J. Cent. South Univ. Technol. (2007)04-0485-05
DOI: 10.1007/s11771-007-0094-0 
Mechanism of gold dissolving in alkaline thiourea solution
CHAI Li-yuan(柴立元), WANG Yun-yan(王云燕)
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
_____________________________________________________________
Abstract:Reaction mechanism of gold dissolving in alkaline thiourea solution was studied by electrochemical methods, such as cyclic voltammetry, chronopotentiometry, AC impedance, linear sweep voltammetry. Apparent activation energy of anodic process of gold electrode dissolving in alkaline thiourea solution is 14.91 kJ/mol. Rate determining step is the process of gold thiourea complex diffusing away from electrode surface to solution. The results of AC impedance and chronopotentiometry indicate that thiourea adsorbs on gold electrode surface before dissolving in solution. There does not exist proceeding chemical reactions. Formamidine disulfide, the decomposed product of thiourea, does not participate the process of gold dissolution and thiourea complex. Species with electro-activity produced in the process of electrode reaction adsorbs on the electrode surface. In alkaline thiourea solution, gold dissolving mechanism undergoes the following courses: adsorption of thiourea on electrode surface; charge transfer from gold atom to thiourea molecule; Au[SC(NH2)2]ads+ receiving a thiourea molecule and forming stable Au[SC(NH2)2]2+; and then Au[SC(NH2)2]2+ diffusing away from the electrode surface to solution, the last step is the rate-determining one.
Key words: gold; alkaline thiourea; dissolution mechanism
_____________________________________________________________
1 Introduction
Dissolution of gold in thiourea solution was reported firstly in 1941[1], and then the theory and process of gold leaching in thiourea solution have been most widely studied throughout the world mainly because thiourea is not detrimental to the environment, non-toxic to humans, and the dissolution of gold in thiourea solutions is much faster than in cyanide solutions[2-9]. However, there is little industrial application of gold leaching in thiourea solution, except higher consumption of thiourea, insufficient investigation on gold leaching in thiourea solution is also the limitation[10-13]. Since gold leaching in the alkaline thiourea solution is still at the developmental stage, as far as studies of mechanism there is little report besides our group’s research. Therefore, a better and further understanding of the foundation will play an important role on guiding application industrially. Firstly, dissolution mechanism of gold electrode in alkaline thiourea solution should be make clear, which can offer some guidance for mechanism of gold leaching from ores in alkaline thiourea solution.
In alkaline thiourea solution, combining the property of thiourea, the process of gold dissolving in alkaline medium may be expressed as: Diffusion of thiourea to electrode surface; Adsorption of thiourea on electrode surface, Au+SC(NH2)2→Au[SC(NH2)2]ads; Oxidization of thiourea into formamidine disulphide, 2SC(NH2)2+2OH--2e=(SCN2H3)2+2H2O; Complex of gold and thiourea losses an electron and is oxidized, Au[(SC(NH2)2)]ads-e=Au[SC(NH2)2]ads+; Transformation of formamidine disulphide into thiourea again, (SCN2H3)2+2H2O+2e=2SC(NH2)2+2OH-; Complex formation, Au[SC(NH2)2]+ads+ SC(NH2)2= Au[SC(NH2)2]2+; Diffusion of Au[SC(NH2)2]2+ from electrode surface to solution. The above characteristics are the theoretical foundations for gold leaching in thiourea solution. However, to describe kinetics of gold dissolution exactly, some questions, such as whether gold dissolution undergoes the above courses, how does formamidine disulfide affect gold dissolution, which step is indeed the rate-determining one, how much is the reaction orders of reactants, and what are the kinetics parameters of reaction process, need to be determined through experiments[14-15]. Accordingly, in this study gold dissolving process in alkaline thiourea solution was investigated by electrochemical methods, such as cyclic voltammetry, chronopotentiometry, AC impedance.
