Ni-20Cr-18W高温合金的熔盐热腐蚀特征
来源期刊:中国有色金属学报(英文版)2015年第11期
论文作者:张铁邦 董瑞峰 胡锐 寇宏超 李金山
文章页码:3840 - 3846
关键词:Ni-20Cr-18W高温合金;热腐蚀;熔盐;腐蚀机制
Key words:Ni-20Cr-18W superalloys; hot corrosion; molten salt; corrosion mechanism
摘 要:研究700和800 °C下Ni-20Cr-18W(质量分数,%)高温合金在混合熔盐(75%Na2SO4-25%NaCl)中的热腐蚀行为。结果表明:合金在混合熔盐环境下受到严重的熔盐热侵蚀。合金的热腐蚀速度随着腐蚀温度的增加迅速加快。热腐蚀层呈现明显的双层结构,并且在内腐蚀层下可明显地观察到贫Cr区的存在。腐蚀过程中所产生的腐蚀产物基本相同,主要包括NiO、Cr2O3和Ni3S2,且在800 °C热腐蚀20 h后,腐蚀产物中检测到少量NiCrO2。此外,提出Ni-20Cr-18W高温合金的热腐蚀机制及腐蚀层的形成机理。
Abstract: The hot corrosion behavior of a Ni-20Cr-18W (mass fraction, %) superalloy in the mixture of 75%Na2SO4-25%NaCl melts at 700 and 800 °C was studied. The results demonstrate that the alloy suffers from serious hot corrosion attack in the mixture molten salt. Meanwhile, the degradation of the substrate accelerates with increasing the corrosion temperature. The corrosion layer has an obvious duplex microstructure, and the Cr-depletion zone is detected obviously nearby the inner corrosion layer. The main corrosion products at 700 and 800 °C are almost the same and mainly include NiO, Cr2O3 and Ni3S2, but a trace amount of NiCrO2 is detected at 800 °C for 20 h. The hot corrosion mechanism and formation mechanism of corrosion scales of the Ni-20Cr-18W superalloy in the molten salt are proposed.
Trans. Nonferrous Met. Soc. China 25(2015) 3840-3846
Tie-bang ZHANG, Rui-feng DONG, Rui HU, Hong-chao KOU, Jin-shan LI
State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi’an 710072, China
Received 9 December 2014; accepted 17 June 2015
Abstract: The hot corrosion behavior of a Ni-20Cr-18W (mass fraction, %) superalloy in the mixture of 75%Na2SO4-25%NaCl melts at 700 and 800 °C was studied. The results demonstrate that the alloy suffers from serious hot corrosion attack in the mixture molten salt. Meanwhile, the degradation of the substrate accelerates with increasing the corrosion temperature. The corrosion layer has an obvious duplex microstructure, and the Cr-depletion zone is detected obviously nearby the inner corrosion layer. The main corrosion products at 700 and 800 °C are almost the same and mainly include NiO, Cr2O3 and Ni3S2, but a trace amount of NiCrO2 is detected at 800 °C for 20 h. The hot corrosion mechanism and formation mechanism of corrosion scales of the Ni-20Cr-18W superalloy in the molten salt are proposed.
Key words: Ni-20Cr-18W superalloys; hot corrosion; molten salt; corrosion mechanism
1 Introduction
Ni-based superalloys have been widely used in high temperature applications. However, a variety of corrosive environments have to be considered for these alloys when they are used in practical applications, such as heat engines and heat exchangers [1-4]. Most of these environments contain oxygen and other reactants such as sulfur, carbon and chlorine. Moreover, the deposits of metallic sulfates and chlorides, such as NaCl and Na2SO4, may also accumulate on the exposed surface. Under certain conditions, these deposited salts give rise to severe hot corrosion attack and accelerate its degradation [3,5]. In recent decades, Ni-based alloys have attracted much attention in the field of heat exchangers and heat engines due to their excellent high temperature strength and good corrosion resistance [3,6]. Haynes 230 and Alloy 617 have been extensively used in various environments such as oxidation, carburization and corrosive conditions owing to their excellent high temperature corrosion resistance [5]. Ni-based superalloy M38G has been designed to meet these stringent conditions by increasing the mass fraction of Cr (16.0%) in alloy’s composition, and found that the protective and compact film of the generated Cr2O3 contributes to the corrosion resistance of the alloy [7].
