联氨和过氧化氢对铝酸钠溶液中沉淀氢氧化铝的影响
来源期刊:中国有色金属学报(英文版)2015年第2期
论文作者:N. K. SAHU C. K. SARANGI B. DASH B. C. TRIPATHY B. K. SATPATHY D. MEYRICK I. N. BHATTACHARYA
文章页码:615 - 621
Key words:aluminium hydroxide; hydrazine; hydrogen peroxide; sodium aluminate; precipitation
摘 要:研究添加联氨和过氧化氢时人工合成铝酸钠溶液中氢氧化铝的结晶行为。添加少量联氨会对氢氧化铝的结晶产生影响,如需获得相同的效果,过氧化氢的用量更多。X射线衍射分析表明生成产物为三水铝石。扫描电镜观察表明,添加联氨时产物为团聚状,而添加过氧化氢时产物为细小弥散颗粒。对有联氨和过氧化氢时存在的结晶机理进行了讨论。
Abstract: Aluminium hydroxide precipitation from synthetic sodium aluminate solution was studied in the presence of hydrazine or hydrogen peroxide. The addition of low concentration of hydrazine is found to be effective, while higher amount of hydrogen peroxide is required to generate similar effect. XRD data confirm the product phase to be gibbsitic by nature. The scanning electron micrographs (SEM) show that agglomerated products form in the presence of hydrazine while fine discrete particles are produced with hydrogen peroxide. The probable mechanism of precipitation in the presence of hydrazine and hydrogen peroxide is also discussed.
Trans. Nonferrous Met. Soc. China 25(2015) 615-621
N. K. SAHU1, C. K. SARANGI2, B. DASH2, B. C. TRIPATHY1,2, B. K. SATPATHY3,
D. MEYRICK4, I. N. BHATTACHARYA1,2
1. Academy of Scientific and Innovative Research, New Delhi 110025, India;
2. Institute of Minerals and Materials Technology, Council of Scientific and Industrial Research, Bhubaneswar 751013, India;
3. National Aluminium Company, Bhubaneswar 751013, India;
4. Chemical and Mathematical Sciences, Murdoch University, Murdoch WA 6150, Australia
Received 25 March 2014; accepted 2 September 2014
Abstract: Aluminium hydroxide precipitation from synthetic sodium aluminate solution was studied in the presence of hydrazine or hydrogen peroxide. The addition of low concentration of hydrazine is found to be effective, while higher amount of hydrogen peroxide is required to generate similar effect. XRD data confirm the product phase to be gibbsitic by nature. The scanning electron micrographs (SEM) show that agglomerated products form in the presence of hydrazine while fine discrete particles are produced with hydrogen peroxide. The probable mechanism of precipitation in the presence of hydrazine and hydrogen peroxide is also discussed.
Key words: aluminium hydroxide; hydrazine; hydrogen peroxide; sodium aluminate; precipitation
1 Introduction
In the Bayer process, bauxite is digested with hot concentrated sodium hydroxide to produce supersaturated sodium aluminate [NaAl(OH)4] solution. Aluminium hydroxide, Al(OH)3(s) (gibbsite), is obtained from the aluminate liquor by seeded precipitation method, and the spent caustic solution is recycled to the digestion process. The productivity of the plant depends upon the gibbsite yield which is low under typical plant operating conditions such as temperature from 60 to 75 °C, m(Al2O3):m(Na2O) ratio of about 1.0, seed addition of 400 to 600 g/L and precipitation period of 48 to 72 h. The wide gap between the quantity of gibbsite precipitates and its equilibrium solubility at a specific precipitation temperature provides scope to enhance the gibbsite yield.
