DOI: 10.11817/j.issn.1672-7207.2020.03.002
铝酸钠溶液净化除锂及其反应机理研究
唐文奇,彭志宏,刘桂华,周秋生,齐天贵,李小斌
(中南大学 冶金与环境学院,难冶有色金属资源高效利用国家工程实验室,湖南 长沙,410083)
摘要:通过合成高活性氢氧化铝净化铝酸钠溶液中的锂,考察不同反应条件对沉锂效果的影响,并对除锂反应机理进行研究。通过XRD和SEM分析,对沉锂产物进行表征。基于不同条件下沉锂率与反应时间的关系,构建Avrami 动力学方程。研究结果表明:在苛性比αk(即溶液中Na2Ok与Al2O3物质的量比)为1.45的铝酸钠溶液中,控制温度为353 K,高活性氢氧化铝添加量为1.3 g/L,反应时间为120 min,沉锂率可达80.04%;高活性氢氧化铝与溶液中锂离子发生沉淀反应,其沉锂产物为Li2Al4(CO3)(OH)12·3H2O;在348~363 K温度范围内,沉锂过程的表观活化能Ea=16.474 kJ/mol,指前因子A=160.355 min-1,速率常数k可以表示为k=160.355× 。
。
关键词:铝酸钠;除锂;铝锂水化合物;沉锂动力学
中图分类号:TF821 文献标志码:A
文章编号:1672-7207(2020)03-0579-08
Lithium removal from sodium aluminate solution and its reaction mechanism
TANG Wenqi, PENG Zhihong, LIU Guihua, ZHOU Qiusheng, QI Tiangui, LI Xiaobin
(National Engineering Laboratory for Efficient Utilization of Refractory Nonferrous Metal Resources,School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: The lithium in sodium aluminate solution was purified by high activity aluminum hydroxide. The effects of different reaction conditions on lithium removal were investigated, and the mechanism of lithium removal reaction was studied. The lithium precipitation products were characterized by XRD and SEM. Based on the relationship between lithium deposition rate and reaction time under different conditions, the Avrami kinetic equation was established. The results show that in sodium aluminate solution with caustic ratio αk (i.e. the molar ratio of Na2Ok and Al2O3) of 1.45, lithium deposition rate can reach 80.04% when the temperature is 353 K, the addition of high active aluminium hydroxide is 1.3 g/L, and the