J. Cent. South Univ. Technol. (2008) 15: 612-616
DOI: 10.1007/s11771-008-0114-8
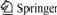
Reaction of erythromycin with dissolved oxygen on gold nanoparticle-modified glassy carbon electrodes
LI Xue(李雪), FU Ying(付颖), WANG Jian-xiu(王建秀),
L? Hui-dan(吕慧丹), XU Mao-tian(徐茂田)
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract: Cyclic voltammetry was used to investigate the reaction of erythromycin (EM) with dissolved oxygen on gold nanoparticle-modified electrodes prepared via electrodeposition. A well-defined reduction peak at -0.420 V and a reoxidation peak at -0.055 V were observed. With the addition of EM into the NaOH solution containing dissolved oxygen, the oxidation peak at -0.055 V was still indiscernible. However, a new oxidation peak at 0.200 V appeared, which suggests the interaction between EM and dissolved oxygen. Therefore, this method can be used for the analysis of EM in tablets. The present method is simple, reproducible, and does not require complex analytical instruments.
Key words: gold nanoparticle-modified electrode; dissolved oxygen; erythromycin; interaction; cyclic voltammetry
1 Introduction
The electrochemistry of oxygen has been considered to be an essential subject for extensive studies of energy conversion, industrial processes and biosensor performance[1-3]. Over the past years, most reports focused on the utilization of conventional substrate electrodes, such as Pt[4], Au[5-6] and glassy carbon (GC)[7] for many electrocatalytic and electroanalytical applications. And the mechanism of the electrocatalytic reduction of oxygen has been elucidated in detail by cyclic voltammetry. Electrocatalytic reduction of oxygen has been reported to carry out in two media, i.e. the acidic and the alkaline media[4, 6]. In the acidic medium, the intermediate and the final product are H2O2 and H2O, respectively. Being different from those in the acidic medium, the intermediate and the final product in the alkaline medium are usually HO2- and OH-, respectively.
Erythromycin (EM) is an important macrolide antibiotic. It can be used to treat streptococcal infections of the throat or the skin and lung infections caused by streptococcal pneumoniae and mycoplasma pneumoniae[8-9]. However, EM has some side-effects, usually causing nausea, vomiting, loss of appetite, diarrhea, and abdominal pain. Therefore, the study on EM is very meaningful for human health. Recently, some experiments indicated that EM can combine with oxygen and has a scavenging activity for superoxide and free radical[10-12]. Superoxide anion (O2-), a univalent reduction product of oxygen, is a potentially cytotoxic species. More and more evidences show that superoxide anion is implicated in the pathology of many human diseases[13]. The investigation of superoxide anion has attracted much attention in many fields[14-15]. O2- can be generated via electrochemical reduction of dissolved oxygen at different potential domains[4, 15], which offers a convenient way to study the reaction of drugs with reactive oxygen species[16-17].
Metal nanoparticles have been extensively studied in the last decade due to[A1] the relative ease of preparation, the variety of the materials, and the unusual physical and chemical properties[18]. Among various metal nanoparticles, gold nanoparticles (AuNPs) are the most commonly used. Modification of electrode surfaces with AuNPs has recently received a wide range of interest as a consequence of the rapid progress in nanotechnology[19]. AuNPs exhibit an outstanding catalytic activity compared with bulk gold. In this work, the electrocatalytic reduction of dissolved oxygen and the interaction of dissolved oxygen with EM on gold nanoparticle-modified GC electrodes were studied in a 0.20 mol/L NaOH solution, providing more information about the pharmacology and scavenging activity of EM for superoxide and free radical.
2 Experiment section
2.1 Instruments
The cyclic voltammetric (CV) measurements were carried out with a CHI 660B electrochemical workstation (CH Instruments, Austin, TX) in a conventional three- electrode cell. The working electrode was a GC disk with a diameter of 3 mm embedded in a Kel-F rod. A platinum electrode and a Ag/AgCl electrode were used as the auxiliary and the reference electrodes, respectively. All experiments were carried out at the ambient temperature.