The decomposition of sodium aluminate to precipitate aluminium hydroxide is possible through either physical or chemical method. Seeding is a physical method by which the decomposition of  takes place to form Al(OH)3(s). Another means of decomposition of
takes place to form Al(OH)3(s). Another means of decomposition of  is by proton (H+) assisted precipitation such as acid neutralization. Carbonisation decomposition is another example of chemical decomposition process [1]. In the present work, the use of hydrazine as an additive was investigated to observe any effect if it can impact the decomposition of aluminate ions since it is a weak base in comparison to highly alkaline sodium aluminate solution. Studies so far reported for enhancing the aluminium hydroxide precipitation have dealt with either thermal or mechanical seed activation [2-6]. Use of additives, mainly organic polymers or surfactants, for promoting productivity, has also been investigated [6-15]. The incorporation of H+ ions in the form of EDTA has been demonstrated for improving the yield [12]. Although hydrogen peroxide has been utilized for regeneration of spent sodium hydroxide from aluminium washed solution [13] and for synthesis of high area alumina particles by precipitating boehmite from sodium aluminate solution [15], its use for precipitating aluminium tri-hydroxide in Bayer precipitation process has not been explored yet. Thus, hydrogen peroxide as an additive has also been studied for enhancing the aluminium hydroxide precipitation.
is by proton (H+) assisted precipitation such as acid neutralization. Carbonisation decomposition is another example of chemical decomposition process [1]. In the present work, the use of hydrazine as an additive was investigated to observe any effect if it can impact the decomposition of aluminate ions since it is a weak base in comparison to highly alkaline sodium aluminate solution. Studies so far reported for enhancing the aluminium hydroxide precipitation have dealt with either thermal or mechanical seed activation [2-6]. Use of additives, mainly organic polymers or surfactants, for promoting productivity, has also been investigated [6-15]. The incorporation of H+ ions in the form of EDTA has been demonstrated for improving the yield [12]. Although hydrogen peroxide has been utilized for regeneration of spent sodium hydroxide from aluminium washed solution [13] and for synthesis of high area alumina particles by precipitating boehmite from sodium aluminate solution [15], its use for precipitating aluminium tri-hydroxide in Bayer precipitation process has not been explored yet. Thus, hydrogen peroxide as an additive has also been studied for enhancing the aluminium hydroxide precipitation.
2 Experimental
2.1 Materials
The chemicals used in this work were analytical grade reagents and obtained from Merck, India. Hydrazine hydrate (N2H4·H2O) containing 64% N2H4 and hydrogen peroxide (H2O2) as 30% by volume of H2O2 in aqueous solution were used. Super saturated sodium aluminate solution was prepared by dissolving measured quantity of aluminium granules in concentrated sodium hydroxide solution. In all the experiments, m(Al2O3):m(Na2O) (A/C) ratio was maintained at either 0.95 or 1.0. The particle size of gibbsite seed was 62.2 μm (d50).
The apparatus used in the precipitation studies was made up of 304 stainless steel of 500 mL capacity. The precipitator is fitted with a digital RPM controller. An anchor type impeller was used for the agitation of the solution. Rotation speed of (190±2) r/min was maintained throughout the time period. The temperature of the system was maintained through a constant thermostatic bath circulator (JULBO, Germany) via an inlet and an outlet port.
2.2 Method
All the experiments were conducted taking 250 mL solution with A/C ratio of 0.95 or 1.0, where caustic concentration was fixed at 150 g/L and Al2O3 concentration was fixed as per the A/C ratio. The temperature was maintained at about 75 °C else otherwise mentioned and the precipitation was continued for 8 or 24 h. The seed and other additions were made once the required temperature was attained. H2O2 was added after mixing it with few drops of NaOH solution to avoid addition of acidic H2O2. At the end of the precipitation period, the slurry was filtered and the residue was washed several times before drying in an oven overnight at 100 °C. The aluminium hydroxide precipitation yield was calculated as follows:
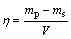 (1)
(1)
where η is the aluminium hydroxide precipitation yield, mp is the mass of precipitate, ms is the mass of seed, and V is the experimental volume of solution.
X-ray diffractograms were recorded for the aluminium hydroxide powders using PANalytical diffractometer (PW 1830, Philips, Japan) with Cu Kα radiation, λ=1.54056  . The scans were recorded in 2θ range of 5° to 65°. A scanning electron microscope (SEM) (JEOL JSM 6510, Japan) was used to examine the surface morphology of the precipitates.