reaction time is 120 min. High active aluminium hydroxide reacts with lithium ions in solution, and the precipitation product is Li2Al4(CO3)(OH)12·3H2O. The apparent activation energy in the range of 348-363 K is 16.474 kJ/mol in lithium deposition process, the pre-exponential factor A is 160.355 min-1, and the rate constant k can be expressed as k=160.355×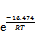 .
.
Key words: sodium aluminate; lithium removal; aluminium lithium hydrate; lithium deposition kinetics
我国铝土矿资源中,大多数铝土矿中锂含量较高(Li2O质量分数为0.016%~0.030%)。在拜耳法溶出过程中,铝土矿中的锂约有80%会进入铝酸钠溶液中[1-3]。进入铝酸钠溶液中的锂在晶种分解时会析出进入氢氧化铝中,这将导致焙烧后的氧化铝产品中锂含量过高。在后续铝电解过程中,锂会在电解槽中积累[4],造成电解质中锂含量升高,大部分生产企业电解质中氟化锂质量分数超过3%,最高达8%~10%[5-7]。电解质体系中的锂含量过高会导致电解槽温度与电解质的熔点下降,氧化铝的溶解度降低,并且会使电解槽炉底沉淀增多,导致电解工艺操作难度增大,铝电解槽稳定性变差,技术条件难以保持,对生产稳定和节能降耗十分不利[8-11],部分电解铝企业甚至被迫弃用高锂含量氧化铝原料[12],所以,研究铝酸钠溶液净化除锂具有重要意义。相关学者研究了锂在铝酸钠溶液中的存在形式及其变化规律,如:VAN STRATEN等[13]发现锂在铝酸钠溶液中以锂离子形式存在,并易与Al(OH)4-反应生成LiA12(OH)7·2H2O沉淀;PRESTIDGE等[14]发现在极稀的铝酸钠溶液中,锂离子能促进拜尔石的析出和长大,并部分与拜尔石一起析出;HUANG等[15]发现升高温度、增大苛性碱质量浓度或减小氧化铝质量浓度都有助于提高铝酸钠溶液中锂离子的质量浓度;在晶种分解 9 h后,铝酸钠溶液中锂离子质量浓度仅为35 mg/L,且随着铝酸钠溶液中锂离子质量浓度增加,溶液的晶种分解率和氢氧化铝中锂含量均增大[16]。而对于铝酸钠溶液中除锂的研究,宁夏[17]在铝酸钠溶液中添加 FeSO4·7H2O和Na3PO4·12H2O与Li+共沉淀,控制Li+,Fe2+和PO43-物质的量比为1:4:4、温度为70 ℃、反应时间为2 h,沉锂率可达90%,但该法同时会给铝酸钠溶液中带入杂质离子;采用高比表面γ-Al2O3吸附铝酸钠溶液中的锂,并将溶液温度控制在80 ℃左右,锂吸附率可达71.14%。而在盐湖卤水提锂过程中,可在卤水中加入无定型氢氧化铝对锂进行高效吸附,获得LiCl·2Al(OH)3 nH2O沉淀,之后再进行铝锂分离,从而达到提取锂的目的[18-20]。本文作者采用高活性氢氧化铝对铝酸钠溶液中的锂进行净化沉淀,考察温度、时间、添加量、苛性比对沉锂效果的影响,确定较适宜的沉锂参数条件,并对沉锂过程的动力学进行分析,探讨净化沉锂的机理,为下一步实现铝酸钠溶液中锂的资源化综合利用提供理论和技术支撑。