2 Experimental
Electrochemical measurements were conducted by CHI660A electrochemical workstation monitored by computer. Three-electrode system was applied, H style cell with sand core glass septum served as electrobath, working electrode was the gold electrode with the purity of 99.99% and area of 1 cm2, a platinum piece with great area compared to working electrode as counter electrode, and Hg/HgO electrode dipped in 1 mol/L NaOH solution as reference electrode[16]. Salt bridge with Luggin capillary was used to eliminate border potential between different solutions and to decrease Ohm resistance of solution. The surface of specimens was polished by 13 μm abrasive paper, dipped successively in acetone and washed by redistilled water before immersion in the test solution. Before polarization measurements commenced, the solution was purged by a stream of purified N2 for at least 15 min in order to remove the dissolved oxygen. The cell was maintained in a thermostated water bath to keep a constant temperature at 25 ℃, The scan rate of steady-state polarization was controlled at 10 mV/s.
The electrolyte was composed of 0.1 mol/L SC(NH2)2 and 0.25 mol/L stabilizing reagent. The conditions were controlled as pH value of 12.5 adjusted by diluent NaOH solution.
3 Overall electrode reaction
Thiourea is a complexing agent with the property of reduction, which can form complexes with many metal ions. For gold ions, thermodynamic data (the stability constant of Au[SC(NH2)2]2+ is 22.1) shows that thiourea is a strong complexing agent. Gold forms only one stable complex with thiourea, the gold-bisthiourea complex.
During the process of gold dissolving in alkaline thiourea solution, gold loses one electron and combines with two thiourea molecules, which indicates that electrode reaction gains one electron. The mainly existing form of gold in solution is Au(SC(NH2)2)2+. So overall electrode reaction can be written as follows:
Au+2 SC(NH2)2-e= Au(SC(NH2)2)2+ (1)
4 Electrode reaction mechanism
4.1 Determination of rate-determining step
Although the result of cyclic voltammetry indicates that gold dissolving in alkaline thiourea solution is irreversible, whether it is controlled by electrochemical polarization or concentration gradient polarization should be testified further. And here activation energy of electrode reaction is efficient[14]. Activation energy is 12-16 kJ/mol when electrode reaction is controlled by species diffusion; while the fact that activation energy is more than 40 kJ/mol indicates that electrochemical reaction is the rate-determining step. However activation energy is similar to that of diffusion process if there exist preceding reactions of chemical transforming, and its value is related to the speed of the preceding reaction and the concentration of the mainly existing ion.
h-lgJη curve is drawn according to linear sweep curves at different temperatures, the relationship between lgJh and 1/T at the same overpotential is made out on the basis of the relationship between activation energy and temperature(shown in Fig.1 where, η is over potential, V; Jη is current density, mA/cm2, T is temperature, K). The slope of the line in Fig.1 is -4 131.26, correlation coefficient is 0.999 5, and the calculated apparent activation energy is 14.91 kJ/mol, which indicates that Au[SC(NH2)2]2+diffusing away from the electrode surface to solution is the rate-determining step during gold dissolving in alkaline thiourea solution.
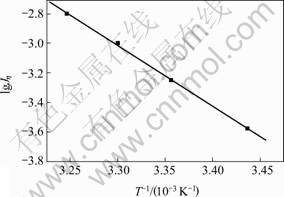
Fig.1 Relationship between current density and temperature
4.2 Determination of thiourea adsorbing on gold electrode surface before reaction
4.2.1 Determination of thiourea adsorption by AC impedance
Thiourea, oxidant, alkali and H2O competitively adsorb on the surface of gold electrode exposed to the alkaline solution composed of thiourea and oxidant. Thiourea molecule adsorbing on the active site of gold surface changes electron cloud distribution of gold atom through the action between gold atom and sulfur atom of thiourea, which makes gold dissolve easily because of potential decline of Au-Au(I).
Adsorption of thiourea on electrode surface, Au+SC(NH2)2=Au[SC(NH2)2]ads, takes place firstly during gold dissolving in alkaline thiourea solution, and then charge transfers from gold atom in Au[(SC(NH2)2)]ads to thiourea molecule, Au[(SC(NH2)2)]ads-e=Au[SC(NH2)2]ads+. Electrode surface process and AC impedance should be used to ascertain the adsorption property of thiourea on gold surface.
Based on AC impedance theory of electrode surface process[15], supposing that thiourea adsorbs on gold electrode surface, non-Faraday adsorption impedance(Z) can be expressed as follows:
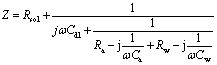 (2)
(2)
where Rsol is solution resistance; Cdl is double layer capacitance; Ra and Ca are adsorption resistance and adsorption capacitance caused by thiourea adsorbing on electrode surface, respectively; Rw and Cw are Warburg resistance and capacitance caused by thiourea diffusing from solution to electrode surface, respectively. ω is angle frequency, j denotes imaginary. The corresponding equivalent circuit sketch and AC impedance diagram are shown in Fig.2.
