纯Ti和Ti-Pd合金在有、无添加H2O2的磷酸盐缓冲溶液中的耐腐蚀性能
来源期刊:中国有色金属学报(英文版)2013年第3期
论文作者:P. HANDZLIK K. FITZNER
文章页码:866 - 875
Key words:titanium; Ti-Pd alloy; biomaterials; corrosion; inflammation; electrochemical impedance spectroscopy
摘 要:采用动电位和电化学阻抗谱技术研究了纯Ti(2级)和Ti-Pd合金(7级)的耐腐蚀性能。实验温度为36.6 °C,实验溶液包括模拟的健康人体条件的pH 7.4 的PBS溶液和添加了H2O2 (0.015 mol/L)的pH 5.2的炎症状态的PBS溶液。Ti-Pd合金(7级Ti),在含H2O2的PBS溶液中,其耐腐蚀性能比纯Ti的好(较低的腐蚀电流密度),表明其是一种很好的骨科植入材料。
Abstract: Corrosion resistance of pure titanium (Grade 2) and Ti-Pd alloy (Grade 7) was studied using the electrochemical techniques of potentiodynamic measurements and electrochemical impedance spectroscopy (EIS). Measurements were performed at the temperature of 36.6 °C in two solutions: PBS solution with pH of 7.4 simulating conditions of healthy human body, and PBS solution with pH of 5.2 and with the addition of hydrogen peroxide (0.015 mol/L) simulating the inflammatory state. It is found that, Ti Grade 7 can be a good candidate as a material for orthopedic implant application, because its corrosion resistance in the PBS solution containing H2O2 is better (lower corrosion current densities) than that of pure titanium.
Trans. Nonferrous Met. Soc. China 23(2013) 866-875
P. HANDZLIK, K. FITZNER
Faculty of Non-Ferrous Metals, AGH University of Science and Technology, 30 Mickiewicza Ave., 30-059 Cracow, Poland
Received 24 February 2012; accepted 5 July 2012
Abstract: Corrosion resistance of pure titanium (Grade 2) and Ti-Pd alloy (Grade 7) was studied using the electrochemical techniques of potentiodynamic measurements and electrochemical impedance spectroscopy (EIS). Measurements were performed at the temperature of 36.6 °C in two solutions: PBS solution with pH of 7.4 simulating conditions of healthy human body, and PBS solution with pH of 5.2 and with the addition of hydrogen peroxide (0.015 mol/L) simulating the inflammatory state. It is found that, Ti Grade 7 can be a good candidate as a material for orthopedic implant application, because its corrosion resistance in the PBS solution containing H2O2 is better (lower corrosion current densities) than that of pure titanium.
Key words: titanium; Ti-Pd alloy; biomaterials; corrosion; inflammation; electrochemical impedance spectroscopy
1 Introduction
Titanium and its alloys are one of the most important materials for orthopedic (articular prostheses, osteosynthesis plates, and screws) and dental applications (crown and bridge) [1]. Titanium exhibits a very good corrosion resistance in various media: acid, alkali, organic compounds, etc. This remarkable resistance is because of the passive oxide film formed instantaneously on the surface of titanium in air or in most aqueous solutions. The physicochemical and electrochemical properties of the oxide film and its long-term stability in biological environments are decisive factors for biocompatibility of titanium and its alloys. The adverse body reaction to the implant is due to the metallic ions released by the implant. The amount of ions released depends on the corrosion rate. For this reason, studies on the corrosion and passivity of implants in various media which simulate fluids of human body, i.e., Ringer’s solution [2,3], phosphate-buffered saline solution [4-6], artificial saliva [6-8], Hank’s solution, [7,9] have been performed.