Recently, a Ni-based superalloy, with the main composition of Ni-20Cr-18W (mass fraction, %), has been developed by increasing the mass fractions of Cr and W in alloy’s composition [8,9]. Ni-20Cr-18W alloys are strengthened by combining the solid-solution strengthening and the carbides precipitation strengthening. The role of W is to provide solid-solution strength. Cr serves two purposes mainly: providing solid-solution strength and improving the corrosion and oxidation resistance. It has good high temperature strength, good oxidation resistance and plasticity [8]. The oxidation behavior of Ni-20Cr-18W superalloys has been studied under different conditions, the results revealed that the alloys exhibited good oxidation resistance [8,10]. From the point of view of application, it is necessary to evaluate the hot corrosion resistance of Ni-20Cr-18W superalloy in corrosive environments.
In the present work, the hot corrosion behaviors of Ni-20Cr-18W superalloys were studied. The mass loss versus time of Ni-20Cr-18W superalloys in molten salt at different temperatures was examined. Then, the effect of test temperature on the hot corrosion behavior was examined. Meanwhile, the products of corrosion scale were systematically characterized. Furthermore, the formation mechanism of the corrosion scales was proposed.
2 Experimental
The chemical composition of the wrought Ni-Cr-W superalloy is (mass fraction): 19.82% Cr, 18.48% W, 1.24% Mo, 0.46% Al, 0.11% C, 0.0028% B, 0.026% La, <0.004% P and S, and balance Ni. The alloy was initially vacuum induction melted (VIM) and then was re-melted twice by vacuum arc remelting (VAR). The ingot was homogenized at 1200 °C for 24 h under vacuum condition. Eventually, the ingot was forged and hot rolled to a sheet with 3 mm in thickness at 1150 °C.
Then, the rectangular specimens with dimensions of 10 mm × 10 mm × 3 mm were cut from the rolled sheet by the electrical-discharge method. The surfaces were polished down to 800-girt abrasive paper. The specimens were cleaned in acetone, ethanol and distilled water in sequence in an ultrasonic bath before the test. A mixture salt of 75%Na2SO4-25%NaCl (mass fraction) was used for hot corrosion study. The Al2O3 crucibles were used to stow the salts and the specimens. The specimens were immersed completely in the mixed salts during the whole process. The crucibles were kept in a muffle furnace at 700 and 800 °C for different time, respectively. Then, the crucibles were taken out from the furnace every 5 h during corrosion up to 20 h and were cooled in air. After the tests, the samples were washed with boiling distilled water to dissolve the remains of Na2SO4-NaCl mixture salts, and to remove the loose corrosion products with hydrochloric acid. Three of the specimens before and after corrosion tests were measured using an electronic balance with an accuracy of 0.1 mg. The morphologies and compositions of the surface of the corroded specimens were investigated by a scanning electron microscope (SEM, VEGA3 TESCAN) equipped with an energy dispersive spectroscope (EDS). The phase compositions of the corroded scales were determined by X-ray diffraction (XRD, DX2700).
3 Results and discussion
3.1 Hot corrosion behavior
Figure 1 shows the mass loss per unit area of the alloys as a function of time in 75%Na2SO4-25%NaCl molten salts at different temperatures. The mass loss is defined as
Δm=(m1-m2)/S (1)
where m1 is the original mass of the specimens, m2 represents the finanl mass of the corroded specimens and S is the original area of the spcimens. The value of Δm less than 0 indicates the mass increase of the spcimens and no spallation of the corrosion scales after corrosion. While the value of Δm greater than 0 suggestes the mass reduction of the specimens and the occurrence of the spallation of the scales after corrosion.

