文章编号:1004-0609(2015)08-2226-10
水相环境中十二胺在石英及磁铁矿表面吸附的分子动力学模拟
刘 安1,樊民强1, 2
(1. 太原理工大学 矿业工程学院,太原 030024;
2. 太原理工大学 煤科学与技术教育部和山西省重点实验室,太原 030024)
摘 要:采用分子动力学模拟方法,研究周期性水环境下十二胺在石英及磁铁矿界面上的吸附过程。考虑到十二胺的水解电离平衡以及矿物表面荷电机理,建立弱酸、中性以及强碱性3种条件下十二胺与石英及磁铁矿的吸附模型。采用微量热仪测量十二胺在石英及磁铁矿表面吸附过程中的吸附热。模拟结果表明:中性环境有利于十二胺在矿物表面的吸附;弱酸性条件下十二胺不能吸附于磁铁矿表面;强碱性环境下十二胺与石英及磁铁矿的相互作用很弱。十二胺与石英作用的吸附焓大于十二胺与磁铁矿的,十二胺更易于吸附在石英表面,且十二胺在矿物表面的吸附为物理吸附。分子动力学模拟与吸附热试验从分子力学及热力学层面上揭示了浮选过程中十二胺对石英及磁铁矿选择性的差异的原因。
关键词:分子动力学模拟;十二胺;石英;磁铁矿;吸附热
中图分类号:TD923 文献标志码:A
Molecular dynamics simulations of dodecylamine adsorption on quartz and magnetite surfaces in aqueous solution
LIU An1, FAN Min-qiang1, 2
(1. College of Mining Engineering, Taiyuan University of Technology, Taiyuan 030024, China;
2. Key Laboratory of Coal Science and Technology of Ministry of Education and Shanxi Province,
Taiyuan University of Technology, Taiyuan 030024, China)
Abstract: The adsorption process of dodecylamine (DDA) on quartz and magnetite surfaces at a molecular level was studied by molecular dynamics (MD) simulations method. In the consideration of hydrolysis ionization equilibrium and surface charge mechanism of DDA, a model close to the realistic flotation system was established to simulate the adsorption behavior under the weak acid, neutral and strong alkali conditions. The adsorption heat in the adsorption process of DDA on quartz and magnetite surface was measured by micro calorimeter. The results indicate that the optimum condition for DDA adsorption on mineral surface is at neutral environment; it is difficult for DDA adsorption on magnetite at weak acid environment; and the interaction of DDA and mineral is weak under strong alkali condition. The interaction energy of DDA and quartz is higher than that of DDA and magnetite. DDA is preferred to adsorb on the surface of quartz, and the adsorption process is physical adsorption. The selectivity phenomenon in molecular mechanics and thermodynamics level was explained by MD simulation and microcalorimetry measurements experiment.
Key words: molecular dynamics simulation; dodecylamine; quartz; magnetite; adsorption heat
随着浮选理论研究的发展,捕收剂吸附机理的研究迫切要求从静态向动态,从小体系向纳米、介观尺度过渡。分子动力学(MD)模拟是解决此类问题的最佳途径。分子动力学模拟是通过计算机建立在原子水平上的分子模型,用以模拟分子及反应体系的结构和行为,与量子化(QM)方法相比,其独有的 MD 约束技术和 SMART 结构优化方法集中了最陡下降法、共轭梯度法及牛顿法的优点。因此,分子动力学模拟有计算量小的优点,适用于大体系的模拟。