. The scans were recorded in 2θ range of 5° to 65°. A scanning electron microscope (SEM) (JEOL JSM 6510, Japan) was used to examine the surface morphology of the precipitates.
In this work, two different precipitation ratios were analyzed and calculated as follows:
 ,
,  (2)
(2)
where RE is the yield enhancement ratio, ηa is the yield with additive, ηb is the yield under blank, “blank” specifies the condition when precipitation was carried out in the absence of additives. It should be kept in mind that yield under blank condition changes with solution concentration, temperature, and A/C ratio. Rs is the precipitation ratio under available supersaturation, η is the yield, and ηs is the supersaturation level.
2.3 Equilibrium solubility
The equilibrium solubility of Al(OH)3 is the saturation point of solution below which level precipitation is improbable at a particular temperature; only the available supersaturation can practically be precipitated at the most under standard seeded precipitation process. The equilibrium solubility of alumina in the sodium aluminate solution was measured using the well known Rosenberg-Healy [16] formula as follows:

 (3)
(3)
where ionic strength I=0.01887c+0.01937cs; c and cs are the caustic and carbonate concentrations in g/L, respectively; ΔG=-30.96 kJ/mol is Gibbs free energy of solid formation; R=8.3145 kJ/mol is the universal gas constant; T is the precipitation temperature, K. Using the above equation, the equilibrium solubility of alumina at 75 °C (A75) was calculated. When the Na2O concentration is 150 g/L, A75=88 g/L. Therefore, for A/C=1.0, where Al2O3 concentration is 150 g/L and ρ(Na2O)=150 g/L, the available supersaturation is 62 g/L as ρ(Al2O3)=95 g/L as aluminium hydroxide. Similarly, for A/C=0.95, where Al2O3 concentration is 142.5 g/L, the available supersaturation is 54.5 g/L as ρ(Al2O3)= 83.35 g/L as aluminium hydroxide.
3 Results and discussion
3.1 Effect of hydrazine
Figure 1 shows the effect of hydrazine concentration on the precipitation yield of aluminium hydroxide. It is observed that at an A/C ratio of 0.95 or 1.0, when hydrazine is added with small quantities, the yield is increased for both 8 h and 24 h precipitation periods, but with increasing the amount of hydrazine, the yield drops almost to its initial value, i.e., that when there is no hydrazine in the solution (“blank”). For example, with A/C ratio of 0.95, the initial yield is observed to be about 32 g/L in 8 h precipitation period when 100 g/L of seed is added in the absence of hydrazine. Under similar conditions, when 24-40 mmol/L of hydrazine is added, the yield increases to 38 g/L and the enhancement with respect to the initial yield is about 20%. However, when the hydrazine concentration is increased step wise to 800 mmol/L, the yield decreases gradually and reaches almost the “blank” value. Beyond this hydrazine amount, the yield is reduced below the “blank” value.

Fig. 1 Variation of aluminium hydroxide yield with hydrazine concentration
Figure 2 shows the variation in yield enhancement ratio of aluminium hydroxide with the hydrazine concentration. It is observed in general that at A/C ratios including 0.95 and 1.0, the yield enhancement ratio increases at low hydrazine concentrations up to 40 mmol/L and further addition of hydrazine results in a reduction of yield enhancement ratio. The comparison of curves (a) and (c) in Fig. 2 indicates that at each level of hydrazine concentration, the yield enhancement ratio is larger for 24 h precipitation period than that of 8 h. In the case of 8 h curve (b), with 150 mmol/L or more hydrazine, the yield enhancement ratio goes below the “blank” value, indicating an inhibition effect of hydrazine at higher concentrations. Whereas for 24 h curve (c), the similar phenomenon is noticed at 800 mmol/L or more hydrazine in the solution. This difference observed in 8 h and 24 h cases may be attributed to the significant growth of the precipitates with prolonged precipitation period.