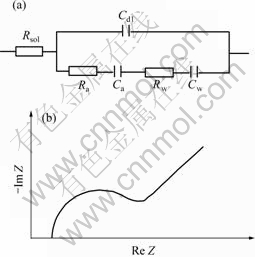
Fig.2 Equivalent circuit sketch(a) and AC impedance diagram(b) of thiourea adsorption
When existing chemical adsorption, AC impedance diagram is made up of a semicircle representing adsorption and a beeline with angle of 45? to abscissa representing diffusion. While without chemical adsorption, AC impedance diagram is only a beeline with angle of 45? to abscissa. So AC impedances of gold dissolving in NaOH solution and alkaline thiourea solution at pH value of 12.5 were measured respectively, the conditions were controlled as frequency of 0.1- 10 000 Hz, potential of 0.58 V(vs SHE), sine wave with Δφ of 5 mV. The results are shown in Fig.3.
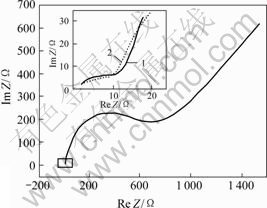
Fig.3 AC impedance diagram of Au electrode dissolving in NaOH solution and alkaline thiourea solution
1—NaOH solution; 2—Alkaline thiourea solution
It is obvious that AC impedance diagram is made up of a semicircle representing adsorption within high frequency range and a beeline representing diffusion in alkaline thiourea solution, which is similar to the above theoretical AC impedance. AC impedance diagram is only a beeline without a semicircle in NaOH solution, which indicates that thiourea firstly adsorbs on the electrode surface before participating reaction. In partial zoom figure, it can be seen that there is a semicircle with a small diameter, which is caused by OH- adsorption on gold surface in two different solutions[16]. Therefore, thiourea adsorbs on gold electrode surface before dissolving in alkaline thiourea solution.
4.2.2 Determination of thiourea adsorption by chronopotentiometry
The total consumed quantity of electric charge can be divided into QC which is used for diffusion of reactant in solution and Qθ which is consumed to form cover layer or adsorption layer when a constant current passes through the electrode, so
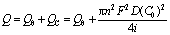 (3)
(3)
where n is the number of electron; F is Faraday constant; D is diffusion coefficient; C0 is the original concentration of reactant; i is current.
Eqn.(3) indicates that there is a linear relationship between Q(=iτ) and i, there exists adsorption when the line does not pass through origin. Contrarily, there is not reactant adsorption on electrode surface if the line has not intercept.
Based on the chronopotentiometry curves measured under different constant currents (Fig.4), transition time and quantity of electric charge were calculated and listed in Table 1. The relationship between Q and 1/i is shown in Fig.5 and the fitted equation is:
Q=0.984 4+0.084 39/i (R=0.995 8) (4)
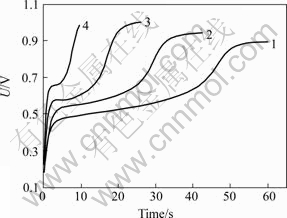
Fig.4 Effect of anodic current on transition time(τ) of Au dissolving in 0.2 mol/L alkaline thiourea solution
1—0.06 mA; 2—0.08 mA; 3—0.12 mA; 4—0.25 mA
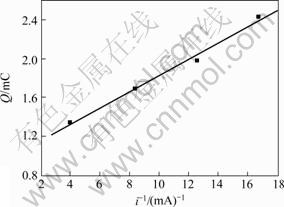
Fig.5 Relationship between Q and 1/i of chronopotentiometry
Table 1 Transition time and charge quantity of chronopotentiometry curves with different currents

From Fig.5 and the fitted equation it can be seen that the line does not pass through origin and Qθ is 0.984 4 mC. Therefore, the result of chronopotent- iometry also illuminates that thiourea adsorbs on gold electrode surface before dissolving in alkaline thiourea solution.