nH2O沉淀,之后再进行铝锂分离,从而达到提取锂的目的[18-20]。本文作者采用高活性氢氧化铝对铝酸钠溶液中的锂进行净化沉淀,考察温度、时间、添加量、苛性比对沉锂效果的影响,确定较适宜的沉锂参数条件,并对沉锂过程的动力学进行分析,探讨净化沉锂的机理,为下一步实现铝酸钠溶液中锂的资源化综合利用提供理论和技术支撑。
1 实验
1.1 实验原料和设备
实验原料为:Al(OH)3和NaOH,均为工业级(中国铝业中州分公司生产);NaHCO3和Li2SO4·H2O,均为分析纯(国药集团化学试剂有限公司生产)。设备为DY-8低压反应群釜(中南大学机械厂制造)。
1.2 含锂铝酸钠溶液的配制
根据溶液中所需苛性比αk(即溶液中Na2Ok与Al2O3物质的量比),将工业级氢氧化铝、工业级氢氧化钠和水按照一定比例加至不锈钢桶内,加热煮沸至溶液清亮,得到浓铝酸钠溶液。使用时,将溶液稀释至苛性碱质量浓度为150~160 g/L,并趁热加入一定量的Li2SO4·H2O,搅拌溶解,得到后续实验所需的含锂铝酸钠溶液,锂的质量分数为50×10-6。
1.3 高活性氢氧化铝的制备
将一定质量浓度的NaHCO3溶液加入已配置好的纯铝酸钠溶液中(NaHCO3溶液与铝酸钠溶液体积比为1:2),在室温下搅拌10 min可获得高活性氢氧化铝。经检测,获得的高活性氢氧化铝的平均粒度d(0.5)=3.55 μm,比表面积为42.074 m2/g,其SEM图如图1所示。从图1可见:颗粒呈不规则形状,表面存在缺陷,具有较多活性点。通过调控不同质量浓度的NaHCO3溶液可合成不同量的高活性氢氧化铝浆液,一般来说,加入的NaHCO3质量浓度越高,获得的Al(OH)3量也越多。将由不同NaHCO3质量浓度制得的Al(OH)3浆液过滤,烘干,称质量,结果如表1所示。
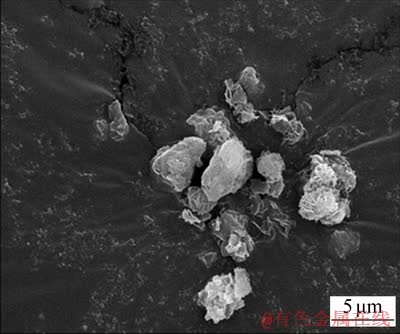
图1 高活性氢氧化铝的SEM图
Fig. 1 SEM image of high activity aluminum hydroxide
1.4 实验方法
实验在低压反应群釜内进行。先在低压弹内加入100 mL已配好的含锂铝酸钠溶液,再往溶液中加入10 mL高活性氢氧化铝浆液。随后,将低压弹放至低压反应群釜,并控制相应的温度以及转动速率。反应一段时间后把低压弹取出,将反应后的溶液进行抽滤。滤液定容稀释后,进行分析检测。抽滤获得的渣烘干后,进行形貌和物相分析。
表1 不同NaHCO3质量浓度对应的高活性Al(OH)3质量
Table 1 Quality of high active Al(OH)3 corresponded to different mass concentrations of NaHCO3

根据反应前后溶液中锂的质量浓度来计算沉锂率ηLi,按下式计算:
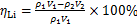 (1)
(1)
式中: 1和
1和 2分别为反应前、后溶液中锂质量浓度,mg/L;V1和V2分别为反应前、后溶液稀释体积,L。
2分别为反应前、后溶液中锂质量浓度,mg/L;V1和V2分别为反应前、后溶液稀释体积,L。
沉锂过程中铝酸钠溶液的水解率可利用反应前后溶液苛性比αk计算,用 表示,
表示,
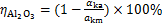 (2)
(2)
式中: 和
和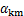 分别为反应前、后铝酸钠溶液的苛性比。
分别为反应前、后铝酸钠溶液的苛性比。
1.5 检测方法
利用滴定法测量溶液中的Na2Ok和Al2O3质量浓度,并计算相应的αk。通过使用电感耦合等离子体发射光谱仪(ICAP7400,美国Radial-赛默飞世尔科技公司)检测稀释滤液中锂的质量浓度。通过使用Rigaku-TTRⅢ型 X线衍射仪(XRD,日本株式会社,铜靶)分析物相成分。通过使用JSM-6360LV 型高低真空扫描电子显微镜(日本电子公司)观察沉锂渣形貌和分布情况。