Alloying titanium with another element is the method to increase the mechanical properties of this metal for medical implants application [10-12]. However, there are also titanium alloys with addition of noble elements which increase corrosion resistance of these materials [13,14]. The improvement in corrosion resistance resulting from the addition of noble metals is based on the principle of cathodic modification which consists in enhanced hydrogen evolution on the alloy surface. This effect raises the cathodic polarization curve above the critical active anodic current so that it intersects the anodic curve at a higher potential in the passive region, and the final corrosion rate is lower than that of pure metal [15]. Titanium-palladium alloys with nominal palladium contents of about 0.2% Pd are used in applications requiring excellent corrosion resistance in chemical processing or storage applications, where the environment is mildly reducing or fluctuates between oxidizing and reducing. In a few works, the titanium-palladium alloy was studied as a promising material for implants. It is known that adding small amount of Pd and other platinum group metals to titanium causes an increase of their corrosion resistance in many media, particularly acids [16-19]. The investigations of the corrosion resistance of Ti-Pd alloys (especially Ti, Grade 7) provide interesting information about electrochemical behavior in different environments. SATOH et al [20] studied crevice corrosion of Ti-Pd alloys and another titanium alloys in chloride solutions at elevated temperatures. The results of this work showed superior corrosion resistance of Ti Grade 7 alloy in comparison with Ti Grade 2 (commercially pure Ti). Authors suggested that this alloy can be used for vessels in the chemical industries, for heat transfer tubes in steam turbine condensers, and in desalination plants. Ti Grade 7 is nowadays widely used in nuclear waste repository environments, so one can find information about problems of corrosion resistance of this alloy under these conditions.
BROSSIA and CRAGNOLINO [21] examined the effects of chloride concentration, fluoride concentration, pH, temperature, and the weldments on the corrosion behavior of Ti-0.15%Pd (Ti Grade 7) alloy. The results showed that, even at elevated temperatures (165 °C), the passivity breakdown and repassivation potentials were well above 1 V (vs SCE) in a solution containing 4 mol/L Cl-. Fluoride addition to Cl– solutions resulted in a significant increase in the potential-independent anodic current density, which was several orders of magnitude greater than typical passive current densities. They also examined the effects of palladium additions on the localized and uniform corrosion of titanium by comparing the corrosion behavior of Ti Grade 2 with Ti Grade 7 [22]. The pitting and repassivation potentials for Ti Grade 2 were significantly lower than the potentials for Ti Grade 7 in the same chloride solutions. It was observed that Pd addition has significant effects on the localized corrosion resistance, but did not reduce the damaging effect of fluoride. HUA et al [23] have presented the review of corrosion performance of Ti Grade 7 and other relevant Ti alloys (Ti Grade 2, 5, 9, 11, 12, 16, 17 and 18) under waste repository conditions. In that work, general corrosion, localized corrosion, stress corrosion cracking, microbiologically influence corrosion and radiation-assisted corrosion were considered. It was concluded that the drip shields made of Ti Grade 7 will not suffer from any of earlier mentioned type of corrosion for 10000 years. VAUGHAN and ALFANTAZI [24] studied the corrosion behavior of titanium alloys (Grade 1, 2—pure Ti, Grade 7—Ti0.15Pd, Grade 12—Ti0.3Mo0.8Ni, and Grade 18— Ti3Al2.5V0.05Pd) in sulfuric acid and the effect of the addition of chlorides because of the application of these materials as the liner and internal components of autoclaves during the high-pressure acid leaching of nickel laterite ores. From the thermodynamic analysis, it was found that the presence of 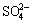 and Cl- ions lessen the region of stability of the passive titanium film. Immersion tests revealed that alloying with Pd enhances the corrosion resistance of Ti significantly; also, higher concentration of Pd in Ti results in lower corrosion rate.
and Cl- ions lessen the region of stability of the passive titanium film. Immersion tests revealed that alloying with Pd enhances the corrosion resistance of Ti significantly; also, higher concentration of Pd in Ti results in lower corrosion rate.