众多的学者通过分子动力学模拟研究过真空中捕收剂在矿物表面的吸附过程[1-9]。LIU等[1]通过分子动力学模拟了季胺盐捕收剂(DRN, DEN及DPN)在高岭石(001)面的吸附过程,对比了亲固原子N原子与矿物表面的距离以及捕收剂与矿物间的静电力,揭示了药剂与矿物的作用规律;孙伟所在课题组[2-5]以分子动力学模拟为手段结合浮选试验,研究了石煤提钒、镍钼矿中回收氧化钼、季铵盐类捕收剂在铝土矿反浮中浮选行为和捕收剂分子吸附机理,模拟结果为宏观试验结果提供了理论依据;杨志超等[6]通过分子动力学模拟考察了菱镁矿浮选过程中调整剂水玻璃以及六偏磷酸钠的活化效果,构建了活化后的矿物表面,模拟结果表明水玻璃是更为有效的调整剂,证实了浮选试验结果;RAI等[7-8]采用UFF力场模拟了羟肟酸盐捕收剂与稀土矿物、含钙矿物的作用,所得结果表明模拟趋势与实验观察到的结果相当吻合;陶坤等[9]从分子动力学模拟的角度研究了有机抑制剂BKY-1 在黄铜矿、黄铁矿表面的吸附,模拟结果表明相互作用能的差异是导致BKY-1选择性抑制的原因。
石英是铁矿选矿中最主要的脉石矿物,阳离子反浮选脱硅工艺已被证明是一种有效提铁降硅工艺[10]。浮选中常用的阳离子捕收剂主要是脂肪胺及其衍生 物[11],研究人员通过原子力显微镜、接触角测量、Zeta电位测量以及红外光谱等技术手段研究了阳离子捕收剂在石英表面的吸附机理,研究认为阳离子捕收剂是通过静电吸引力作用吸附在荷负电的石英表面,并且这一过程属于物理吸附,是一种可逆的非特性吸附过程[12-14]。分子模拟在铁矿浮选中的研究尚在起步阶段,SWAGAT等[15]通过分子动力学模拟对比了油酸在磁铁矿、赤铁矿及针铁矿表面的吸附规律,WENG等[16]通过分子动力学模拟考察了酯基季胺盐捕收剂(M302)在磁铁矿、石英表面的吸附行为。
总的来说,分子动力学模拟药剂与矿物作用的过程能够预测矿物实际浮选行为,研究者通常模拟的是真空条件下药剂与矿物的相互作用,也得到了许多有意义的结论。但是实际矿物浮选是在矿浆中进行的,溶剂化效应必然导致矿物表面荷电,药剂的赋存状态也与矿浆的pH相关,所以模拟水相环境中捕收剂与矿物的吸附过程更接近于真实的吸附过程。
本文作者以石英及磁铁矿作为研究对象,借助分子动力学模拟手段,考虑到溶剂化效应,模拟了水相环境中典型的阳离子捕收剂十二胺与矿物作用的微观过程,并通过微量热仪测量了十二胺在石英及磁铁矿表面吸附过程中的吸附热,从分子力学及热力学层面揭示了捕收剂在矿物表面的吸附机理。
1 实验
1.1 矿物样品及试剂
实验所用的纯石英取自河北灵寿,纯度为99.05%。将纯矿物破碎,用瓷磨机磨细至粒径小于74 μm的颗粒占90%以上。实验所用的纯磁铁矿取自太钢尖山铁矿,品位为71.05%,该矿物的粒度较细,粒径小于38 μm的颗粒含量高达80%。
实验所用的捕收剂为十二胺(DDA),pH调整剂为分析纯盐酸(HCl)和氢氧化钠(NaOH)。十二胺盐酸盐溶液是将等摩尔的盐酸与十二胺混合配制而成,该捕收剂的浓度为1%。为了减小粒度效应对吸附热测试结果的影响,实验过程中采用的是窄粒级的纯矿物,石英纯矿物粒径在30~40 μm范围内,比表面积为0.2225 m2/g;磁铁矿纯矿物粒径在20~30 μm范围内,比表面积为0.2637 m2/g。
1.2 实验方法
1.2.1 浮选实验
单矿物浮选实验在XFGⅡ型挂槽式浮选机上进行,主轴转速为1500 r/min。每次称取20 g矿物加入100 mL浮选槽中,搅拌调浆时间为2 min;加入捕收剂之后的调浆时间为3 min,浮选时间为5 min。
1.2.2 Zeta电位测试
Zeta电位测试是采用的Zeta Plus电位分析仪对矿物吸附药剂前后动电位的变化进行测量。测试前将矿物磨至粒径为5 μm以下,以1×10-3 mol/L KCl水溶液做支持电解质,将捕收剂配置成一定浓度的溶液,加入纯矿物将其配置成0.01%(质量分数)的悬浮液,采用HCl与NaOH溶液调pH值,每个试样测定3次取平均值。
1.2.3 分子模拟
矿物原胞以及解理面的表面能计算采用的是Materials Studio 4.4 软件中的CASTEP 模块包对矿物晶体截面进行优化计算,计算中以广义梯度近似法GGA-PBESOL 为交换相关函数。平面波截至能量Energy cutoff为260 eV,自洽场收敛精度为1×10-6。磁铁矿的(111)、(001)和(110)面的表面能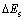 的表达式如式(1)所示[15]:
的表达式如式(1)所示[15]:
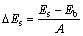 (1)
(1)
式中:Es为切割解理面后磁铁矿的总能量;Eb为相同数目(磁铁矿解离面)磁铁矿原胞的总能量;A为解理面的表面积。
采用分子动力学模拟方法研究捕收剂分子与矿物界面的相互作用。模拟过程中采用周期性边界条件,计算模型选用二层结构模型,构建过程如下:首先,构建矿物表面,选择磁铁矿解理面(1 1 1)面和石英的 (001)面为研究对象,模拟过程中冻结矿物表面体系中的所有原子;其次,利用Amorphous Cell 模块构建溶剂层(包含多个水分子和1 个捕收剂分子)用于研究单分子的吸附行为。分子动力学模拟采用的是Materials Studio 软件中的DISCOVER模块,力场为COMPASS 力场,通过DISCOVER模块下的Smart Minimizer 方法对体系进行优化,采用正则系综( NVT)进行分子动力学模拟。模拟温度为298 K,各分子起始速度由Maxwell-Boltzmann 分布随机产生,牛顿运动方程的求解建立在周期性边界条件、时间平均等效于系综平均等基本假设之上,运用Velocity Verlet 法进行积分求和。计算体系非键作用时,采用Atom based 方法计算Van der Waals,截断半径为9.5 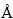 ,截断距离之外的分子间相互作用按平均密度近似方法进行校正;库仑相互作用采用Ewald算法,Ewald 加和方法比较适合于结晶固体。模拟时间为500 ps,步长为1.0 fs。