2 结果和讨论
2.1 温度和时间对沉锂的影响
首先研究温度对沉锂反应的影响,控制溶液初始αk为1.45,高活性氢氧化铝添加量为1.3 g/L,结果见图2。由图2可知:沉锂率均随着反应时间延长而增大;温度越高,沉锂率反而越低;当温度为348 K时对应沉锂率最高,在反应180 min后达到87.17%,而在363 K时沉锂率最低;反应180 min后,沉锂率只有74.17%。
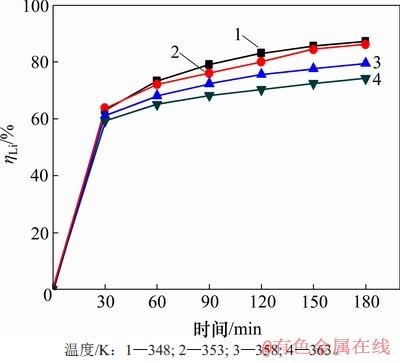
图2 温度对沉锂率ηLi的影响
Fig. 2 Effect of temperature on lithium deposition rate
但低αk溶液不稳定,在温度较低时易产生水解。沉锂过程中水解率变化见图3。由图3可知:温度越低,时间越长,水解程度越大;当温度为348 K时,反应180 min后溶液中水解率 达14.7%。这是因为当温度较低并且随着时间的延长,铝酸钠溶液的不稳定性导致溶液中铝酸根离子水解生成Al(OH)3,并且Al(OH)3的产生也有助于沉锂反应的进行。
达14.7%。这是因为当温度较低并且随着时间的延长,铝酸钠溶液的不稳定性导致溶液中铝酸根离子水解生成Al(OH)3,并且Al(OH)3的产生也有助于沉锂反应的进行。
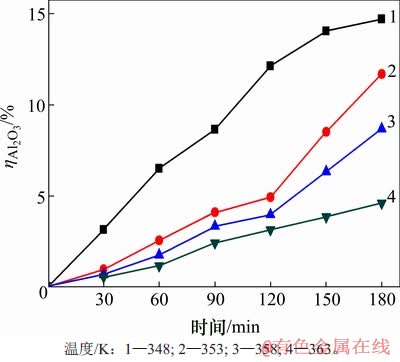
图3 沉锂过程中水解率ηAl2O3变化
Fig. 3 Change of hydrolysis rate during lithium deposition
在除锂的过程中,若铝酸根离子产生过多水解会造成铝酸钠溶液中铝的损失加大,且不利于后续铝锂分离。而在反应时间120 min内,353,358和363 K这3种温度所对应的 较接近,且均小于5%,说明在这个范围内铝酸根离子水解较少。因此,结合反应中的沉锂率和水解率,经综合权衡,当可控制温度为353 K以及反应时间为120 min时,可获得较高沉锂率,同时,能使溶液中铝酸根离子产生较少的水解。
较接近,且均小于5%,说明在这个范围内铝酸根离子水解较少。因此,结合反应中的沉锂率和水解率,经综合权衡,当可控制温度为353 K以及反应时间为120 min时,可获得较高沉锂率,同时,能使溶液中铝酸根离子产生较少的水解。
2.2 高活性氢氧化铝添加量对沉锂的影响
当温度为353 K时,在αk为1.45的铝酸钠溶液中,高活性氢氧化铝添加量(即加入的高活性氢氧化铝浆液中对应的干基质量与含锂铝酸钠溶液体积的比值)与时间对沉锂率的影响如图4所示。从图4可见:随着添加量加大,反应时间延长,沉锂率均随之增大。需要指出的是,在反应120 min后,所有不同添加量所对应的沉锂率均变化缓慢,随后保持稳定,其中,当添加量为1.3,1.9和2.7 g/L时,沉锂率较接近。这是因为添加量较高时,溶液中高活性氢氧化铝较多,在短时间内结晶成核速率快,从而使得沉锂率高。而理论上只需1.1 g/L高活性氢氧化铝即可与质量分数为50×10-6锂离子反应完全,因此,随着时间延长,过量的高活性氢氧化铝对成核将无显著效果。经综合考虑,可选取高活性氢氧化铝添加量为1.3 g/L来沉淀溶液中的锂。
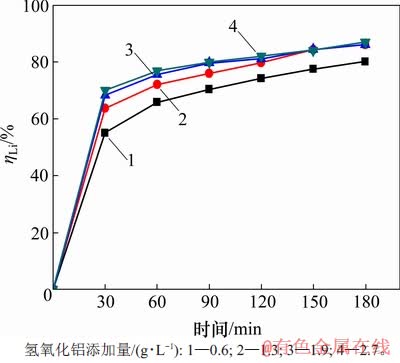
图4 高活性氢氧化铝添加量对沉锂率ηLi的影响
Fig. 4 Effect of addition of high active aluminium hydroxide on lithium deposition rate