Fig. 1 Mass loss as function of corrosion time of Ni-20Cr- 18W superalloy in molten salt at 700 and 800 °C
It can be seen that the mass loss of the alloys exhibits different stages with increasing the exposure time. At 800 °C, the mass loss increases with the extension of corrosion time, which is closely associated with the spallation of the corrosion scales. At 700 °C, however, the mass loss slightly decreases with increasing the corrosion time during the first 7 h, which can be attributed to no corrosion scales spalling off in this period. Moreover, the mass loss of the specimens at 700 °C is obviously lower than that at 800 °C. Compared with the corrosion at 800 °C during the first 7 h, the mass loss of the specimens corroed at 700 °C is not significant and the corrosion rate is slow, and then the mass loss starts to increase with increasing the corrosion time. The mass loss is ~2.10 mg/mm2 after hot corrosion at 700 °C for 20 h, while the mass loss at 800 °C reachs up to ~7.70 mg/mm2, which indicates that the spallation of specimens corroded at 800 °C is serious. The corrosion rate of the experimental alloy at 800 °C reaches as high as ~0.385 mg/(mm2·h), which is much higher than that at 700 °C (~0.105 mg/(mm2·h)). It is concluded that the corrosion process accelerates extremely with increasing the temperature. ZHAO et al [11] indicated that the oxidation kinetic curve of the Inconel 740 without Na2SO4 deposit followed the parabolic law at 950 °C, while the kinetic curve of the alloy with Na2SO4 deposit at 950 °C did not follow a parabolic law owing to the corrosion scale spallation. Obviously, the spallation of the scale occurs during the whole process and the kinetic curves of the specimens do not obey the parabolic law Figure 2 shows the XRD patterns of the specimens after corrosion for 1 h. The results reveal that the corrosion products on the specimen surface are similar at different temperatures, including mainly NiO, Ni3S2 and Cr2O3. In addition, the peak intensities of the substrate corroded at 700 °C for 1 h are much stronger than those treated at 800 °C, suggesting the formation of a thinner corrosive scale on the specimen corroded at 700 °C for 1 h [12]. Figure 3 shows the XRD patterns of the corrosion products spalled from the substrate after corrosion for 20 h. Compared with the XRD pattern of the specimen corroded at 700 °C for 20 h, the intensities of Bragg peaks of the Ni3S2 corroded at 800 °C for 20 h are obviously stronger, indicating the increased content of Ni3S2. Furthermore, a trace amount of NiCrO2 forms after corroded at 800 °C for 20 h. The difference of the products may be related to the difference of the diffusion of elements at different temperatures [13]. Ni-20Cr- 18W superalloy contains a high content of Cr, and the previous work has demonstrated that the alloy exhibited good oxidation resistance due to the formation of the compact film of Cr2O3 [10]. The recent research has indicated that the compact film of Cr2O3 on the surface of Haynes 230 also contributed to the corrosion resistance [5]. ZHAO et al [14] demonstrated that the elements of Co and Cr improved the corrosion resistance of the Inconel alloy 740, and the rapid degradation of corrosion resistance of the alloy could be attributed to the dissolution of the protective oxide on the surface. However, in this work, a small amount of Cr2O3 is detected on the corrosion scales. This is possibly associated with the fact that the generated Cr2O3 is immediately consumed at the relatively high temperature in the molten salts.

Fig. 2 XRD patterns of corroded scales on Ni-20Cr-18W alloys after corrosion in molten salt at 700 °C (a) and 800 °C (b) for 1 h

Fig. 3 XRD patterns of corroded scales on Ni-20Cr-18W alloys after corrosion in molten salt at 700 °C (a) and 800 °C (b) for 20 h
3.2 Morphology of corrosion scale
The surface morphologies and corresponding EDS analysis of the specimens are presented in Fig. 4 and Table 1, respectively. It can be seen that the surface morphologies of the specimens exhibit different features after corrosion at 700 and at 800 °C for 1 h in mixed molten salts (Figs. 4(a) and (b)). At 700 °C, its surface is composed of many fine grains and there are no obvious corrosion scales spalling from the specimen. The surface of the scales is compact and flat (Fig. 4(a)). At 800 °C, it is shown that its surface consists of many grains in the same way, but its size is big and fluctuant. The surface of the specimen corroded at 800 °C is loose and uneven. Meanwhile, the cracks can be detected on the surface (Fig. 4(b)). The EDS results reveal that the big grains are rich in Ni and S (marked as Spectrum 4 in Fig. 4(b)). It can be deduced that there are some corrosion products spalled off, which is consistent with the analysis of the kinetics of hot corrosion.