,截断距离之外的分子间相互作用按平均密度近似方法进行校正;库仑相互作用采用Ewald算法,Ewald 加和方法比较适合于结晶固体。模拟时间为500 ps,步长为1.0 fs。
矿物与药剂的相互作用能Ei如式(2)所示[17]:
Ei=Et-(Ef +Ec) (2)
式中:Et为矿物与药剂优化之后的总能量;Ef和Ec分别为矿物表面自由能以及药剂分子的能量。值得指出的是,相互作用能负值越大表明药剂越容易吸附在矿物表面。
1.2.4 吸附热测试
药剂与矿物作用过程中的吸附热测试采用的是Setaram C80型微量热仪,测量了在不同pH下十二胺与石英以及磁铁矿纯矿物的吸附热。试验中称取100 mg矿样置于量热池下部,在混合池的上部加入2 mL的待测捕收剂溶液,用聚四氟乙烯薄膜来分隔矿物与捕收剂溶液。待量热计基线完全稳定后,通过移动杆划破薄膜使捕收剂溶液进入量热池与矿物作用。每次试验会产生一个热效应Qr,扣除了相应的润湿热效应Qw(矿物与纯溶剂作用的热效应),即得到吸附过程中的吸附热。当吸附平衡后,测量上清液的的浓度ceq以及药剂的吸附量nads。
根据文献[18]可知,在pH为4.3的HAc-NaAc缓冲溶液中,阳离子表面活性剂能与曙红Y通过静电、疏水作用和荷电转移形成离子缔合物,使曙红Y溶液褪色。试验采用的是尤尼柯UV-2012紫外分光光度计,在516 nm波长处,以曙红Y溶液为参比液,测定不同浓度的十二胺和曙红Y的吸光度。
药剂吸附量采用式(3)计算[17]:
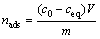 (3)
(3)
式中:nads为单位质量矿物所吸附的十二胺的量;c0为捕收剂的初始浓度;ceq为吸附之后的捕收剂浓度;V为捕收剂的体积;m为矿物的质量。
2 结果与讨论
2.1 矿物表面荷电机理及磁铁矿和石英的浮选行为
图1所示为氧化矿在水中的荷电机理[19]。矿物受力破裂后,磁铁矿的解理面暴露出大量的铁原子和氧原子,石英的断裂面出现硅原子和氧原子,断口上有残留的共价键,具有较强的亲水性。根据矿物表面荷电机理[19],大部分氧化矿和硅酸盐矿物在水中首先形成羟基化表面(M—OH),在不同pH下,矿物表面H+的吸附或解离,发生质子化或去质子化反应,进一步形成质子化面(M—OH2+)或去质子化面(M—O-),使得表面荷正电或负电。文献[20]表明磁铁矿的零电点在pH为6左右,石英的零电点在pH为3左右[21]。这说明当溶液pH小于6时,磁铁矿表面荷正电;当pH大于6时,磁铁矿表面荷负电;当矿浆pH大于3时,石英表面荷负电,当pH小于3时,石英表面荷正电。
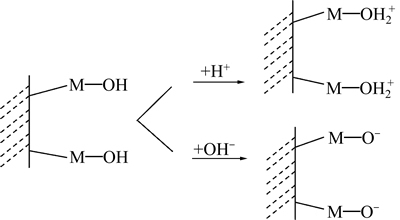
图1 矿物表面荷电机理
Fig. 1 Charging mechanism of mineral edges
十二胺是反浮选脱硅工艺中典型的阳离子捕收剂,根据WANG等[22]计算的 DDA 在水溶液中的水解平衡常数(见式(4)~(7)),得到DDA在水溶液中各组分的摩尔分配系数(见图2)。
RNH2(s) = RNH2(aq)
S=1×10-4.69 (4)
RNH3+ = RNH2(aq)+H+
Ka=c(H+)·c(RNH2(aq))/c(RNH3+)=1×10-10.63 (5)
2RNH3+ = (RNH3)22+
Kd= c((RNH3)22+)/c(RNH3+)2=1×102.08 (6)
RNH3++RNH2(aq) = [RNH3+·RNH2(aq)],
Kim=c[RNH3+·RNH2(aq)]/(c(RNH3+)c(RNH2(aq)))
=1×103.12 (7)
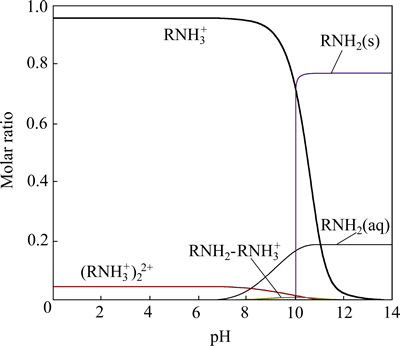
图2 水溶液中十二胺各组分的摩尔浓度分配系数
Fig. 2 Species distribution diagram of DDA as function of pH in solution (cDDA=1.0×10-4 mol/L)
由图2可知,当 pH<9.0 时,溶液中主要为带正电的C12H25NH3+ 组分;当 9.0<pH<12.0时,十二胺溶液中的主要组分是C12H25NH2 分子,同时溶液中出现大量C12H25NH2分子沉淀;当8.0<pH<11.0时,有极少量的C12H25NH2·C12H25NH3+存在;而在pH<11.0的广泛范围内有少量的(C12H25NH3+)22+ 存在。
图3所示为石英以及磁铁矿与DDA作用前后的ζ电位与溶液pH的关系。在不加浮选药剂条件下,石英的等电点在pH为3 附近,说明在pH为3~12的广泛范围内,石英的表面都带负电,易于与带正电的阳离子药剂吸附;而磁铁矿的等电点则在pH为5左右,即在pH为5~12的范围内,磁铁矿都带负电,也可以通过静电引力与阳离子捕收剂作用。矿浆pH会影响矿物表面荷电性质及捕收剂的组分。图4所示为pH对矿物浮选行为的影响。从图4可知,强酸及强碱环境下石英和磁铁矿的回收率均很低,矿物最适宜的浮选环境为pH为6~9之间,在pH为6~9的范围内,石英的回收率均高于85%,而磁铁矿的回收率接近30%。