2.2 Reagents
EM was purchased from National Institute of Pharmaceutical and Biological Products (Beijing, China) with purity higher than 99%. The stock solution of EM (1.00 mmol/L) was prepared by dissolving 0.073 4 g EM in absolute ethanol and diluted to 100 mL. Other reagents involved were all from commercial sources with analytical purity and used as received. All solutions were prepared with deionized water treated with a Millipore water purification system (Millipore Corp).
2.3 Procedures
Prior to each measurement, GC electrodes were polished with alumina slurry down to 0.3 μm, followed by sonicating in water. The procedure for deposition of gold nanoparticles onto GC electrodes was according to the previously published report[20]. Briefly, the gold nanoparticles were electrodeposited onto the polished GC electrode from a 0.50 mol/L H2SO4 solution containing 0.10 mmol/L AuCl4- by applying a 3 s-potential step from 1.100 to 0 V (vs Ag/AgCl). Then, the gold nanoparticle-modified GC electrode was transferred into a 0.20 mol/L NaOH solution and measured with CV in the potential range from 0.600 to -0.800 V. Deposition time, deposition potential and concentration of AuCl4- had a great influence on the construction of gold nanoparticles on the GC electrode. The formation of the gold nanoparticles on the electrode surfaces with typical AFM images or SEM micrographs has been reported[20-22]. To get the optimum surface coverage of gold nanoparticles, we chose the multi-potential step method with the deposition potential from 1.100 to 0 V and the deposition time of 3 s.
3 Results and discussion
3.1 CV response of dissolved oxygen
CV measurements were carried out in different supporting electrolytes such as HAc-NaAc, KH2PO4- K2HPO4, NH3×H2O-NH4Cl, KClO4, NaOH and KCl solutions. However, well-defined redox peaks of dissolved oxygen were obtained for the modified electrode in a NaOH solution. Different concentrations of NaOH solution from 0.10 to 1.00 mol/L were tested. The results indicate that strong and stable voltammetric responses could be obtained in 0.10-0.30 mol/L NaOH solutions. Therefore, 0.20 mol/L NaOH solution was chosen as the supporting electrolyte.
The gold nanoparticle-modified GC electrode possesses an electrocatalytic activity towards the dissolved oxygen. As shown in Fig.1 (curve b), two reduction peaks at 0.071 and -0.420 V are observed for the gold nanoparticle-modified GC electrode. And two oxidation peaks appeare at 0.370 and -0.055 V. The peaks at 0.071 V and 0.370 V are also obtained for the gold nanoparticle-modified electrode in the 0.20 mol/L NaOH solution in the absence of dissolved oxygen (curve c), suggesting that the two peaks are originated from the background signals. As a consequence, only the reduction peak at -0.420 V and the oxidation peak at -0.055 V correspond to the reduction and the reoxidation of dissolved oxygen on the gold nanoparticle-modified electrode. In contrast, the reduction peak of dissolved oxygen at -0.460 V is poor with a larger background for the bare GC electrode (curve (a)). Based on the fact that the reduction peak of dissolved oxygen for the bare GC electrode shifts positively to -0.420 V for modified electrode, we can conclude that electrocatalytic reduction toward dissolved oxygen can be realized with the aid of gold nanoparticles.
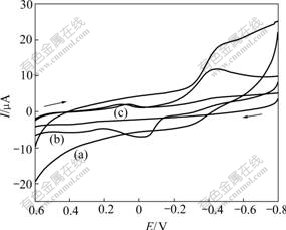
Fig.1 CVs of dissolved oxygen on bare GC electrode (a), on gold nanoparticle-modified GC electrode in 0.20 mol/L NaOH solution (b) and on gold nanoparticle-modified GC electrode in 0.20 mol/L NaOH solution degased with N2 (c) (Scan rate employed was 0.1 V/s and arrows indicate scan directions)
To demonstrate that the reduction peak at -0.420 V and the reoxidation peak at -0.055 V are attributable to the redox reaction of dissolved O2, we examined the influence of the deaeration time on the voltammetric behavior of dissolved O2 degased with N2. Fig.2 depicts the influence of deaeration time on the intensity of the peaks at -0.420 and -0.055 V. As can be seen, the reduction peak at -0.420 V and the reoxidation peak at -0.055 V of dissolved oxygen decrease gradually with the increase of the deaeration time. The inset shows the plots of the peak current vs the deaeration time. These plots exhibit two regions with different slopes. The upper curve (deaeration time from 0 to 60 s) has a slope that is much steeper than that of the lower section (deaeration time from 60 to 240 s). The variation in the slopes (sensitivities) between the two regions may be interpreted on the basis of different O2 concentrations in the solution. At about 60 s, two curves begin to plateau. This suggests that most of the dissolved oxygen in 0.20 mol/L NaOH solution has been removed.