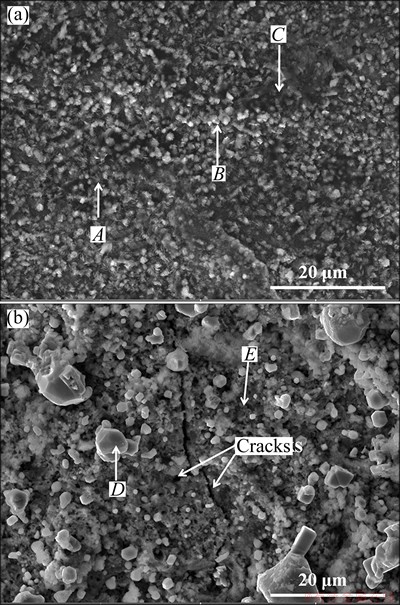
Fig. 4 Surface morphologies of specimens after hot corrosion in mixture melts at 700 °C (a) and 800 °C (b) for 1 h
Table 1 EDS analysis results of points shown in Fig. 4


Fig. 5 Surface morphologies (a, b), corresponding cross-section images (c-f) and linear analysis of specimens (g, h) after hot corrosion in mixture melts at 700 °C (a, c, e, g) and 800 °C (b, d, f, h) for 5 h
Figure 5 shows the surface morphologies and corresponding cross-section morphologies of the specimens corroded in the mixed molten salts at 700 and 800 °C for 5 h. As the corrosion processes at 700 °C, the surface morphology gradually becomes loose and porous, along with the increase of the grain size. Moreover, it can be seen from Fig. 5(a) that a small amount of corrosion scales spalls from the substrate in the form of particles. However, the surface morphology of specimen corroded at 800 °C for 5 h, as shown in Fig. 5(b), presents obvious cracks and the corrosion scales spall off in the form of large lamella. It is revealed that a relatively thick corrosion layer forms on the surface of the specimen shown in Fig. 5(b). The thickness of specimen corroded at 800 °C (Fig. 5(d)) is thinner than that corroded at 700 °C (Fig. 5(c)), which can be related to the severe spallation of the corrosion scales at 800 °C, and it is insignificant at 700 °C. As shown in Figs. 5(e) and (f), the corrosion scales exhibit a duplex structure, consisting of outer layer and inner layer. The inner layer is thinner than the outer one. The outer layer contains more cracks and inclusions than the inner one, which indicates that the corrosion resistance of the inner layer is superior to that of the outer layer. Compared with the scales formed at 700 °C, the loose scales formed at 800 °C contain more cracks and inclusions. The inner layer formed at 700 °C is thicker than that formed at 800 °C, while the thickness of outer layer formed at two different temperatures is reverse. As can be seen from Fig. 5(f), the outer layer stacks in the form of a lamellar structure. Combining the results of XRD and EDS analysis (as indicated in Fig. 5(f)), the inclusions are Ni3S2, and the cracks derive from these inclusions, which accounts for the spallation of corrosion scales [7,13]. A large quantity of cracks in the remain corrosion scales are presented in Fig. 5(f), and which is rare in Fig. 5(e). It can be seen that a thin corrosion affected zone (CAZ) [1] appears beneath the corrosion layer in Figs. 5(e) and (f). Figures 5(g) and (h) show the line distribution of different elements along the arrow marked in Figs. 5(e) and (f), respectively. It is clearly seen that a trace amount of sulfur is detected in the substrate, which implies that internal sulfidation occurs. The detected Cr-depletion zone [14], as shown in Figs. 5(g) and (h), consists well with the CAZ region as indicated by dashed line in Figs. 5(e) and (f), respectively.
Figure 6 shows the surface morphologies and corresponding high magnification images of specimens corroded at 700 and 800 °C for 20 h. At 700 °C (Fig. 6(a)), the cracks are presented and some inclusions are exposed. Combining the results of XRD (Fig. 3) and EDS analysis (Table 2), it can be deduced that the inclusions are Ni3S2. The cracks originate from the inclusions, as shown Figs. 5(f) and 6(a), which eventually leads to the corrosion scales spalling off. Compared with the corrosion at 700 °C, the inclusions formed at 800 °C are big, and the specimen suffers from serious corrosion attack, as shown in Figs. 6(b) and (d).