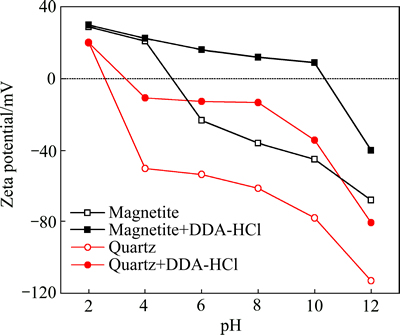
图3 石英与磁铁矿在DDA水溶液中ζ电位与溶液pH值的关系
Fig. 3 Relationship between zeta potential of quartz and magnetite and pH in presence of DDA (cDDA=1.0×10-4 mol/L)
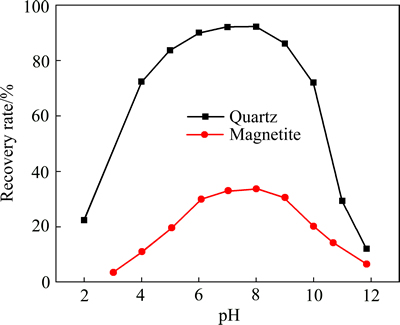
图4 pH对石英和磁铁矿矿物可浮性的影响
Fig. 4 Effect of pH on flotation recovery of quartz and magnetite (cDDA=1.0×10-4 mol/L)
阳离子反浮选脱硅工艺中,浮选矿浆pH值一般控制在中性或弱碱性条件下,在这个范围内,石英表面荷负电,而磁铁矿表面同样荷负电,从静电力作用物理吸附的角度看,在此pH范围内,药剂的选择性会很弱,事实上石英会优先上浮(见图4和5),磁铁矿也会上浮但其上浮所需要的药剂量远高于石英所需的,为了研究造成这种选择性的差异的原因,在分子力学及热力学层面探讨了十二胺与石英及磁铁矿相互作用的强弱。
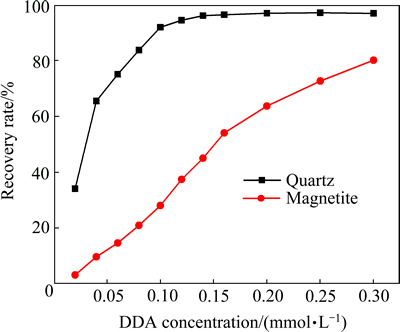
图5 自然pH下石英和磁铁矿浮选回收率与十二胺浓度的关系
Fig. 5 Relationship between recovery of quartz and magnetite and DDA concentration at natural pH
2.2 十二胺在石英及磁铁矿表面吸附的分子动力学模拟
模拟捕收剂分子在矿物表面吸附过程的第一步是选择最为稳定的解理面作为药剂的吸附面。石英晶体属于三方晶系,每个硅原子与周围的4个氧原子都以原子键结合,4个单键分别连接4个氧原子构成硅氧四面体,硅与周围的4个氧原子都以原子键结合,且各方向的键力相等,具有各向同性的结构特点,本研究中选择石英的(001)面与捕收剂作用[23-24]。
十二胺与石英(001)面模拟过程中采用周期性边界条件,构建了一个尺寸为14.73 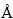 ×34.37
×34.37 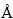 ×42.18
×42.18 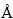 、包含矿物和1个捕收剂及420个水分子的水盒子。表1所列为模拟过程中各个条件下的原子数。从十二胺的浮选溶液化学平衡(见图2)以及石英矿物的荷电机理(见图1)可知,在水溶液中,无论是十二胺还是石英其赋存状态会随着矿浆的pH的改变而变化。从图2可以看出,酸性及中性环境下DDA主要以质子化的C12H25NH3+的形式存在,而在碱性条件下则以分子C12H25NH2形式存在。为了直观地描述这种变化以及客观反映矿浆中药剂及矿物的真实状态,模拟了弱酸、中性以及强碱性这3种环境下十二胺与石英的相互作用情况。为了描述矿物表面荷电效应,参照文献[25-26],在低pH的环境下矿物表面主要以Si—OH或Si—OH2+形式存在,在pH高的环境下矿物表面主要以Si—O-形式存在。具体而言,在pH为4时,矿物表面主要是羟基(Si—OH),矿物表面的电荷密度约为-2 μC/cm2,具体来说,表面有41个羟基以及1个裸露的氧原子,而捕收剂则以质子化状态形式存在;在pH为8时,矿物表面以裸露的氧原子(Si—O-)形式存在,捕收剂同样以质子状态存在;在pH为12时,矿物表面同样以裸露的氧原子形式存在,而捕收剂则以分子状态存在。
、包含矿物和1个捕收剂及420个水分子的水盒子。表1所列为模拟过程中各个条件下的原子数。从十二胺的浮选溶液化学平衡(见图2)以及石英矿物的荷电机理(见图1)可知,在水溶液中,无论是十二胺还是石英其赋存状态会随着矿浆的pH的改变而变化。从图2可以看出,酸性及中性环境下DDA主要以质子化的C12H25NH3+的形式存在,而在碱性条件下则以分子C12H25NH2形式存在。为了直观地描述这种变化以及客观反映矿浆中药剂及矿物的真实状态,模拟了弱酸、中性以及强碱性这3种环境下十二胺与石英的相互作用情况。为了描述矿物表面荷电效应,参照文献[25-26],在低pH的环境下矿物表面主要以Si—OH或Si—OH2+形式存在,在pH高的环境下矿物表面主要以Si—O-形式存在。具体而言,在pH为4时,矿物表面主要是羟基(Si—OH),矿物表面的电荷密度约为-2 μC/cm2,具体来说,表面有41个羟基以及1个裸露的氧原子,而捕收剂则以质子化状态形式存在;在pH为8时,矿物表面以裸露的氧原子(Si—O-)形式存在,捕收剂同样以质子状态存在;在pH为12时,矿物表面同样以裸露的氧原子形式存在,而捕收剂则以分子状态存在。
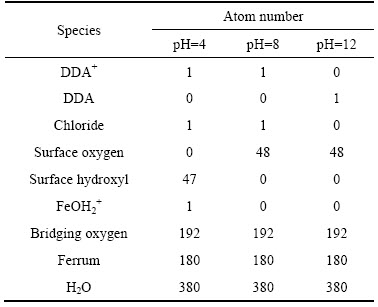
表1 DDA在石英表面吸附模拟过程中各组分的原子数
Table 1 Atom numbers of all species in simulation of DDA adsorption on quartz surface