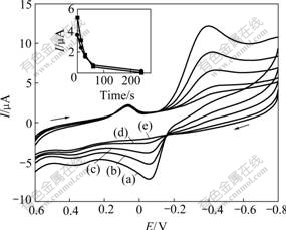
Fig.2 Effect of deaeration time on GC electrode modified with gold nanoparticles in 0.20 mol/L NaOH solution: (a) 0 s; (b) 15 s; (c) 30 s; (d) 60 s; (e) 240 s (Other conditions are the same as those in Fig.1. Inset shows plots of peak current vs deaeration time)
3.2 CV response of dissolved oxygen in the presence of EM
EM, an important macrolide antibiotic, has a great influence on the voltammetric response of dissolved oxygen on the gold nanoparticle-modified electrode (Fig.3). In comparison with curve (b) in the absence of EM, the reduction peak of dissolved oxygen at -0.420 V increases in the 0.20 mol/L NaOH solution containing 40.00 ?mol/L EM, and simultaneously the oxidation peak at -0.055 V becomes indiscernable (curve (a)). However, a new oxidation peak appeares at 0.200 V with larger peak intensity. While in the case of 40.00 ?mol/L EM, for the gold nanoparticle-modified electrode in the absence of dissolved oxygen (curve (c)), no those peaks are observed at all. The indiscernibility of the oxidation peak at -0.055 V and the occurrence of the new oxidation peak at 0.200 V demonstrate the interaction of EM with dissolved oxygen.
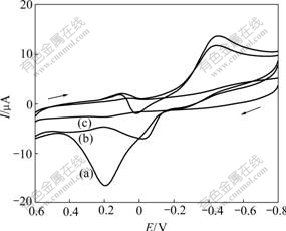
Fig. 3 CVs of dissolved oxygen on GC electrode modified with gold nanoparticles in 0.20 mol/L NaOH solution: (a) In the presence of 40.00 ?mol/L EM (b) In the absence of EM (c) Containing 40.00 ?mol/L EM in the absence of oxygen (Other conditions are the same as those in 1)
The interaction of EM and dissolved oxygen could be modulated by different deaeration time. As shown in Fig.4 and the inset, the oxidation peak current at 0.200 V decreases sharply with the deaeration time prolonging from 0 to 5 min. When a longer deaeration time is used, for example, 20 min, the oxidation peak almost disappears, suggesting that the concentration of dissolved oxygen has an extraordinary effect on the interaction between EM and dissolved oxygen.
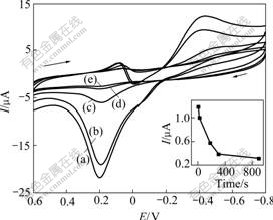
Fig. 4 Effect of deaeration time on gold nanoparticle-modifid GC electrodes in 0.20 mol/L NaOH solution containing 40.00 ?mol/L EM: (a) 0 s (b) 5 s (c) 60 s (d) 300 s (e) 900 s (Other conditions are the same as those in Fig1. Inset shows plots of peak current with deaeration time)
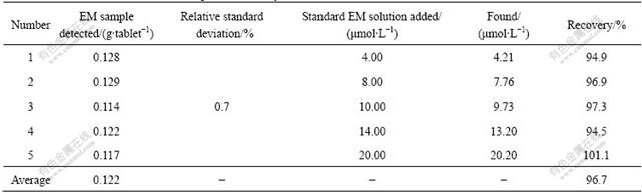
Table 1 Determination of EM in EM sample and recovery data for that added with different concentrations of EM
Nevertheless, due to complexity involved in this process, the exact nature of the interaction mechanism is still unclear.