2.3 溶液αk对沉锂率的影响
配置不同苛性比αk的含锂铝酸钠溶液,在温度为353 K,高活性氢氧化铝添加量为1.3 g/L时,考察溶液中苛性比αk对沉锂率的影响,如图5所示。从图5可见:溶液中αk越大,沉锂率也越大。这表明溶液中游离碱含量升高,有利于沉锂反应的进行。但结合氧化铝生产实践[21],大部分铝土矿溶出后的铝酸钠溶液αk较低,通常在1.45左右。
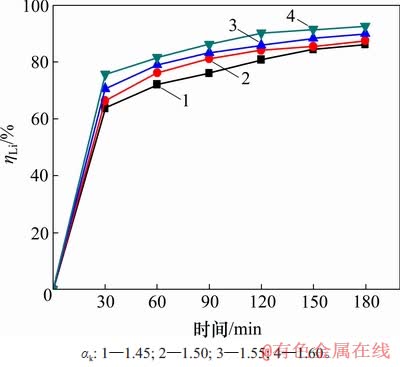
图5 溶液αk对沉锂率的影响
Fig. 5 Effect of caustic ratio on lithium deposition rate
3 沉锂反应机理
3.1 沉锂产物表征
在较适宜沉锂条件(即溶液初始αk为1.45,温度为353 K,高活性氢氧化铝添加量为1.3 g/L,反应时间为120 min)下,将获得的沉锂渣干燥后进行XRD分析,结果如图6所示。从图6可见:沉锂产物的主要物相成分为Li2Al4(CO3)(OH)12·3H2O,且具有较好的结晶形态。沉锂渣的SEM图如图7所示。从图7可观察到沉锂渣主要以片状交叉或片状重叠形式富聚在一起。经检测,沉锂渣平均粒度d(0.5)为28.36 μm,比表面积为3.91 m2/g。因此,结合图1、图6和图7,可判定沉锂反应主要是由高活性氢氧化铝浆液中的Al(OH)3以及CO32-与含锂铝酸钠溶液中的Li+以非均相成核方式结晶长大,最终形成化合物沉淀。含锂铝酸钠溶液在加入高活性氢氧化铝后的化学反应式可表示为
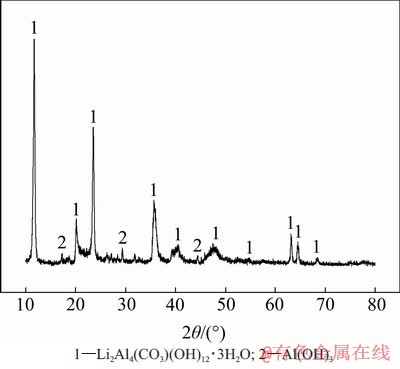
图6 沉锂渣XRD图
Fig. 6 XRD pattern of lithium slag

图7 沉锂渣的SEM图
Fig. 7 SEM image of lithium slag
2Li++4Al(OH)3+CO32-+3H2O→Li2Al4(CO3)(OH)12·3H2O (3)
3.2 动力学模型的选择
氢氧化铝沉锂过程的等温结晶动力学可用 Avrami 动力学模型描述:
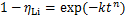 (4)
(4)
式中:k为结晶速率常数,min-n;t为反应时间,min;ηLi为沉锂率;n为Avrami指数,与成核机理和生长方式有关。对式(4)两边取对数,可得
lg[-ln(1-ηLi)]=lnk+nlgt (5)
将lg[-ln(1-ηLi)]对lgt作图,根据所得直线的斜率和截距可确定Avrami指数n和动力学速率常数k。利用不同条件下的沉锂率与时间关系,可构建相关沉锂过程动力学模型[22-23]。
3.3 动力学模型的验证
将不同温度、高活性氢氧化铝添加量、溶液的苛性比条件下的沉锂率分别与反应时间采用 Avrami动力学方程进行拟合,其拟合曲线分别如图8、图9和图10所示,根据各曲线的斜率和截距可计算出各动力学参数,其结果及拟合程度分别如表2~4所示。从图8~10可看出:在实验时间内,沉锂反应均符合Avrami方程,能很好地对结晶成核过程进行描述。Avrami指数n理论上为整数1,2,3等,通常n=1为棒状和柱状晶粒,n=2为盘状晶粒,n=3为球状晶粒。但许多实验表明,实际结晶过程较复杂,成核不可能完全按一种方式进行。因此,在一般情况下,Avrami指数不为整数,通常为小数。
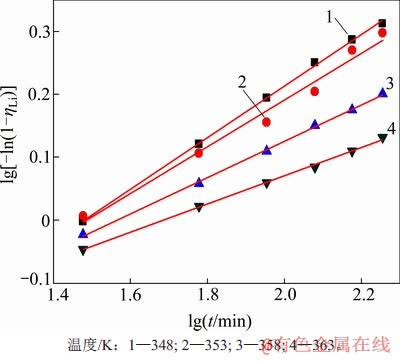
图8 不同反应温度下lg[-ln(1-ηLi)]与lgt的关系
Fig. 8 Relationship between lg[-ln(1-ηLi)] and lgt at different temperatures