Fig. 2 Variation of yield enhancement ratio with hydrazine concentration

Fig. 3 Variation of precipitation ratio under available supersaturation with hydrazine concentration
The variation in precipitation ratio under available supersaturation (Section 2.3) with hydrazine concentration is shown in Fig. 3. It indicates that in all the cases, the precipitation ratio under available supersaturation increases with the addition of hydrazine in the aluminate liquor up to 40 mmol/L in a similar manner as observed with yield enhancement ratio. However, for the period of precipitation of 8 h with A/C ratio of 1.0, precipitation ratios are comparatively much higher than that with A/C ratio of 0.95. At A/C ratio of 1.0, with increased period of precipitation (24 h), considerable amount of growth of the precipitates leads to an increase in the precipitation ratio which is confirmed from the comparison of 8 h and 24 h cases curves (b) and (c) in Fig. 3. In the case of 8 h, with 40 mmol/L hydrazine addition, about 63% precipitation ratio is observed but increasing the time period to 24 h, the precipitation ratio increases to 82%, leaving only 18% of supersaturation. Furthermore, this precipitation ratio value of 82% is found to be higher than that of “blank” by about 13%.
The effect of temperature from 65 to 80 °C on aluminium hydroxide yield enhancement ratio was studied and the results are shown in Fig. 4. It is observed that with 40 mmol/L of hydrazine in the aluminate liquor, the yield enhancement ratios increase with the increase of temperature. The yield enhancement ratio has increased from 10% at 65 °C to about 27% when precipitation temperature is raised to 80 °C. Thus, it is concluded that temperature has a positive role in aluminium hydroxide precipitation process at low hydrazine concentration.

Fig. 4 Variation of yield enhancement ratio with temperature in presence of hydrazine
It is worthy to mention here that, two major effects of hydrazine have been observed during aluminium hydroxide precipitation. Firstly, aluminium hydroxide yield increases with the addition of low amount (24- 40 mmol/L) of hydrazine and the inhibiting effect is observed when hydrazine is added in huge amount (800 mmol/L or more). Secondly, increase in temperature also possesses beneficial effect on aluminium hydroxide precipitation. Although the accurate mechanism for the observed phenomena is not well understood due to its complexity, a possible mechanism has been explained as follows. When hydrazine is added into an aqueous solution system, it hydrolyses to form hydrazinium ion  and hydroxyl ion (OH-) as shown in Eq. (4).
and hydroxyl ion (OH-) as shown in Eq. (4).
N2H4+H2O=N2H5++OH- (4)
These hydrazinium ions possibly enhance the nucleation phenomenon by assisting the coalescence of aluminate ions to form clusters so that the concentration zone develops for the formation of critical crystallite sites to form nuclei. Once the nuclei form, they promote further precipitation of aluminium hydroxide from the solution. This may be categorized as heterogeneous nucleation assisted by some foreign ions. The effect is found to be more pronounced at higher temperatures due to the improved mobility of ions, which increase the possibility of coalescences. KABDASLI et al [17] observed the role of different ions during struvite precipitation. They found that sodium ions retard induction time significantly while sulphate ions increase it. WATLING et al [18] found that low concentrations (1-3 mmol/L) of gluconate enhance the secondary nucleation. However, higher amounts of gluconate suppressed nucleation and leads to poisoning of the liquor as well [19]. In the present case, the inhibiting effect of hydrazine at higher concentrations may be attributed to the covering of the seed surface area by hydrazinium ions which may prevent aluminate ions to coalesce onto the seed surface and thereby reduce the secondary nucleation.
X-ray diffraction patterns (Fig. 5) reveal that crystalline gibbsite is the only phase presented in all the cases, with no evidence of bayerite, boehmite or pseudo- boehmite in the precipitates. As visualized from the SEM images (Fig. 6), the precipitate obtained with only seed (Fig. 6(a)) is composed of very fine crystals and there is little agglomeration of the particles. It seems that delayed nucleation with limited growth and lack of availability of time for agglomeration are responsible for the formation of fine precipitates. A marked improvement in morphology is observed when hydrazine is added together with seed (Fig. 6(b)). This produces well agglomerated gibbsite crystals of larger size, which indicates that in the presence of hydrazine, early nucleation occurs and time for agglomeration followed by cementation of the crystallites is also available.