The problem of the application of the Ti-Pd alloy as a biomaterial was analyzed only in a few works. RAMIRES and GUASTALDI [25] studied the electrochemical behavior of Ti-Pd and Ti-6Al-4V alloys in a 0.9% NaCl solution by using single triangular potential sweep, potentiodynamic polarization curves and electrochemical impedance spectroscopy (EIS). The results of experiments indicate that the alloying element has an effect on the corrosion resistance of titanium in the NaCl solution. The complicated corrosion mechanism can be interpreted at least one electro- dissolution process and the posterior formation of a corrosion films. The results from EIS indicate that the corrosion layer is formed by different mechanisms on each alloy. NAKAGAWA et al [26] investigated the corrosion of pure titanium, Ti6Al4V and Ti6Al7Nb alloys, and experimental alloys Ti-(0.1%-2.0%)Pt and Ti-(0.1%-2.0%)Pd using anodic polarization and corrosion potential measurements in aerated and deaerated artificial saliva containing 0.2% NaF solution. The surfaces of the Ti-Pt and Ti-Pd alloys were not affected by an acidic environment with fluoride, but the surfaces of pure titanium, Ti6Al4V alloy, and Ti6Al7Nb alloy were markedly roughened by corrosion. Authors suggested that the new Ti-Pt and Ti-Pd alloys are expected to be used in dental work. However, in the work of YOKOYAMA et al [27] the fracturing of Ti-0.2Pd alloy in acidic and neutral fluoride solutions by a sustained tensile-loading test was considered. In 2.0% acidulated phosphate fluoride (APF) and neutral 2.0% NaF solution, the time to fracture the Ti-0.2Pd alloy is shorter compared with commercial pure titanium for the same applied stress. It was concluded that the susceptibility to fracturing of Ti-0.2Pd alloy is higher than that of commercial pure titanium in earlier mentioned solutions, and the corrosion behavior of this alloy is very sensitive to the applied stress.
Although most in vitro studies show that oxide layer on titanium and its alloys after 30-60 d of immersion in artificial physiological solutions has a few nanometers [28,29], some clinical observations deny this fact. It seems that the oxide layer can interact with the body fluids and as a result of this interaction it can grow. It was found by MCQUEEN et al [30] that the thickness of the oxide layer on titanium implants placed in human body may reach values above nanometric scale after few years. Additionally, there exists an evidence that Ti is released into and accumulated in tissues neighboring to implants [5]. Accumulation of Ti in tissues adjacent to the implant could not be connected only with wear process [31]. TENGVALL et al [32,33] suggested that possible explanation of the high corrosion rate of Ti in vivo consisted in the inflammatory response caused by the surgical trauma after implants inserting into the living tissues. During the wound healing process, pH values of tissues adjacent to implant may decrease to 3.5 [34]. During the inflammatory state, the hydrogen peroxide is produced by bacteria and leucocytes. Hydrogen peroxide is dismuted from superoxide O2·- (free radical) in mitochondrion [35]. H2O2 may be a driving force for the enhanced titanium oxide dissolution and growth. PAN et al [5,36,37] investigated titanium exposed to a phosphate buffered saline (PBS) solutions without and with 100 mmol/L H2O2 addition respectively. They found that H2O2 addition led to a significant decrease in corrosion resistance of titanium. FONSECA and BARBOSA [38] studied the electrochemical behavior of Ti in PBS solutions with and without 50 or 150 mmol/L of H2O2 using electrochemical impedance spectroscopy (EIS) for 3 weeks. After 1 week, the PBS/H2O2 solution was replaced by fresh PBS solution in order to simulate the end of the inflammatory process and recovery of the system. They concluded that the corrosion resistance of titanium is strongly affected by the presence of H2O2. When the peroxide is removed, the metal displays a sharp resistance increase. MABILLEAU et al [39] confirmed that hydrogen peroxide causes a significant corrosion of titanium surface. They used atomic force microscopy (AFM) and scanning electron microscopy (SEM) to investigate the corrosion resistance of titanium disks after 9 d immersion in different solutions based on artificial saliva containing F- (0.5% and 2.5%), H2O2 (0.1% and 10%) and/or lactic acid. Recently, ASHWORTH et al [40] reported the method of fabrication of Ti-alloy parts with enhanced corrosion resistance. Commercial purity Ti powders modified with Pd were hot-pressed and compacted. For obtained materials the effect of powder size on the corrosion resistance was investigated. The corrosion resistance was found to be equivalent to commercial Grade 7 Ti alloys.