3.3 Formation mechanism of corrosion scale
Similar to many alloys [4,11,15], the scales formed on the alloys have a duplex microstructure during the hot corrosion (Figs. 5(e) and (f)). The outer layer consists of coarse grains with many pores and cracks, the inner one is composed of fine grains and is compact, and the thickness of the inner layer is thinner than that of the outer one. The difference between two layers can be described as oxide dissolution/reprecipitation mechanism [16,17]. According to the reaction: 4M+ =MS+ 3MO+O2-, the corrosion scales form on the substrate surface firstly [12]. This inner layer is compact and dense. Secondly, the formed scales dissolve into the molten salt simultaneously, according to reaction: MO=M2++O2-, owing to the concentration gradient of O2-. Subsequently, the dissolved oxides diffuse outward through molten salt, and reprecipitate according to reaction: M2++O2-=MO [12], to form the outer oxide layer. As a result, the microstructure of outer layer would be porous and incomplete, as shown in Figs. 5(a) and (b). In addition, the molten salt penetrates through the defects, e.g., pores and cracks, and
=MS+ 3MO+O2-, the corrosion scales form on the substrate surface firstly [12]. This inner layer is compact and dense. Secondly, the formed scales dissolve into the molten salt simultaneously, according to reaction: MO=M2++O2-, owing to the concentration gradient of O2-. Subsequently, the dissolved oxides diffuse outward through molten salt, and reprecipitate according to reaction: M2++O2-=MO [12], to form the outer oxide layer. As a result, the microstructure of outer layer would be porous and incomplete, as shown in Figs. 5(a) and (b). In addition, the molten salt penetrates through the defects, e.g., pores and cracks, and  is reduced by the chemical equilibrium:
is reduced by the chemical equilibrium:  =2O2-+3O2+2S. Then, the released S attacks the substrate, leading to internal sulfidation and forming the sulfide. The corrosion process of the Ni-20Cr-18W superalloy can be considered to follow a sequence presented in Fig. 7. Firstly, S and O diffuse inward, and metal elements, such as Ni, Cr and W, diffuse outward through the molten salts. Secondly, the oxides nucleate at the substrate/salt interface, such as M1O (its vapor pressure is high and can evaporate easily) and M2O (its vapor pressure is relatively low and can dissolve and precipitate easily). As the corrosion progresses, S diffuses inward through the salt, meanwhile M2O will dissolve in the scales and M2 diffuses outward, eventually leading to the formation of sulfide (such as Ni3S2 [14]) in the corrosion scales. Finally, as the vapor pressure of M1O is high enough that some M1O evaporates, meanwhile, M2O reprecipitates on the surface of the scales and its morphology is porous and incompact. The inclusion of the sulfide is generated in the scales. And it can be deduced that the cracks originate from the inclusion, e.g., Ni3S2, which consists with the analysis results presented in Fig. 5. The outer layer peels off easily from the substrate, and the residual outer layer is divided into a layer structure by those inclusions.
=2O2-+3O2+2S. Then, the released S attacks the substrate, leading to internal sulfidation and forming the sulfide. The corrosion process of the Ni-20Cr-18W superalloy can be considered to follow a sequence presented in Fig. 7. Firstly, S and O diffuse inward, and metal elements, such as Ni, Cr and W, diffuse outward through the molten salts. Secondly, the oxides nucleate at the substrate/salt interface, such as M1O (its vapor pressure is high and can evaporate easily) and M2O (its vapor pressure is relatively low and can dissolve and precipitate easily). As the corrosion progresses, S diffuses inward through the salt, meanwhile M2O will dissolve in the scales and M2 diffuses outward, eventually leading to the formation of sulfide (such as Ni3S2 [14]) in the corrosion scales. Finally, as the vapor pressure of M1O is high enough that some M1O evaporates, meanwhile, M2O reprecipitates on the surface of the scales and its morphology is porous and incompact. The inclusion of the sulfide is generated in the scales. And it can be deduced that the cracks originate from the inclusion, e.g., Ni3S2, which consists with the analysis results presented in Fig. 5. The outer layer peels off easily from the substrate, and the residual outer layer is divided into a layer structure by those inclusions.