Fig. 5 XRD patterns of aluminium hydroxide precipitates obtained in presence of blank (a), hydrazine (b) and hydrogen peroxide (c)
3.2 Effect of hydrogen peroxide
Figure 7 shows the effect of hydrogen peroxide on the yield of aluminium hydroxide for A/C ratio of 0.95 and 1.0 in 8 h and 24 h precipitation periods. In all cases, the yields are found to increase with the increase in concentrations of H2O2 up to a level of 350 mmol/L, and beyond this level, the enhancement in yield is found to be minimal.

Fig. 6 SEM images of aluminium hydroxide precipitates

Fig. 7 Variation of aluminium hydroxide yield with H2O2 concentration
The variation in yield enhancement ratio of aluminium hydroxide with H2O2 concentration is shown in Figure 8. For 8 h case, it is observed that the yield enhancement ratio with A/C ratio of 0.95 is higher than that with A/C ratio of 1.0. Around 35% to 38% yield enhancement is observed in the case of A/C ratio of 1.0 and around 80% enhancement is observed with a A/C ratio of 0.95. However, enhancement in yield with A/C ratio of 1.0 is found to be lower when the precipitation time period is increased to 24 h. Around 15% reduction in yield is observed in 24 h case compared to that in 8 h.
With regard to the available supersaturation, the precipitation ratios are increased considerably with the increase in H2O2 concentration (Fig. 9). However, beyond 350 mmol/L of H2O2, the change in precipitation ratio is negligible. Precipitation ratios in 8 h case are found to be about 75% and 69% for A/C ratios of 1.0 and 0.95, respectively. Furthermore, when the time period is increased to 24 h, precipitation ratio under available supersaturation increases to 82%, thereby leaving only around 18% supersaturation to be precipitated. This is almost equivalent to that observed with the addition of hydrazine.

Fig. 8 Variation of yield enhancement ratio with H2O2 concentration

Fig. 9 Variation of precipitation ratio under available supersaturation with H2O2 concentration
A possible mechanism for the enhancement in yield due to the presence of hydrogen peroxide has been explained as follows. In strong alkaline solution, H2O2 is deprotonated to give hydroperoxide ion ( ) and hydrogen ion (H+) (Eq. (5)) [13]. The proton (H+) generated from hydrogen peroxide decomposes tetrahydroxoaluminate ion
) and hydrogen ion (H+) (Eq. (5)) [13]. The proton (H+) generated from hydrogen peroxide decomposes tetrahydroxoaluminate ion  giving rise to aluminium hydroxide (Eq. (6)). The sodium ions released after the decomposition of sodium aluminate species would attach to OH- ions which are generated during the decomposition of hydroperoxide ions, keeping the electroneutrality and alkalinity of the sodium aluminate solution intact. In conventional precipitation process, OH- ion released from aluminate ion dissociation during seeded precipitation will get attached to the released Na+ ion and form NaOH. While in this case, as proton reacts with the OH- ion and forms water molecule during aluminate ion decomposition, the hydroxyl ion required for the regeneration of soda is available from the dissociation product of hydroperoxide ion as shown in Eq. (7).
giving rise to aluminium hydroxide (Eq. (6)). The sodium ions released after the decomposition of sodium aluminate species would attach to OH- ions which are generated during the decomposition of hydroperoxide ions, keeping the electroneutrality and alkalinity of the sodium aluminate solution intact. In conventional precipitation process, OH- ion released from aluminate ion dissociation during seeded precipitation will get attached to the released Na+ ion and form NaOH. While in this case, as proton reacts with the OH- ion and forms water molecule during aluminate ion decomposition, the hydroxyl ion required for the regeneration of soda is available from the dissociation product of hydroperoxide ion as shown in Eq. (7).