However, there is still a lack of the detailed knowledge about electrochemical behavior and corrosion resistance of Ti Grade 7 (Ti-0.2Pd) alloy in simulating inflammatory state (PBS with hydrogen peroxide addition).
Consequently, the purpose of this work is to study the electrochemical behavior of commercially pure titanium and Ti-Grade 7 alloy in simulated conditions of healthy human body (PBS pH=7.4), and in the inflammatory state (solution with hydrogen peroxide addition and lower pH=5.2).
2 Experimental
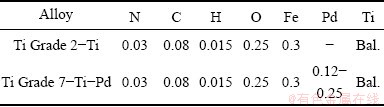
Titanium Grade 2 and titanium Grade 7 rods were purchased from Bibus Metals, Poland and the compositions of these metals are presented in Table 1. The pieces of rods were placed in Duracryl plus acrylic resin. Next, samples were mechanically polished to a mirror-like surface by a 0.25 mm alumina abrasive. After polishing, they were cleaned with ethanol in an ultrasonic cleaner and rinsed with deionized water and used as a working electrode.
Table 1 Composition of titanium alloys (mass fraction, %)
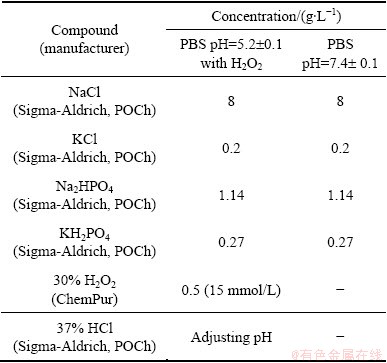
All measurements were performed using the phosphate-buffered saline PBS (pH 7.4) simulating conditions of healthy human body [4,38,41] and PBS solution (pH 5.2) with addition of 15 mmol/L H2O2 simulating the inflammatory state [38,42,43]. The compositions of solutions are presented in Table 2. All compounds were analytically pure. Electrolytes were not de-aerated to simulate the better conditions of human body fluids. The pH of each solution was measured before and after each experiment using UPM 2000-pH/mV pH-meter (Tel-Eko) with the electrode ESA AgP-301W (Eurosensor). The value of pH did not change during experiments. To prepare the solutions, deionized water (0.05 μS, Hydrolab HLP 30 system, Hydrolab Polska) was used. Experimental technique used in this work is similar to that reported in Refs. [44,45].
Table 2 Composition of solutions used in experiments
Experiments were performed in a standard three-electrode electrochemical cell with thermostatic jacket containing about 80 mL of electrolyte. A saturated calomel electrode (SCE, Eurosensor EKK-312) and a platinum sheet were used as the reference and counter electrode, respectively. Total surface area of the working electrode was 0.196 cm2 for Ti Grade 2 and 0.316 cm2 for Ti Grade 7. Additionally, in all EIS measurements a platinum wire was used. It was immersed in the solution close to the SCE and connected through a capacitor (0.1- 1 mF) to the reference electrode connector of the potentiostat. This was done to reduce possible problems at high frequencies caused by the reference electrode. The electrolyte in the cell was maintained at (36.6±0.2) °C throughout the tests using thermostat Medingen E5 (PD Group).
A potentiostat-galvanostat with the built-in FRA2 module (AUTOLAB PGSTAT 30, Eco Chemie BV, The Netherlands) was used to perform all the tests. The potentiostat was controlled by GPES and FRA software (Eco Chemie BV) installed on PC. The following tests were performed: 1) open-circuit potential (OCP) measurements for 1 h before each test to stabilize the conditions (this rest potential is in fact true corrosion potential); 2)potentiodynamic tests; 3) electrochemical impedance spectroscopy (EIS) measurements.