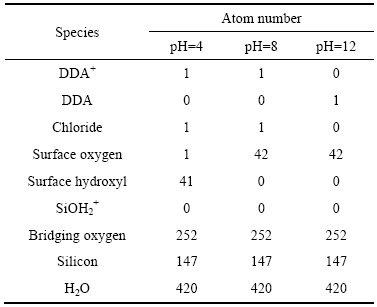
图6所示为经几何、能量优化和动力学模拟后,液相条件下捕收剂分子在石英(001)表面的平衡吸附构型。从图6中可以看出,捕收剂分子的极性头部(胺基)由于较强的化学反应活性比烷基链优先吸附于石英(001)表面,而烃基碳链在溶剂水的作用下发生扭转并偏离矿物表面以一定倾角指向溶剂。矿物的疏水化是通过吸附捕收剂以减少矿物与水分子的相互接触作用面而达到的,捕收剂的扭转程度越大,阻隔水分子与矿物作用的能力就越强。由此可知,对于石英而言,矿浆的pH为12时,捕收剂几乎是垂直矿物表面,而溶液的pH为4和8时,十二胺分子在水相中出现了不同程度的扭转现象,这说明强碱性条件下不利于十二胺与石英之间的相互作用。
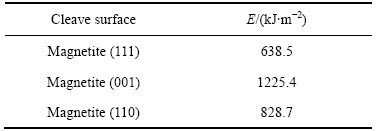
磁铁矿属等轴晶体系,为尖晶石结构的立方体矿物,氧离子形成面心立方体格架,Fe2+和Fe3+充填在晶格空隙之中,一个晶胞有4个小单元组成,在每个晶胞中,每个阳离子被4个O2-包围形成四面体结构。对于磁铁矿而言,有研究表明其(111)面最为稳定,也有研究指出其(001)面或(110)面更为稳定[27-28]。为了确定磁铁矿最为稳定的解理面,用CASTEP 模块GGA-PBESOL为交换相关函数计算了其(111),(001)以及(110)面的表面能。表2所列为磁铁矿解理面的表面能。由表2可知,各个解理面的表面自由能大小顺序为:E(111)<E(110)<E(001)。基于此,选择磁铁矿的(111)面作为与捕收剂作用的吸附面。
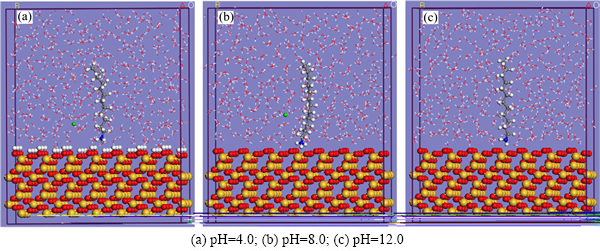
图6 不同pH的液相中DDA在石英(001)表面的平衡吸附构型
Fig. 6 Equilibrium configurations of DDA on quartz (001) surface in aqueous under different pH (Color representation is as follows: red, oxygen atoms; yellow, silicon; white, hydrogen atoms; blue, nitrogen atoms; light green, chloride ions; and gray, carbon atoms. For clarity, only skeletons of water molecules are shown.)
表2 磁铁矿解理面的表面能
Table 2 Surface energy of magnetite cleave plane
十二胺与磁铁矿(1 1 1)面模拟过程中采用周期性边界条件,构建了1个11.87  ×35.61
×35.61  ×40.54
×40.54  范围的包含矿物、1个捕收剂及380个水分子的水盒子。磁铁矿的零电点在pH为5左右,所以pH为4时矿物表面是荷正电的,磁铁矿表面应该会有少量的FeOH2+。表3所列为模拟过程中各个条件下各组分的原子数。图7所示为不同pH条件下捕收剂分子在磁铁矿(111)面的平衡吸附构型。从图7可以看出,在pH为4的条件下,捕收剂分子有远离矿物表面的趋势,并且捕收剂倾向于垂直吸附在磁铁矿表面,捕收剂在磁铁矿表面的扭转程度弱于石英的。
范围的包含矿物、1个捕收剂及380个水分子的水盒子。磁铁矿的零电点在pH为5左右,所以pH为4时矿物表面是荷正电的,磁铁矿表面应该会有少量的FeOH2+。表3所列为模拟过程中各个条件下各组分的原子数。图7所示为不同pH条件下捕收剂分子在磁铁矿(111)面的平衡吸附构型。从图7可以看出,在pH为4的条件下,捕收剂分子有远离矿物表面的趋势,并且捕收剂倾向于垂直吸附在磁铁矿表面,捕收剂在磁铁矿表面的扭转程度弱于石英的。
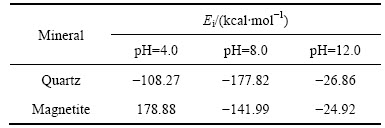
捕收剂分子与矿物之间相互作用能能够在一定程度上反映捕收性能的强弱,其绝对值越大,表明捕收剂与矿物表面的结合越稳定。表4所列为不同pH下矿物与捕收剂的相互作用能。由表4可知,十二胺与石英的相互作用能数据均为负值,表明捕收剂在石英(001)表面的吸附是体系放出热量、自由能降低的过程,即体系为稳定状态。此外,在不同矿浆环境下捕收剂与矿物之间的相互作用能顺序为:E(pH=12)<E(pH=4)<E(pH=8),而且pH为12时数值很小,说明在该条件下DDA不易吸附在矿物表面,这与实际情况相符(见图4)。而十二胺与磁铁矿作用的相互作用能小于石英的,表明十二胺更易于吸附在石英表面;此外,在pH为4时,相互作用能数值为正值,说明在这种情况下十二胺不会吸附在磁铁矿表面,它们之间的作用是需要外界提供能量的过程。
表3 DDA在磁铁矿表面吸附模拟过程中各组分的原子数
Table 3 Atom numbers of all species in simulation of DDA adsorption on magnetite surface
2.3 十二胺在石英及磁铁矿表面的吸附热测试
从模拟结果可知,十二胺更易于吸附于石英表面,为了研究实际情况中药剂与矿物之间的相互作用,采用微量热法测量了药剂吸附在矿物表面过程中体系与环境的热交换变化。众所周知,所有的物理、化学反应过程都伴随着热交换,恒压环境下反应过程中的焓变是一项描述反应过程释放或吸收热量的基本热力学参数[29]。