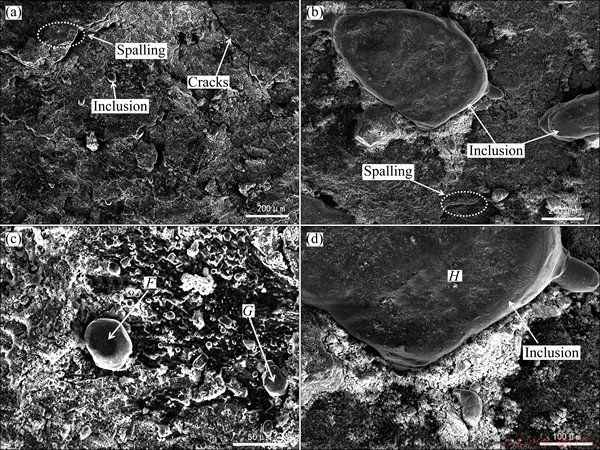
Fig. 6 Surface morphologies and corresponding high magnification images of specimens after hot corrosion in mixture melts at 700 °C (a, c) and 800 °C (b, d) for 20 h

Fig. 7 Schematic diagram showing transition of corrosion scales on substrate during hot corrosion in molten salts
Table 2 EDS analysis results of points shown in Fig. 6
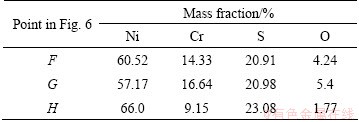
It is also noticed that the difference of surface microstructures of the scales forms at different temperatures. At 700 °C, the precipitate grains are fine and granular, but at 800 °C, the shape is irregular and the size of precipitate grains increases significantly. It can be ascribed to the difference of the diffusion rates of elements and molten salts at different temperatures. At 800 °C, they grow rapidly due to the high diffusion rates of the elements, resulting in a considerably large size.
4 Conclusions
1) The Ni-20Cr-18W superalloy suffers from severe hot corrosion attack in the mixture of 75%Na2SO4-25%NaCl melts, and the corrosion rate at 800 °C (~0.385 mg/(mm2·h)) is obviously higher than that at 700 °C (~0.105 mg/(mm2·h)).
2) The corrosion products at different temperatures are similar, including NiO, Ni3S2 and Cr2O3, but a trace amount of NiCrO2 is detected at 800 °C for 20 h.
3) The corrosion layer has duplex microstructure. The outer layer consists of coarse grains with many pores and cracks, the inner layer is composed of fine grains and is compact. The outer layer is divided into a layer structure by those inclusions. Some cracks exist in the outer layer and they derive from the formation of granulated Ni3S2.
4) The Cr-depletion zone is detected obviously nearby the inner corrosion layer. The difference between two layers can be described as the oxide dissolution/ reprecipitation mechanism.
References
[1] ELIAZ N, SHEMESH G, LATANISION R M. Hot corrosion in gas turbine components [J]. Engineering Failure Analysis, 2002, 9(1): 31-43.
[2] MISRA A K. Studies on the hot corrosion of a nickel-base superalloy, Udimet 700 [J]. Oxidation of Metals, 1986, 25(3-4): 129-161.
[3] ZINKLE S J, WAS G S. Materials challenges in nuclear energy [J]. Acta Materialia, 2013, 61(3): 735-758.
[4] SIDKY P S, HOCKING M G. The hot corrosion of Ni-based ternary alloys and superalloys for application in gas turbines employing residual fuels [J]. Corrosion Science, 1987, 27(5): 499-530.
[5] KIM D, KIM D, LEE H J, JANG C, YOON D J. Corrosion characteristics of Ni-base superalloys in high temperature steam with and without hydrogen [J]. Journal of Nuclear Materials, 2013, 441(1-3): 612-622.
[6] JANG C H, KIM D J, KIM D H, SAH I, RYU W S, YOO Y S. Oxidation behaviors of wrought nickel-based superalloys in various high temperature environments [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(7): 1524-1531.