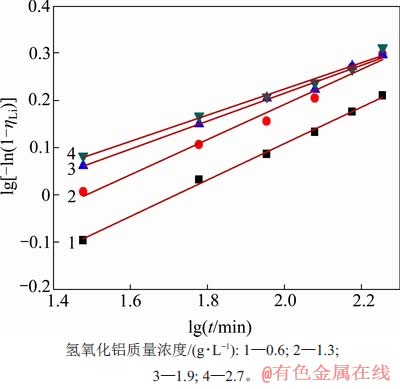
图9 不同高活性氢氧化铝质量浓度下lg[-ln(1-ηLi)]与lgt的关系
Fig. 9 Relationship between lg[-ln(1-ηLi)] and lgt with different mass concentration of high active aluminium hydroxid
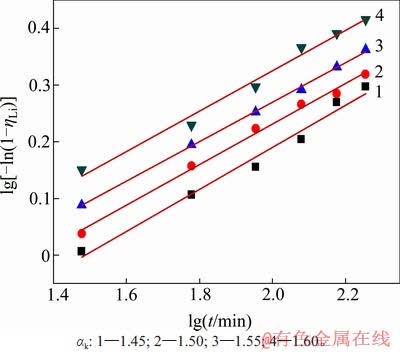
图10 不同溶液苛性比下lg[-ln(1-ηLi)]与lgt的关系
Fig. 10 Relationship between lg[-ln(1-ηLi)] and lgt corresponding to different caustic ratios
表2 不同温度下的动力学参数
Table 2 Kinetic parameters at different temperatures
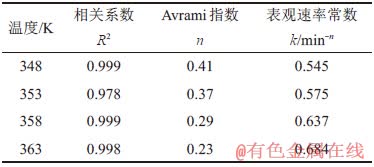
表3 不同添加量下的动力学参数
Table 3 Kinetic parameters at different additions

表4 不同苛性比αk下的动力学参数
Table 4 Kinetic parameters at different caustic ratios

从表2~4可看出:随着反应温度、高活性氢氧化铝添加量、溶液苛性比增大,结晶速率常数k不断增大;而 Avrami指数n最高为0.41,小于1,可以说明反应中的晶体为一维生长[24],并且反应温度与表观速率常数的关系通常可用Arrhenius经验式[25]表示:
 (6)
(6)
式中:k为温度为T时反应的表观速率常数,min-1;R为摩尔气体常数,J/(mol·K);T为反应温度,K;A为指前因子,min-1;Ea为表观活化能,J/mol。
对式(6)两边取对数,可得
 (7)
(7)
根据表2数据,将lnk与T -1作图,如图 11所示。由图11中拟合直线的斜率和截距可计算得出反应表观活化能Ea=16.474 kJ/mol,指前因子A=160.355 min-1,反应表观速率常数可表示为k=160.355×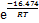 。
。
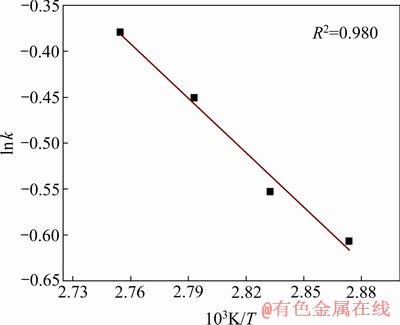
图11 反应表观速率常数与温度的关系
Fig. 11 Relationship between apparent rate constant and temperature
4 结论
1) 在苛性比为1.45的铝酸钠溶液中,控制温度为353 K,高活性氢氧化铝添加量为1.3 g/L,反应时间为120 min,除锂率能达80.04%,且溶液中铝酸根离子的水解率仅为4.9%。
2) 高活性氢氧化铝能与铝酸钠溶液中的锂离子反应形成Li2Al4(CO3)(OH)12·3H2O沉淀,且主要以片状交叉或片状重叠形式聚集。
3) 随着温度升高和溶液中αk增大以及高活性氢氧化铝添加量增加,反应速率常数也随之增大。在温度为348~363 K时,反应表观活化能Ea=16.474 kJ/mol,指前因子A=160.355 min-1,Avrami指数最高可达0.41,速率常数可用方程k=160.355×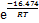 表示。
表示。
参考文献:
[1] 曹阿林, 李春焕. 拜耳法氧化铝生产过程中锂的富集机制研究[J]. 有色金属(冶炼部分), 2017(9): 23-26.