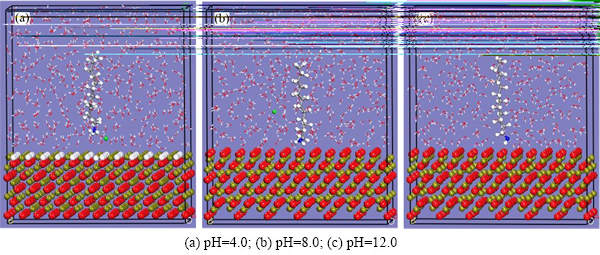
图7 不同pH的液相中DDA在磁铁矿(111)表面的平衡吸附构型
Fig. 7 Equilibrium configurations of DDA on magnetite (111) surface in aqueous under different pH (Color representation is as follows: red, oxygen atoms; yellow and green, iron atoms; white, hydrogen atoms; blue, nitrogen atoms; light green, chloride ions; and gray, carbon atoms. For clarity, only skeletons of water molecules are shown.)
表4 不同pH下矿物与捕收剂的相互作用能
Table 4 Values of interaction energies of mineral surfaces with collector at different pH
十二胺溶液与矿物之间的吸附过程与均质溶剂(水和乙醇等)的作用是不同的,这是因为十二胺溶液本身就是一个混合物(见图2),其中包括RNH3+,(RNH3)22+,RNH2·RNH3+和RNH2 分子,十二胺的赋存状态与溶液的pH紧密相关。吸附热试验所测得的热值实际上包括整个过程中的热变化,其中包括润湿热及吸附热,所以每次试验过程中会扣除纯溶剂(去离子水)的润湿热。通过测量吸附过程中的吸附热以及吸附量得到反应焓变(ΔadsH,kJ/mol)的计算公式如式(8)所示:
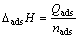 (8)
(8)
式中:Qads为吸附过程中的净能量变化,计算公式如式(9)所示:
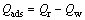 (9)
(9)
图8所示为十二胺在石英表面吸附过程中的典型微量热曲线。从图8中可知,十二胺达到吸附平衡的时间大约为60 min,不对称的曲线表明捕收剂溶液是非均质溶液,其热值是对该曲线的积分。吸附过程中的吸附焓变化反映了捕收剂与矿物作用的强弱,捕收剂的吸附量以及吸附过程中的能量变化与溶液的pH密切相关,研究了不同pH下十二胺与石英及磁铁矿作用过程的热变化。
表5所列为十二胺在石英及磁铁矿表面吸附过程中的吸附热及吸附量。由表5可知,所有的热力学参数(吸附热与吸附焓)均为负值,表明该过程为放热的过程,放热过程意味着矿物与捕收剂之间主要以吸引力为主,捕收剂的吸附过程是自发进行的;并且十二胺与石英作用的吸附焓大于磁铁矿的,由此可知石英与十二胺的作用程度强于磁铁矿的。
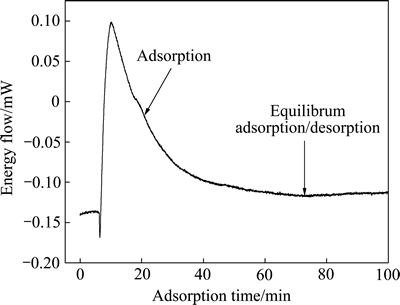
图8 十二胺在石英表面吸附过程中的典型微量热曲线
Fig. 8 Typical microcalorimetric plot for adsorption of DDA on quartz (pH=8.0, c0=5.0×10-2 mol/L, t=30 ℃)
表5 DDA与石英及磁铁矿作用的吸附热试验结果
Table 5 Calorimetric results of DDA adsorption on quartz and magnetite
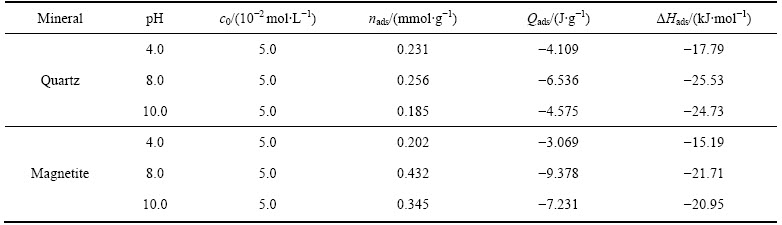
对于两种矿物而言,pH为4时的吸附热均小于pH为8以及pH为10条件下的,这是因为pH为4时矿物与药剂主要以氢键相互作用,这种N—H…O弱氢键的能量值小于20 kJ/mol[30],与模拟结果不同的是十二胺在pH为4时能够吸附在磁铁矿表面,这是因为模拟中并没有考虑到氢键作用。捕收剂在矿物表面的吸附分为物理吸附和化学吸附,反应过程中焓变可以作为区别物理或者化学吸附的度量,一般来说,焓变小于84 kJ/mol的过程为物理吸附[31],基于此可知十二胺在石英及磁铁矿表面的吸附过程均为物理吸附。此外,对于石英及磁铁矿而言,在pH为8.0时的吸附量均高于pH为4.0及pH为10.0时的吸附量,这是因为不同pH环境下阳离子捕收剂的赋存状态不同;在pH小于10时十二胺是以RNH3+的形式存在的,而在强碱性环境下十二胺以分子或沉淀状态形式存在(这是选择pH为10.0的原因,在强碱性环境下会产生沉淀),这种改变阻碍了其在矿物表面的吸附。对比十二胺在石英及磁铁矿表面的吸附量可知,在pH为8和10的条件下十二胺在磁铁矿表面的吸附量高于石英的,这说明在同样的捕收剂浓度下(试验条件是高浓度下),磁铁矿表面的吸附量是高于石英的,造成这种现象的原因是矿物的比表面积不同,磁铁矿的比表面积高于石英的。吸附过程中吸附量的大小并不能反映吸附能力的强弱,而焓判据是判断反应进行方向的判据之一,焓减小有利于反应自发,它是自发反应的一个内在推动力[29],基于此可知十二胺更易于吸附在石英表面。
3 结论
1) 以典型的阳离子捕收剂十二胺作为研究对象,通过单矿物的浮选试验,研究了石英及磁铁矿的浮选行为差异,结果表明矿物最适宜的分选环境为pH在6~9之间,并且石英的回收率始终高于磁铁矿的。