3.3 Analytical applications
The above method could be used for the determina- tion of EM. As shown in Fig.3 and Fig.4, a stable and well-defined oxidation peak at 0.200 V is obtained in the presence of EM in oxygen-saturated NaOH solution, and there is a linear relationship between the peak current Ip (?A) and EM concentration in the range of 4.00-50.00 ?mol/L. The linear regression equation is expressed as Ip = 0.464 8 + 1.309 9c, with a regression coefficient, r = 0.999 5 (n = 8 c is molar concentration, mol/L). The error bar shown in the calibration plot is 2.8%, which is acceptable for most analytical techniques. When the signal-to-noise ratio (SNR) is 3, the detection limit is 2.00 ?mol/L. The modified electrode has a good stability with the peak current remaining almost constant over 4 h, thus sample analysis could be conducted at for the gold nanoparticle-modified electrode in 0.20 mol/L NaOH solution.
10 pieces of EM tablets were weighed and grounded to fine powder in a mortar. The resultant powder was accurately weighed and dissolved in absolute ethanol with the aid of sonication. The above solution was then transferred into a 1 000 ml mL volumetric flask to a final concentration of 1.25 mg/ The content of EM in the sample solution was then analyzed with the calibration plot method. The average value for 5 determinations iwas 0.122 g/tablet. Recovery was studied for a diluted EM sample added with varying amounts of standard EM solution, 4.00, 8.00, 10.00, 14.00 and 20.00 μmol/L, respectively. Deducting the amount, of EM in EM sample, the corresponding amount of 4.21, 7.76, 9.73, 13.20 and 20.20 μmol/L were detected with an acceptable recovery of 94.9%, 96.9%, 97.3%, 94.5%, and 101.1%, respectively. The above results are listed in Table 1.
4 Conclusions
1) The reaction of EM with dissolved oxygen is investigated on the gold nanoparticle-modified GC electrode. With the addition of EM in the NaOH solution containing dissolved oxygen, a new oxidation peak at 0.200 V originated from the interaction of EM and oxygen is observed for the modified electrode. And at the same time, the oxidation peak of dissolved oxygen at -0.055 V becomes indiscernible. The above results provide the possibility of investigation of the pharmacology and scavenging activity of EM for superoxide and free radical.
2) The developed method can be used for the determination of EM in troches. The peak current at 0.200 V increases linearly with the increase of concentra- tion of EM in the range of 4.0050.00 ?mol/L, and the detection limit is 2.00 ?mol/L. The modified electrode may be used in biosensors to further study the pharmacology of EM.
References
[1] ZHANG Y R, ASAHINA S, YOSHIHARA S, SHIRAKASHI T. Oxygen reduction on Au nanoparticle deposited boron doped diamond films [J]. Electrochim Acta, 2003, 48(6): 741-747.
[2] GLASSPOOL W, ATKINSON J. A screen-printed amperometric dissolved oxygen sensor utilising an immobilised electrolyte gel and membrane [J]. Sens Actuators B, 1998, 48(1/3): 308-317.
[3] HU S S. Electrocatalytic reduction of molecular oxygen on a sodium montmorillonite-methyl viologen carbon paste chemically modified electrode [J]. J Electroanal Chem, 1999, 463(2): 253-257.
[4] El-DEAB M S, OHSAKA T. Electrocatalysis by nanoparticles: Oxygen reduction on gold nanoparticles-electrodeposited platinum electrodes [J]. J Electroanal Chem, 2003, 553(1): 107-115.
[5] El-DEAB M S, OHSAKA T. Hydrodynamic voltammetric studies of the oxygen reduction at gold nanoparticles-electrodeposited gold electrodes [J]. Electrochim Acta, 2002, 47(26): 4255-4261.
[6] El-DEAB M S, OHSAKA T. An extraordinary electrocatalytic reduction of oxygen on gold nanoparticles-electrodeposited gold electrodes [J]. Electrochem Commun, 2002, 4(4): 288-292.