CAO Alin, LI Chunhuan. Enrichment mechanism of lithium in bayer process of alumina production[J]. Nonferrous Metals(Extractive Metallurgy), 2017(9): 23-26.
[2] 李春潮, 黄健. 锂在氧化铝生产过程中的存在行为[J]. 轻金属, 2005(6):17-19.
LI Chunchao, HUANG Jian. The existing state of lithium in the process of alumina production[J]. Light Metals, 2005(6): 17-19..
[3] WANG Denghong, LI Peigang, QU Wenjun, et al. Discovery and preliminary study of the high tungsten and lithium contents in the Dazhuyuan bauxite deposit, Guizhou, China[J]. Science China (Earth Sciences), 2013, 56(1):145-152.
[4] 胡清韬, 梁玉冬, 王成智, 等. 高锂电解质对铝电解生产的影响及改善措施[J]. 有色金属(冶炼部分), 2018(1): 34-38, 43.
HU Qingtao, LIANG Yudong, WANG Chengzhi, et al. Effect of high lithium electrolyte on aluminum electrolysis production and improvement measures[J]. Nonferrous Metals(Extractive Metallurgy), 2018(1): 34-38, 43.
[5] LU Xiaojun, CHEN Shiyue, LAI Yanqing, et al. Effect of LiAlO2 and KF on physicochemical properties for industrial aluminum electrolyte[C]. TMS 2013 142nd Annual Meeting & Exhibition. San Antonio, USA: TMS Light Metals, 2013: 705-709.
[6] 黄海波, 邱仕麟. 富锂氧化铝对铝电解生产的影响[J]. 轻金属, 2014(8): 26-28.
HUANG Haibo, QIU Shilin. Influences of rich-lithium alumina on aluminum reduction production[J]. Light Metals, 2014(8): 26-28.
[7] 周云峰, 李昌林, 柴登鹏, 等. 富锂氧化铝电解过程中锂平衡研究[J]. 轻金属, 2016(1): 30-33.
ZHOU Yunfeng, LI Changlin, CHAI Dengpeng, et al. Balance of lithium on lithium-rich aluminum electrolytic process [J]. Light metals, 2016 (1): 30-33.
[8] LU Xiaojun, SHUANG Yajing, LI Jie, et al. Physicochemical properties of industrial aluminum electrolytes enriching Li and K: the liquidus temperature[J]. Metallurgical and Materials Transactions B, 2017, 48(2): 1315-1320.
[9] SAITOV A V, BAZHIN V Y, POVAROV V G. On the application of lithium additives in the electrolytic production of primary aluminum[J]. Russian Metallurgy(Metally), 2017, 2017(12): 1018-1024.
[10] MORISHIGE T, HAARBERG G M, GUDBRANDSEN H, et al. Effects of composition and temperature on current efficiency for aluminium electrolysis from cryolite-based molten alumina electrolytes[J]. ECS Transactions, 2017, 77(11): 997-1002.