2) 采用分子动力学模拟方法研究十二胺与矿物界面的相互作用,建立了弱酸、中性以及强碱性三种环境下十二胺与石英及磁铁矿的吸附模型。结果表明:十二胺更易于吸附在石英表面;pH对矿物的吸附行为有显著的影响,在弱酸性条件下十二胺不能吸附于磁铁矿表面,中性环境有利于十二胺在石英及磁铁矿表面的吸附,在强碱性环境下十二胺与矿物的相互作用很弱。
3) 采用微量热法测量了十二胺在矿物表面吸附过程中的吸附热,以研究实际情况中药剂与矿物之间的相互作用。结果表明:捕收剂在矿物表面的吸附过程为放热的过程,药剂的吸附过程是自发进行的,十二胺与石英作用的吸附焓大于磁铁矿的,基于化学反应焓判据原则可知十二胺与石英的作用强于磁铁矿的。
REFERENCES
[1] LIU C M, FENG A S, GUO Z X, CAO X F, HU Y H. Dynamics simulation of tertiary amines adsorbing on kaolinite (001) plane[J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 1874-1879
[2] 孙 伟, 王 丽, 曹学锋, 刘润清, 徐龙华, 韩海生. 石煤提钒的浮选工艺及吸附机理[J]. 中国有色金属学报, 2012, 22(7): 2069-2074.
SUN Wei, WANG Li, CAO Xue-feng, LIU Rui-qing, XU Long-hua, HAN Hai-sheng. Flotation technology and adsorption mechanism of vanadium extraction from stone coal[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(7): 2069-2074.
[3] 岳 彤, 孙 伟, 陈 攀. 季铵盐类捕收剂对铝土矿反浮选的作用机理[J]. 中国有色金属学报, 2014, 24(11): 2872-2878.
YUE Tong, SUN Wei, CHEN Pan. Mechanism of reverse flotation desilication for bauxite by quaternary ammonium salt collector[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(11):2872-2878.
[4] 刘建东, 孙 伟. 捕收剂CSU-M对镍钼矿中氧化钼的浮选工艺及吸附机理[J]. 中南大学学报(自然科学版), 2014, 45(12): 4105-4110.
LIU Jian-dong, SUN Wei. Flotation technology and adsorption mechanism of collector CSU-M to molybdenum oxide in Ni-Mo ore[J]. Journal of Central South University (Science and Technology), 2014, 45(12): 4105-4110.
[5] 王 振, 孙 伟, 徐龙华, 肖军辉, 刘若华. CPC在氧化钼表面吸附行为及分子动力学模拟[J]. 中南大学学报(自然科学版), 2013, 44(8): 3102-3107.
WANG Zhen, SUN Wei, XU Hua-long, XIAO Jun-hui, LIU Ruo-hua. Adsorption behavior of CPC on molybdite surface and molecular dynamics simulation[J]. Journal of Central South University (Natural Science), 2013, 44(8): 3102-3107.
[6] 杨志超, 冯雅丽, 李浩然, 刘欣伟, 蔡震雷. 菱镁矿煅烧-浮选除钙及其机理[J]. 中国有色金属学报, 2012, 22(10): 2944-2950.
YANG Zhi-chao, FENG Ya-li, LI Hao-ran, LIU Xin-wei, CAI Zhen-lei. Separability of calcium from calcining magnesite by flotation and its mechanism[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(10): 2944-2950.
[7] RAI B, PRADIP. Molecular modeling and rational design of flotation reagents[J]. International Journal of Mineral Processing, 2003, 72: 95-110.
[8] RAI B, PRADIP, RAO T K, KRISHNAMURTHY S, VETRIVEL F R, MIELCZARSKI J. Molecular modeling of interactions of diphosphonic acid based surfactants with calcium minerals[J]. Langmuir, 2002, 18: 932-940.
[9] 陶 坤, 魏明安. 新型铜硫分离有机抑制剂BKY-1的机理研究[J]. 有色金属(选矿部分), 2013(5): 73-77.