4.3 Determination of preceding chemical reaction
Whether the decomposed product of thiourea participates the electrode reaction can be justified by the existing of proceeding chemical reaction. Chronopotentiometry experiment is performed at different currents for estimating preceding chemical transforming reaction undergoes or not before gold dissolution.
The experimental data abide by the follow equation if there is a preceding reaction[14]
 (5)
(5)
where K1 and K-1 are rate constants of positive and reverse reaction, respectively, K=K1/K-1, D is the diffusion coefficient, r=aO0+aR0, a is activity, A is electrode area, τ is transition time. The relationship between iτ1/2 and i is linear when i is low, while iτ1/2 is independent of i when i is high.
Fig.6 gives the relationship between iτ1/2 and i. There is a linear relationship between iτ1/2 and i with a positive slope. The results do not agree with the theoretical equation, which indicates that preceding chemical transforming reaction does not exist before thiourea reacts with gold.
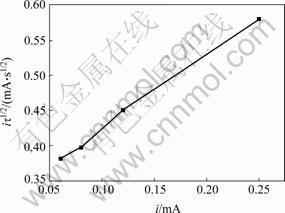
Fig.6 Relationship between iτ1/2 and i of chronopotentiometry
4.4 Determination of whether formamidine disulphide participates reaction
In chronopotentiometry curves measured under different constant currents (Fig.5) there is only one transition time, which indicates that there is only one electron that transfers during gold dissolving in alkaline thiourea solution. So it can be concluded that formamidine disulfide, the decomposed product of thiourea, does not participate the process of gold dissolution and thiourea complex.
4.5 Determination of adsorption of species with electro-activity
There is a linear relationship between peak current density Jp and scan rate ν when species with electro-activity adsorbs on electrode surface. There is a linear relationship between peak current density Jp and ν1/2 when diffusion species adsorbs on electrode surface.
Cyclic voltammetrical curves of gold electrode in alkaline thiourea solution at different scan rates are shown in Fig.7. The relationship between scan rate and peak current density is shown in Fig.8, Fig.9 and Table 2. Fig.8 indicates that the diffusion species adsorbs on electrode surface. Approximate linear relationship in Fig.9 indicates that there is a kind of species with electro-activity adsorbing on electrode surface. Smaller current density is caused by great effect of passivation of gold electrode surface on gold dissolving in alkaline thiourea when scan rate is as slow as 10 mV/s.
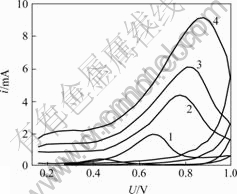
Fig.7 Cyclic voltammetrical curves of gold electrode in 0.05 mol/L alkaline thiourea solution at different scan rates
Scan rate/(mV·s-1): 1—10; 2—50; 3—100; 4—200
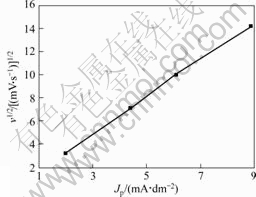
Fig.8 Relationship between v1/2 and Jp
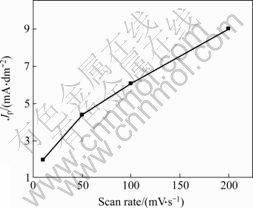
Fig.9 Relationship between v and Jp
Table 2 Relationship between scan rate and peak current density

4.6 Determination of electrode reaction mechanism
From the above results it can be concluded that in alkaline thiourea solution, gold dissolution mechanism undergoes the following courses: adsorption of thiourea on electrode surface; charge transfer from gold atom to thiourea molecule; Au[SC(NH2)2]ads+ receiving a thiourea molecule and forming stable Au[CS(NH2)2]2+; and then Au[SC(NH2)2]2+ diffusing away from the electrode surface to solution, the last step is the rate-determining step.
5 Conclusions
1) Apparent activation energy of anodic process of gold electrode dissolving in alkaline thiourea solution is 14.91 kJ/mol. Rate determining step is the process of gold thiourea complex diffusing away from electrode surface to solution. The results of AC impedance and chronopotentiometry indicate that thiourea adsorbs on gold electrode surface before dissolving in alkaline thiourea solution. There does not exist proceeding chemical reaction. Formamidine disulfide, the decomposed product of thiourea does not participate the process of gold dissolution and thiourea complex. Species with electro-activity produced in the process of electrode reaction adsorbs on the electrode surface.
