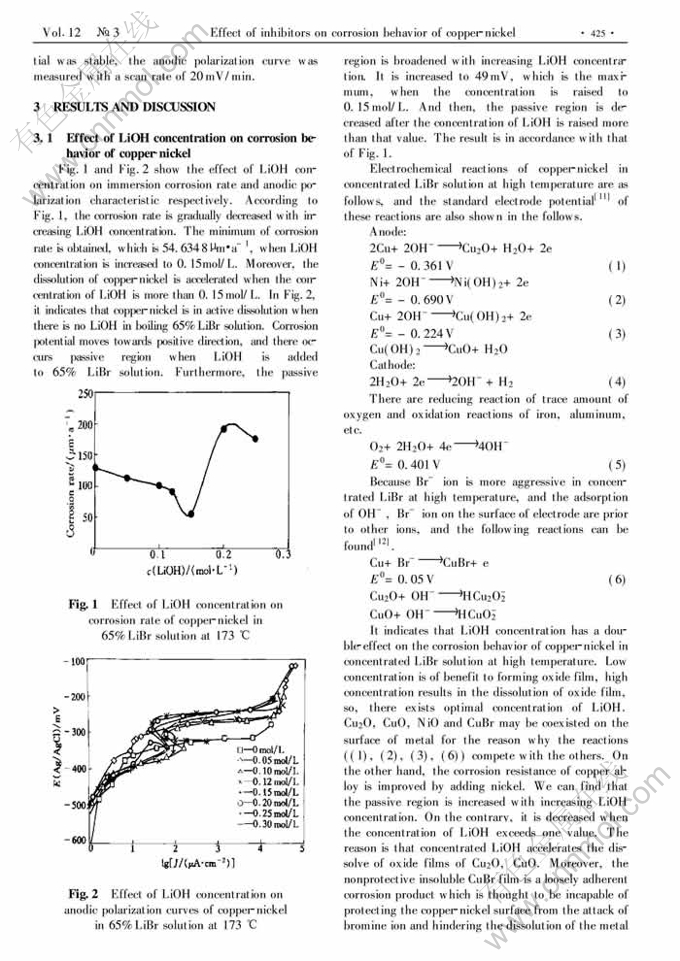
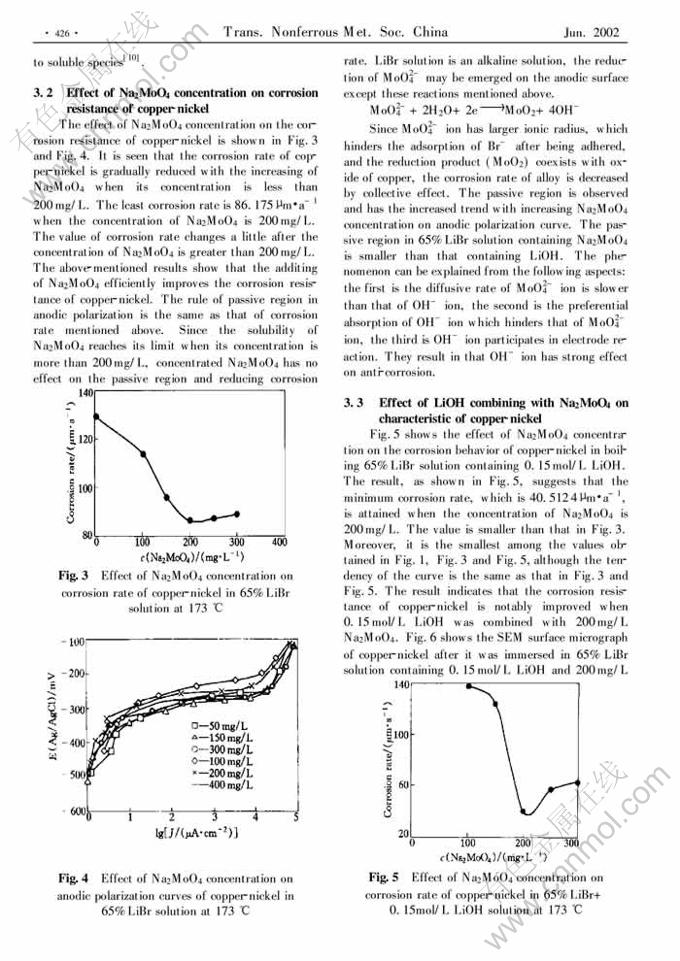
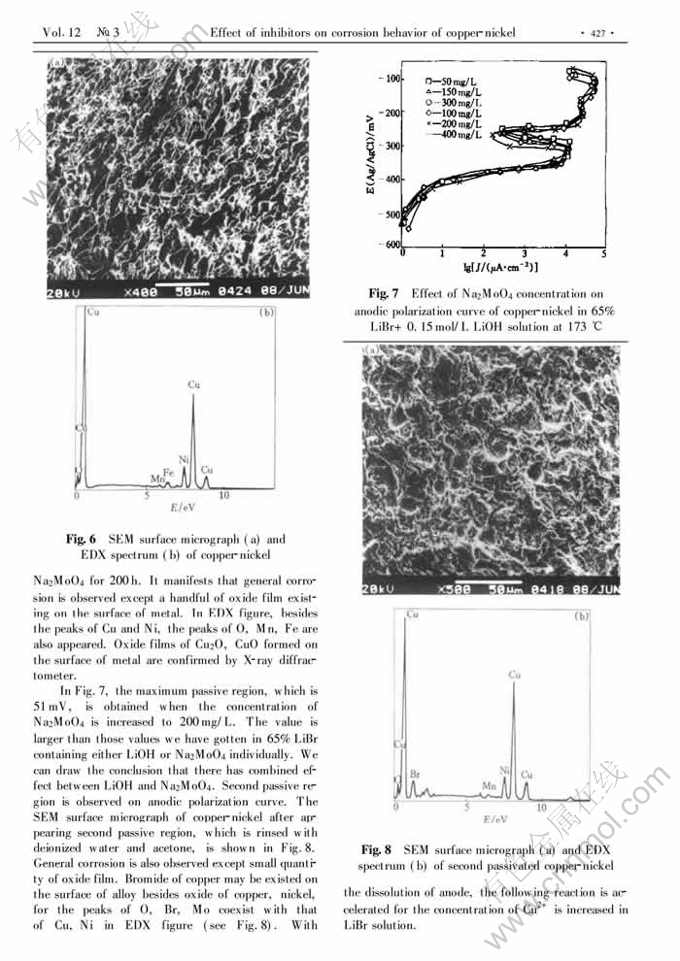
Effect of inhibitors on corrosion behavior of copper-nickel in concentrated lithium bromide solution at high temperature
来源期刊:中国有色金属学报(英文版)2002年第3期
论文作者:黄乃宝 梁成浩 佟大维
文章页码:424 - 428
Key words:inhibitor; copper-nickel; lithium bromide; corrosion
Abstract: The conventional mass-loss tests and the electrochemical techniques were used t o study the inhibition action of LiOH and Na2MoO4 either individually or in different combination for copper-nickel alloy in boiling 65%LiBr solution. It indicates that the corrosion rate of copper-nickel is decreased when LiOH or Na2MoO4 is added to the solution individually. LiOH concentration has a double-effect on the corrosion behavior of copper-nickel. Low concentration is benefit to forming oxide film. High concentration results in dissolution of oxide film. The optimal concentration of LiOH is 0.15mol/L. The dissolution of copper-nickel is effectively prevented when adding 200mg/L Na2MoO4 to boiling 65%LiBr solution with 0.15mol/L LiOH. The inhibition mechanism is considered that the films of Cu, Ni, Mo oxides and deposited nonprotective in soluble CuBr on the surface of metal could prevent Br- ion from absorption, which prevent alloy dissolving.