[7] LIU G M, YU F, TIAN J H, MA J H. Influence of pre-oxidation on the hot corrosion of M38G superalloy in the mixture of Na2SO4-NaCl melts [J]. Materials Science and Engineering A, 2008, 496(1-2): 40-44.
[8] HU R, BAI G H, LI J S, ZHANG J Q, ZHANG T B, FU H Z. Precipitation behavior of grain boundary M23C6 and its effect on tensile properties of Ni–Cr–W based superalloy [J]. Materials Science and Engineering A, 2012, 548: 83-88.
[9] BAI G H, LI J S, HU R, TANG Z W, XUE X Y, FU H Z. Effect of temperature on tensile behavior of Ni–Cr–W based superalloy [J]. Materials Science and Engineering A, 2011, 528(4-5): 1974-1978.
[10] MA Jian, HU Rui, CHANG Hui, BAI Guang-hai, LIU Jun, ZHOU Lian. Evolution of oxidation scale for a Ni-22Cr-20Co-18W alloy at 1100 °C [J]. Rare Metal Materials and Engineering, 2012, 41(3): 486-489. (in Chinese)
[11] ZHAO S, XIE X, SMITH G D. The oxidation behavior of the new nickel-based superalloy Inconel 740 with and without Na2SO4 deposit [J]. Surface and Coatings Technology, 2004, 185(2-3): 178-183.
[12] GUO Jian-ting. Materials science and engineering for superalloys [M]. Beijing: Science Press, 2008: 622-631. (in Chinese)
[13] LI Wei-jie, LIU Yong, WANG Yan, HAN Chao, TANG Hui-ping. Hot corrosion behavior of Ni–16Cr–xAl based alloys in mixture of Na2SO4–NaCl at 600 °C [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(12): 2617-2625.
[14] ZHAO S Q, XIE X S, SMITH G D, PATEL S J. The corrosion of INCONEL alloy 740 in simulated environments for pulverized coal-fired boiler [J]. Materials Chemistry and Physics, 2005, 90(2-3): 275-281.
[15] YANG X, PENG X, WANG F. Hot corrosion of a novel electrodeposited Ni-6Cr-7Al nanocomposite under molten (0.9Na,0.1K)2SO4 at 900 oC [J]. Scripta Materialia, 2007, 56(10): 891-894.
[16] LIU G, LI M, ZHOU Y, ZHANG Y. Influence of pre-oxidation on the hot corrosion of Ti3SiC2 in the mixture of Na2SO4–NaCl melts [J]. Corrosion Science, 2006, 48(3): 650-661.
[17] LIU G, LI M, ZHOU Y. Hot corrosion of Ti3SiC2-based ceramics coated with Na2SO4 at 900 and 1000 oC in air [J]. Corrosion Science, 2003, 45(6): 1217-1226.
张铁邦,董瑞峰,胡 锐,寇宏超,李金山
西北工业大学 凝固技术国家重点实验室,西安 710072
摘 要:研究700和800 °C下Ni-20Cr-18W(质量分数,%)高温合金在混合熔盐(75%Na2SO4-25%NaCl)中的热腐蚀行为。结果表明:合金在混合熔盐环境下受到严重的熔盐热侵蚀。合金的热腐蚀速度随着腐蚀温度的增加迅速加快。热腐蚀层呈现明显的双层结构,并且在内腐蚀层下可明显地观察到贫Cr区的存在。腐蚀过程中所产生的腐蚀产物基本相同,主要包括NiO、Cr2O3和Ni3S2,且在800 °C热腐蚀20 h后,腐蚀产物中检测到少量NiCrO2。此外,提出Ni-20Cr-18W高温合金的热腐蚀机制及腐蚀层的形成机理。
关键词:Ni-20Cr-18W高温合金;热腐蚀;熔盐;腐蚀机制
(Edited by Mu-lan QIN)
Foundation item: Project (51171150) supported by the National Natural Science Foundation of China
Corresponding author: Tie-bang ZHANG; Tel: +86-29-88491764; E-mail: tiebangzhang@nwpu.edu.cn
DOI: 10.1016/S1003-6326(15)64031-6