Open-circuit potential was measured and recorded for 1 h during stabilization of the samples in the electrolyte. The potentiodynamic scans (after 1 h immersion of sample in the solution) were acquired in the potential ranging from -0.8 V to 0.4 V, towards anodic direction with a scan rate of 1 mV/s. These scans were used to obtain passive current density (Jpass) which describes well the behavior of passivated alloys. These experiments were performed 3 times. Each measurement was done using fresh solution and new working electrode.
The corrosion behavior for the passive film formed on the surface was also studied using the electrochemical impedance spectroscopy (EIS). The impedance spectra were acquired at the OCP and 0 V (vs SCE) potential. This second potential was chosen on the basis of Fig. 2 to check the behavior of the passive layer in the range of potentials in which the current density is almost constant. Experiments were run with AC amplitude of 10 mV over the frequency range of 105-5×10-3 Hz (50 points per spectrum). Each sample was immersed in the solution for 1 h before starting the EIS test to stabilize the conditions. The EIS experiments were repeated three times.
3 Results
3.1 Open circuit potential and potentiodynamic measurements
Figure 1 shows the variations of the open-circuit potential over 1 h for the titanium alloy specimens immersed in PBS solutions. The electrical potential on the metal-electrolyte surface is closely dependent on the conditions of the experiment, namely the nature of the electrolyte, the concentration, the temperature, the pH, the surface state of the metal. As a result, the electrochemical reactions at the metal-electrolyte interface vary with time.
Potentiodynamic tests revealed that well defined Tafel slopes were not observed in the anodic region (Fig. 2), which suggests that samples display a passive behavior. In such a case, corrosion rate is defined by the passive current density [46]. Passivation current densities were determined from the plateau regions of anodic curves shown in Fig. 2 and listed in Table 3.
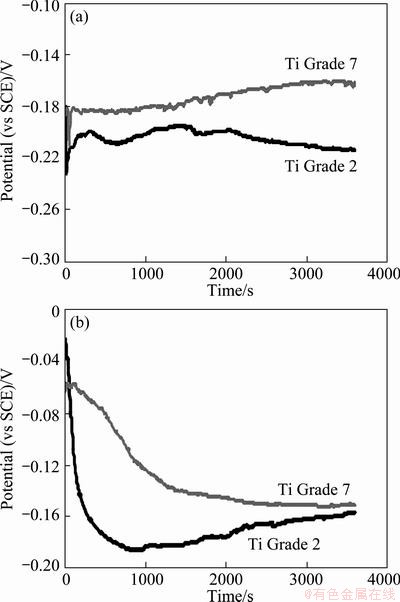
Fig. 1 Open circuit potential as function of time for Ti Grade 2 and Ti Grade 7 immersed in PBS solution at pH 7.4 (a) and pH 5.2 with 15 mmol/L of H2O2 (b)
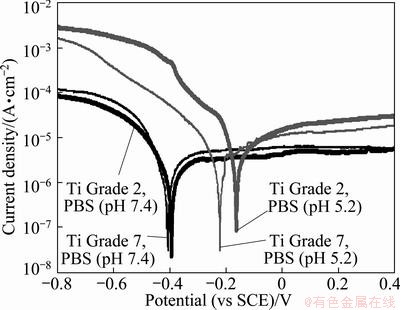
Fig. 2 Potentiodynamic curves for studied metals in PBS solution used for determination of passivation current densities
Table 3 Average values of parameters describing corrosion process for titanium alloys in PBS solutions
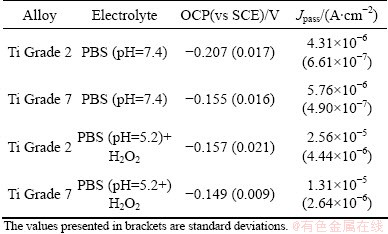
Table 3 summarizes the results of OCP and potentiodynamic measurements. It shows the mean values of open-circuit potentials calculated from the values recorded at the end of each graph of potential variation after 1 h of immersion. In each case the average value was calculated using at least 10 measurements. It can be observed that OCP shifts slightly towards more anodic potentials when the solution becomes more acidic.