[7] FINOT M O, BRAYBROOK G D, MCDERMOTT M T. Characterization of electrochemically deposited gold nanocrystals on glassy carbon electrodes [J]. J Electroanal Chem, 1999, 466(2): 234-241.
[8] LIN J T, CONNELLY M B, AMOLO C, OTANI S, YAVER D S. Global transcriptional response of Bacillus subtilis to treatment with subinhibitory concentrations of antibiotics that inhibit protein synthesis [J]. Antimicrob Agents Chemother, 2005, 49(5): 1915-1926.
[9] ANTZELEVITCH C, SUN Z Q, ZHANG Z Q, YAN G X. Cellular and ionic mechanisms underlying erythromycin-induced long QT Intervals and torsade de pointes [J]. J Am Coll Cardiol, 1996, 28(7): 1836-1848.
[10] MURANAKA H, SUGA M, SATO K, NAKAGAWA K, AKAIKE T, OKAMOTO T, MAEDA H, ANDO M. Superoxide scavenging activity of erythromycin-iron complex [J]. Biochem Biophys Res Commun, 1997, 232(1): 183-187.
[11] CROSS J B, CURRIER R P, TORRACO D J, VANDERBERG L A, WAGNER G L, GLADEN P D. Killing of bacillus spores by aqueous dissolved oxygen, ascorbic acid, and copper ions [J]. Appl Environ Microbiol, 2003, 69(4): 2245-2252.
[12] KARBOWSKI M, KURONO C, WOZNIAK M, OSTROWSKI M, TERANISHI M. Free radical-induced megamitochondria formation and apoptisis [J]. Free Radical Biol Med, 1999, 26(3/4): 396-409.
[13] FRIDOVICH I. How innocuous is superoxide [J]. Acc Chem Res, 1982, 15(7): 200.
[14] WEI Y L, WU K B, WU Y H, HU S S. Electrochemical characterization of a new system for detection of superoxide ion in alkaline solution [J]. Electrochem Commun, 2003, 5(9): 819-824.
[15] SAWYER D T. How super is superoxide [J]. Acc Chem Res, 1981, 14(12): 393-400.
[16] BUSHELL M E, DUNSTAN G L, WILSON G C. Effect of small scale culture vessel type on hyphal fragment size and erythromycin production in Saccharopolyspora erythraea [J]. Biotechnol Lett, 1997, 19(9): 849-852.
[17] KEMPF M, THEOBALD U, FIEDLER H P. Influence of dissolved O2 on the fermentative production of gallidermin by Staphylococcus gallinarum [J]. Biotechnol Lett, 1997, 19(11): 1063-1065.
[18] BILJANA ?, RONAN B, CHRIS S, ALISON C, RICHARD G C. Combinatorial electrochemistry using metalnanoparticles: from proof-of-concept topracticalrealisation for bromidedetection [J]. Anal Chim Acta, 2007, 590(1): 67-73.
[19] NATH N, CHILKOTI A. Label-free biosensing by surface plasmon resonance of nanoparticles on glass: Optimization of nanoparticle size [J]. Anal Chem, 2004, 76(18): 5370-5378.
[20] DAI X, NEKRASSOVA O, HYDE M E, COMTON R G. Anodic stripping voltammetry of arsenic(III) using gold nanoparticle- modified electrodes [J]. Anal Chem, 2004, 76(19): 5924-5929.
[21] ZHANG Y, SURYANARAYANAN V, NAKAZAWA I, YOSHIHARA S, SHIRAKASHI T. Electrochemical behavior of Au nanoparticle deposited on as-grown and O-terminated diamond electrodes for oxygen reduction in alkaline solution [J]. Electrochim Acta, 2004, 49(28): 5235-5240.
[22] DANIEL M C, ASTRUC D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related proerties, and applications toward biology, catalysis, and nanotechnology [J]. Chem Rev, 2004, 104(1): 293-346.
(Edited by YANG Hua)
Foundation item: Project(2005037207) supported by Postdoctoral Science Foundation of China
Received date: 2008-03-10; Accepted date: 2008-05-12
Corresponding author: XU Mao-tian, Professor; Tel: +86-731-8836954; E-mail: xumaotian@163.com