In the present case the following reactions therefore take place
H2O2→ +H+ (5)
+H+ (5)
NaAl(OH)4+H+→Al(OH)3+H2O+Na+ (6)
 →OH-+(1/2)O2 (7)
→OH-+(1/2)O2 (7)
Na++OH-→NaOH (8)
The overall reaction thus becomes
2NaAl(OH)4+2H2O2→2Al(OH)3+2NaOH+2H2O+O2 (9)
When H2O2 is added into the sodium aluminate solution initially, a localized white precipitate of aluminium hydroxide forms and subsequently it dissolves in the solution while stirring. This has got beneficial effect on precipitation as it initiates early nucleation thereby producing enhanced yield with 80%- 85% precipitation under available supersaturation.
The XRD patterns shown in Fig. 5 confirm the formation of gibbsite particles at the experimental temperature. The addition of hydrogen peroxide did not change the product phase (Fig. 5(c)). SEM micrographs (Figs. 6(a) and (c)) show the morphologies of the gibbsite particles in the absence and presence of hydrogen peroxide, respectively. The morphology in both cases is similar, indicating no adverse effect of H2O2 on gibbsite precipitation.
4 Conclusions
1) The presence of either hydrazine or hydrogen peroxide in the sodium aluminate liquor is found to be effective in improving the process of precipitation substantially. Lower amount of hydrazine of 24- 40 mmol/L is beneficial whereas higher dose of about 350 mmol/L is required for a significant yield enhancement in the case of hydrogen peroxide.
2) Yield enhancement ratios of aluminium hydroxide are higher with increased temperatures under the studied range of 65-80 °C. About 82% precipitation of available supersaturation, which is about 13% more than that of “blank”, is observed when both the additives are added separately, leaving only 18% for precipitation.
3) XRD patterns of the precipitated aluminium hydroxide confirms their gibbsitic nature irrespective of hydrazine or hydrogen peroxide content. SEM images show that the agglomerated particles are formed in the presence of hydrazine. However, particles produced in the presence of hydrogen peroxide are found to be discrete and of finer size.
4) A further study on this aspect of utilizing weak base and other additives will definitely open up the avenue for finding a long wanted solution for obtaining aluminium hydroxide precipitation equivalent to 100% supersaturation in Bayer precipitation process.
Acknowledgements
The authors would like to thank Prof. B. K. MISHRA, Director, CSIR-IMMT for his keen interest in the work. They are also thankful to M/s NALCO, Bhubaneswar for the partial financial support to carry out this work. One of the authors would like to thank DST, New Delhi for providing him INSPIRE fellowship.
References
[1] LI X. A preliminary discussion on the carbonisation decomposition process [J]. Light Metals, 1988: 135-142.
[2] HECTOR J M, MERCED J M R, MANUEL J R L, ORIANO A, VARGAS P, JUAN S R. Aluminium oxide and hydroxide from non-bauxite sources [J]. American Ceramic Society Bulletin, 1997, 76(6): 55-59.
[3] ANJIER J L, BREUER R G, BUTLER H L. Utilization of partially calcined alumina as precipitation aid in the Bayer process: US4568527 [P]. 1986
[4] HALFON A, KALIAGUINE S. Alumina trihydrate crystallization Part 1: Secondary nucleation and growth rate kinetics [J]. Canadian Jounal of Chemical Engineering, 1976, 54(3): 160-167.
[5] ZHANG B, LI J, CHEN Q, CHEN G. Precipitation of Al(OH)3 crystals from supersaturated sodium aluminate solution irradiated with ultrasonic sound [J]. Minerals Engineering, 2009, 22(9-10): 853–858.
[6] ZENG J, YIN Z, CHEN Q. Intensification of precipitation of gibbsite from seeded caustic aluminate liquor by seed activation and addition of crown ether [J]. Hydrometallurgy, 2007, 89(1-2): 107-116.
[7] ROE W J, PERISH J L. Use of polymers in alumina precipitation in Bayer process of bauxite beneficiation: US4608237 [P]. 1986.
[8] OWEN D O, DAVIS D C. Use of surfactants in alumina precipitation in Bayer process: US4737352 [P]. 1988.