2) Mechanism of gold dissolving in alkaline thiourea is: adsorption of thiourea on electrode surface; charge transfer from gold atom to thiourea molecule; Au[SC(NH2)2]ads+ receiving a thiourea molecule and forming stable Au[SC(NH2)2]2+; and then Au- [SC(NH2)2]2+ diffusing away from the electrode surface to solution, the last step is the rate-determining one.
References
[1] GRONEWALD T. Potential application of thiourea in the processing of gold[J]. J S African Inst M&M, 1977, 77(11): 217-223.
[2] ORGUL S, ALTALAY U. Reaction chemistry of gold leaching in thiourea solution for a Turkish gold ore[J]. Hydrometallurgy, 2002, 67(1/3): 71-77.
[3] DENG T L, LIAO M X, WANG M H, et al. Enhancement of gold extraction from biooxidation residues using an acidic sodium sulphite-thiourea system[J]. Mineral Engineering, 2001, 14(2): 263-268.
[4] KUMAR V, MURTHY D S R, RAO K V. Extraction of gold from an Indian low-grade refractory gold ore through physical beneficiation and thiorea leaching[J]. Hydrometallurgy, 2003, 68(1/3): 125-130.
[5] GONEN N. Leaching of finely disseminated gold ore with cyanide and thiourea solutions[J]. Hydrometallurgy, 2003, 69(1/3) 169-176.
[6] SENANAYAKE G.. Gold leaching in non-cyanide lixiviant systems: Critical issues on fundamentals and applications[J]. Minerals Engineering, 2004, 17(6): 785-801.
[7] SENANAYAKE G.. Analysis of reaction kinetics, speciation and mechanism of gold leaching and thiosulfate oxidation by ammoniacal copper(II) solutions[J]. Hydrometallurgy, 2004,75(1/4): 55-75.
[8] UBALDINI S, FORNARI P, Massidda R, et al. Innovative thiourea gold leaching process[J]. Hydrometallurgy, 1998, 48(1): 113-124.
[9] NINAE M, OBOSO A. TAKENAKA Y, et al. Synergistic extraction of gold from sulfuric acid solution containing thiourea[J]. Nippon Kinzoku Gakkaishi/Journal of the Japan Institute of Metals, 1991, 55(8): 867-873.
[10] GASPAR V, MEJEROVICH A S, MERETUKOV M A. et al. Practical application of potential-pH diagrams for Au-CS(NH2)2-H2O and Ag-SC(NH2)2-H2O systems for leaching gold and silver with acidic thiourea solution[J]. Hydrometallurgy, 1994, 34(3): 369-381.
[11] CHANDRA I, JEFFREY M I. An electrochemical study of the effect of additives and electrolyte on the dissolution of gold in thiosulfate solutions[J]. Hydrometallurgy, 2004, 73(3/4): 305-312.
[12] HUANG Zi-qing. Theory Introduction to Electrolyte Solutions[M]. Beijing: Science Press, 1983: 84-86. (in Chinese)
[13] BARD A J, PARSONS R, JORDAN J. Standard Potentials in Aqueous Solution[M]. International Union of Pure and Applied Chemistry. New York: Basel Press, 1985: 287-321.
[14] HU Yu-de, CHEN Bai-zhen. Investigating Methods of Metallurgical Electrochemistry[M]. Changsha: Central South University of Technology Press, 1990. (in Chinese)
[15] CAO Chu-nan. Electrochemical Principles of Corrosion[M]. Beijing: Chemical Industry Press, 1985: 252-261.(in Chinese)
[16] BARDER A J, FUKNA L R. Principle and Application of Electrochemistry Technique[M]. GU Lin-ying, L? Ming-xiang, SONG Shi-zhe, et al translate. Beijing: Chemical Industry Press, 1986: 615. (in Chinese)
_______________________
Foundation item: Project(50004009) supported by the National Natural Science Foundation of China
Received date: 2006-10-20; Accepted date: 2006-12-23
Corresponding author: WANG Yun-yan, PhD, Associate professor; Tel: +86-731-8830875; E-mail: wyy@mail.csu.edu.cn
(Edited by ZHAO Jun)