[11] 陈富强.锂盐、钾盐对预焙槽生产的影响[J]. 材料与冶金学报, 2010, 9(增刊): 148-149, 151.
CHEN Fuqiang. Effect of lithium and potassium salts on the production of prebaked cell[J]. Journal of Materials and Metallurgy, 2010, 9(Suppl): 148-149, 151.
[12] 刘冬喜. 富锂电解质对铝电解工艺条件的影响及对策[J]. 轻金属, 2014(2): 30-33.
LIU Dongxi. Influence of the Li rich electrolyte on aluminum reduction process and countermeasures[J]. Light Metals, 2014(2): 30-33.
[13] VAN STRATEN H, SCHOONEN M, DE BRUYN P. Precipitation from supersaturated aluminate solutions. III. Influence of alkali ions with special reference to Li+[J]. Journal of Colloid and Interface Science, 1985, 103(2): 493-507.
[14] PRESTIDGE C A, AMETOV I. Cation effects during aggregation and agglomeration of gibbsite particles under synthetic Bayer crystallisation conditions[J]. Journal of Crystal Growth, 2000, 209(4): 924-933.
[15] HUANG Wenqiang, LIU Guihua, LIU Peng, et al. Equilibrium concentration of lithium ion in sodium aluminate solution[J]. Journal of Central South University, 2019, 26(2): 304-311.
[16] HUANG Wenqiang, LIU Guihua, JU Jinbin, et al. Effect of lithium ion on seed precipitation from sodium aluminate solution[J]. Transactions of Nonferrous Metals Society of China, 2019, 29(6): 1323-1331.
[17] 宁夏. 铝酸钠溶液除锂的研究[D]. 长沙: 中南大学冶金与环境学院, 2018: 15-26.
NING Xia. Study on lithium removal from sodium aluminate solution[D]. Changsha: Central South University. School of Metallurgy and Environment, 2018: 15-26.
[18] LIU Xuheng, CHEN Xingyu, HE Lihua, et al. Study on extraction of lithium from salt lake brine by membrane electrolysis[J]. Desalination, 2015, 376: 35-40.
[19] HEIDARI N, MOMENI P. Selective adsorption of lithium ions from Urmia Lake onto aluminum hydroxide[J]. Environmental Earth Sciences, 2017, 76(16): 551.
[20] WILLIAMS G R, O'HARE D. A kinetic study of the intercalation of lithium salts into Al(OH)3[J]. The Journal of Physical Chemistry B, 2006, 110(22): 10619-10629.
[21] 毕诗文. 氧化铝生产工艺[M]. 北京: 化学工业出版社, 2006: 32-36.
BI Shiwen. Alumina production process[M]. Beijing: Chemical Industry Press, 2006: 32-36.
[22] YANG Jiao, MCCOY B J, MADRAS G. Distribution kinetics of polymer crystallization and the Avrami equation[J]. The Journal of Chemical Physics, 2005, 122(6): 064901.
[23] HUBBES S, DANZL W, FOERST P. Crystallization kinetics of palm oil of different geographic origins and blends thereof by the application of the Avrami model[J]. LWT-Food Science and Technology,2018,93:189-196.
[24] HAY J N. Application of the modified avrami equations to polymer crystallisation kinetics[J]. British Polymer Journal, 1971, 3(2): 74-82.
[25] 李洪桂. 冶金原理[M]. 北京: 科学出版社, 2010: 307-310.
LI Honggui. Metallurgical principle[M]. Beijing: Science Press, 2010: 307-310.
(编辑 陈灿华)
收稿日期: 2019 -10 -10; 修回日期: 2019 -12 -22
基金项目(Foundation item):国家自然科学基金资助项目(51874366)(Project(51874366) supported by the National Natural Science Foundation of China)
通信作者:齐天贵,博士,副教授,从事氧化铝生产理论与工艺研究;E-mail: qitiangui@csu.edu.cn