[9] GONTIJO G S, de ARAUJO A C B, PRASAD S, VASCONCELOS L G S, ALVES J J N, BRITO R P. Improving the Bayer process productivity: An industrial case study [J]. Minerals Engineering, 2009, 22(13): 1130-1136.
[10] ZENG J S, YIN Z L, CHEN Q Y. Effect of tetracarbon additives on gibbsite precipitation from seeded sodium aluminate liquor [J]. Journal of Central South University of Technology, 2008, 15(5): 622-626.
[11] ZHANG Y, ZHENG S, DU H, XU H, WANG S, ZHANG Y. Improved precipitation of gibbsite from sodium aluminate solution by adding methanol [J]. Hydrometallurgy, 2009, 98(1-2): 38-44.
[12] LU B L, CHEN Q Y, YIN Z L, HU H P. Effects of Na4EDTA and EDTA on seeded precipitation of sodium aluminate solution [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(1): 37-41.
[13] BARAKAT M A, EL-SHEIKH S M, FARGHLY F E. Removing Al and regenerating caustic soda from spent washing liquor of Al etching [J]. Journal of Metals, 2005, 57(8): 34-38.
[14] BARAKAT M A, EL-SHEIKH S M, FARGHLY F E. Regeneration of spent alkali from aluminium washing [J]. Separation and Purification Technology, 2005, 46(3): 214-218.
[15] EL-KATATNY E A, HALAWY S A, MOHAMED M A, ZAKI M I. A novel synthesis of high area alumina via H2O2-precipitated boehmite from sodium aluminate solution [J]. Journal of Chemical Technology and Biotechnology, 1998, 72(4): 320-328.
[16] ROSENBERG S P, HEALY S J. A thermodynamic model for gibbsite solubility in Bayer liquor [C]//Proceedings of the Fourth Alumina Quality Workshop. Darwin, Australia, 1996: 301-310.
[17] KABDASZLI I, PARSONS S A, TUNAYA O. Effect of major ions on induction time of struvite precipitation [J]. Croatica Chemica Acta, 2006, 79(2): 243-251.
[18] WATLING H, LOH J, GATTER H. Gibbsite crystallization inhibition: Effects of sodium gluconate on nucleation, agglomeration and growth [J]. Hydrometallurgy, 2000, 55(3): 275-288.
[19] ROSSITER D S, ILIEVSKI D, SMITH P G, PARKINSON G M. The mechanism of sodium gluconate poisoning of gibbsite precipitation [J]. Chemical Engineering Research and Design, IChemE, 1996, 74A: 828-834.
N. K. SAHU1, C. K. SARANGI2, B. DASH2, B. C. TRIPATHY1,2, B. K. SATPATHY3,
D. MEYRICK4, I. N. BHATTACHARYA1,2
1. Academy of Scientific and Innovative Research, New Delhi 110025, India;
2. Institute of Minerals and Materials Technology, Council of Scientific and Industrial Research, Bhubaneswar 751013, India;
3. National Aluminium Company, Bhubaneswar 751013, India;
4. Chemical and Mathematical Sciences, Murdoch University, Murdoch WA 6150, Australia
摘 要:研究添加联氨和过氧化氢时人工合成铝酸钠溶液中氢氧化铝的结晶行为。添加少量联氨会对氢氧化铝的结晶产生影响,如需获得相同的效果,过氧化氢的用量更多。X射线衍射分析表明生成产物为三水铝石。扫描电镜观察表明,添加联氨时产物为团聚状,而添加过氧化氢时产物为细小弥散颗粒。对有联氨和过氧化氢时存在的结晶机理进行了讨论。
关键词:氢氧化铝;联氨;过氧化氢;铝酸钠;结晶
(Edited by Yun-bin HE)
Corresponding author: C. K. SARANGI; Tel: +91-674-2379386; E-mails: sarangi.ck@gmail.com; cksarangi@immt.res.in
DOI: 10.1016/S1003-6326(15)63644-5

