catalyst concentration on the photocatalytic degradation of pollutants[J]. Catalysis Today, 2002, 76(2): 177-188.
[29] Torniainen K, Tammilehto S, Ulvi V. The effect of pH, buffer type and drug concentration on the photodegradation of ciprofloxacin[J]. International Journal of Pharmaceutics, 1996, 132: 53-61.
[30] Boreen A L, Arnold W A, McNeill K. Photochemical fate of sulfa drugs in the aquatic environment: Sulfa drugs containing five-membered heterocyclic groups[J]. Environment Science and Technology, 2004, 38: 3933-3940.
[31] 姜艳玲, 王九思, 孔爱平, 等. 用复合固体超强酸SO42-/TiO2- Fe2O3催化剂光降解甲基橙[J]. 石化技术与应用, 2009, 27(1): 28-31.
JIANG Yan-ling, WANG Jiu-si, KONG Ai-ping, et al. Photocatalytic degradation solid superacid of methyl orange by composite SO42-/TiO2-Fe2O3[J]. Petrochemical Technology & Application, 2009, 27(1): 28-31
[32] 胡昌勤, 杨亚莉. 喹诺酮类药物的光促反应特征[J]. 国外医药:抗生素分册, 2001, 22(6): 259-261.
HU Chang-qin, YANG Ya-li. Photocatalysis properties of fluoroquinolone antibacterial[J]. World Notes on Foreign Pharmaceutical Antibiotics, 2001, 22(6): 259-261.
[33] 白政忠, 张秋生, 盛龙生. 喹诺酮类抗菌药光降解动力学及光降解物研究进展[J] .中国药事, 2000, 14(5): 322-325.
BAI Zhong-zheng, ZHANG Qiu-sheng, SHENG Long-sheng. Review of fluoroquinolone antibiotics photodegradation and the metabolites[J]. Chinese Pharmaceutical Affairs, 2000, 14(5): 322-325.
[34] 徐冰冰, 陈忠林, 齐飞, 等. 紫外光降解水中痕量NDMA的效能研究[J]. 环境科学, 2008, 9(7): 1909-1913.
XU Bing-bing, CHEN Zhong-lin, QI Fei, et al. Efficiency of photodecomposition of trace NDMA in water by UV irradiation[J]. Environmental Science, 2008, 9(7): 1909-1913.
[35] 尤宏, 吴东海, 姚杰, 等. 水中硝基苯光降解研究[J]. 安全与环境学报, 2008, 8(2): 16-19.
YOU Hong, WU Dong-hai, YAO Jie, et al. Photo-degradation of the nitrobenzene in water[J]. Journal of Safety and Environment, 2008, 8(2): 16-19.
[36] 褚明杰, 岳永德, 花日茂, 等. 几种物质对苯噻草胺在水中光降解的影响[J]. 应用生态学报, 2006, 17(1): 155-158.
ZHU Ming-jie, YUE Yong-de, HUA Ri-mao, et al. Effects of dissolved compounds on photodegradation of mefenacet in water[J]. Chinese Journal of Applied Ecology, 2006, 17(1): 155-158.
(编辑 赵俊)
收稿日期:2011-08-25;修回日期:2011-10-30
基金项目:国家自然科学基金资助项目(41173101);广东省科技计划项目(2010B020311006);惠州市科技计划项目(2009B010001009);广东省高校高层次人才项目(2010-794);广州市科技计划项目(2010A82070466)
通信作者:莫测辉(1965-),男,广西柳州人,博士,教授,博士生导师,从事环境有机污染与控制研究;电话:15361778109;E-mail:tchmo@jnu.edu.cn