TAO Kun, WEI Ming-an. Mechanism research on a new type of organic depressant BKY-1 for the separation of chalcopyrite and pyrite[J]. Nonferrous Metals (Mineral Processing Section), 2013(5): 73-77.
[10] WANG L K, SHAMMAS N K, SELKE W A, AULENBACH D B. Flotation technology[M]. New York: Humana Press Inc, 2010.
[11] 余新阳, 钟 宏, 刘广义. 阳离子反浮选脱硅捕收剂研究现状[J]. 轻金属, 2008, 6: 6-10.
YU Xing-yang, ZHONG Hong, LIU Guang-yi. Current research status on cationic collector of reverse flotation desilication[J]. Light Metals, 2008, 6: 6-10.
[12] NOVICH B E, RING T A. A predictive model for the alkylamine–quartz flotation system[J]. Langmuir, 1985, 1: 701-708.
[13] ZHANG J H, YOON R H, ERIKSSON J C. AFM surface force measurements concluded with silica in CnTACl solutions: effect of chain length on hydrophobic force[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2007, 300: 335-345.
[14] CHERNYSHOVA I V, RAO K H, VIDYADHAR A. Mechanism of adsorption of long chain alkylamines on silicates. A spectroscopic study. 1. Quartz[J]. Langmuir, 2000, 16: 8071-8084.
[15] SWAGAT S R, NISHANT S, HRUSHIKESH S, BISWESWAR D, BARADA K M. Molecular modeling studies of oleate adsorption on iron oxides[J]. Applied Surface Science, 2014, 295: 115-122.
[16] WENG X, MEI G, ZHAO T, ZHU Y. Utilization of novel ester-containing quaternary ammonium surfactant as cationic collector for iron ore flotation[J]. Separation and Purification Technology, 2013, 103: 187-194.
[17] LIU A, FAN J C, FAN M Q. Quantum chemical calculations and molecular dynamics simulations of amine collector adsorption on quartz (001) surface in the aqueous solution[J]. International Journal of Mineral Processing, 2015, 134: 1-10.
[18] 秦宗会, 谭 蓉. 曙红Y分光光度法测定阳离子表面活性剂及其机理研究[J]. 分析试验室, 2006, 25: 110-114.
QIN Zong-hui, TAN Rong. Spectrophotometric method for determination of cationic surfactants with eosin Y and its reaction mechanism[J]. Chinese Journal of Analysis Laboratory, 2006, 25: 110-114.
[19] HUANG Z Q, ZHONG H, WANG S, XIA LY, ZHAO G, LIU G Y. Gemini trisiloxane surfactant: Synthesis and flotation of alum inosilicate minerals[J]. Minerals Engineering, 2014, 56: 145-154.
[20] ERDEMOGLU M, SARRIKAYA M. Effects of heavy metals and oxalate on the zeta potential of magnetite[J]. Journal of Colloid and Interface Science, 2006, 300: 795-804.
[21] FUERSTENAUA D W, PRADIP. Zeta potentials in the flotation of oxide and silicate minerals[J]. Adv in Colloid and Interface Sci, 2005, (114/115): 9-26.
[22] SOMASUNDARAN P, WANG D. Solution chemistry: minerals and reagents[M]. Netherlands: Elsevier Science, 2006: 20-22.
[23] BALAKIREV V G. On the oriented cleavage of quartz[J]. International Geology Review, 1977, 19(4): 482-490.
[24] 严奉林. 石英中有害杂质元素分布特征和赋存状态及提纯方法[J]. 地质学刊, 2009, 33(3): 277-279.
YAN Feng-lin. Distribution properties and hosting conditions and purification methods of baneful impurity elements in quartz[J]. Journal of Geology, 2009, 33(3): 277-279.
[25] ZHENG J, BEHRENS S H, BORKOVEC M, POWERS S E. Predicting the wettability of quartz surfaces exposed to dense nonaqueous phase liquids[J]. Environmental Science & Technology, 2001, 35: 2207-2213.
[26] JIA R. Adsorption of cationic surfactants and their effects on the interfacial properties of quartz[J]. Surface Chemistry and Colloids, 2004, 64(9): 45-64.
[27] MARTIN G J, CUTTING R S, VAUGHAN D J, WARREN M C. Bulk and key surface structures of hematite, magnetite, and goethite: A density functional theory study[J]. American Mineralogist, 2009, 94: 1341-1350.
[28] YANG T, WEN X, REN J, LI Y, WANG J, HUO C. Surface structures of Fe3O4 (111), (110), and (001): A density functional theory study[J]. Journal of Fuel Chemistry Technology, 2010, 38: 121-128.
[29] 王正烈, 周亚平. 物理化学[M]. 4版. 北京: 高等教育出版社, 2001.
WANG Zheng-lie, ZHOU Ya-ping. Physical chemistry[M]. 4th ed. Beijing: Higher Education Press, 2001.
[30] VIDYADHAR A, HANUMANTHA RAO K, CHERNYSHOVA I V, PRADIP, FORSSBERG K S E. Mechanisms of amine– quartz interaction in the absence and presence of alcohols studied by spectroscopic methods[J]. Journal of Colloid and Interface Science, 2002, 256: 59-72.
[31] ROSELIN L S, LIN M S, LIN P H, CHANG Y, CHEN W Y. Recent trends and some applications of isothermal titration calorimetry in biotechnology[J]. Biotechnology Journal, 2010, 5: 85-98.
(编辑 王 超)
收稿日期:2014-11-16;修订日期:2015-03-09
通信作者:樊民强,教授,博士;电话:0351-6014776;E-mail: fanminqiang@tyut.edu.cn