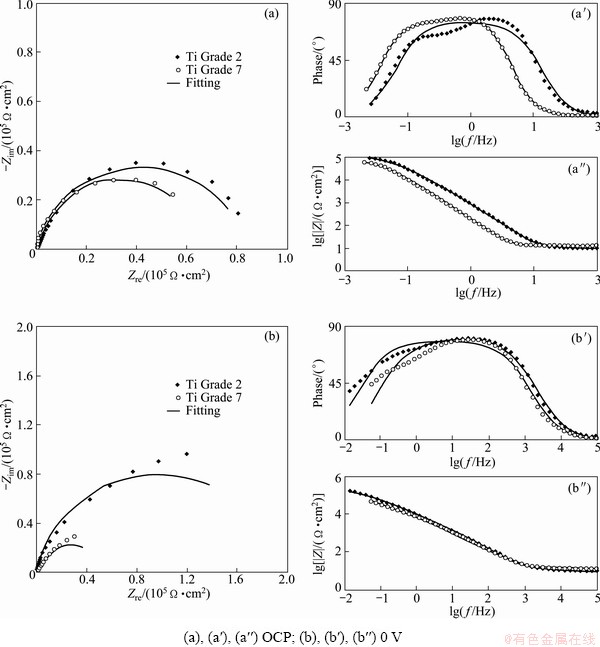
Fig. 3 Results of impedance measurements performed on studied metals in PBS solution at pH=7.4 under different potentials
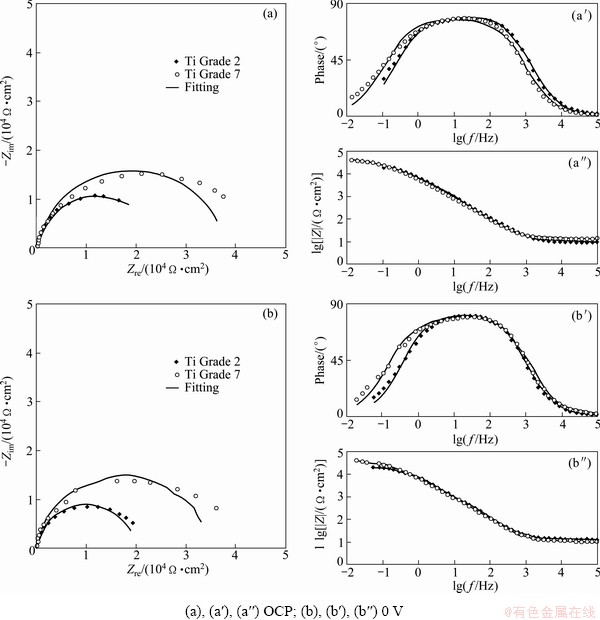
Fig. 4 Results of impedance measurements performed on studied metals in PBS solution at pH=5.2 with H2O2 addition under different potentials
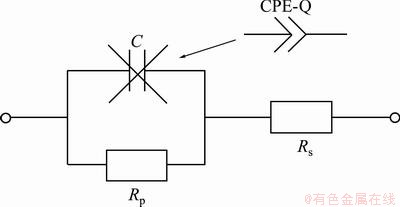
Fig. 5 Modified Randles equivalent circuit used to impedance spectra analysis
3.2 EIS measurements
Since polarization measurements gave approximate information about corrosion of titanium alloys in PBS solutions, and EIS technique was used. The results obtained from impedance measurements in the form of Nyquist and Bode plots are presented in Figs. 3 and 4. For each choice of the experimental conditions, measurements were performed 3 times. These results were analyzed using ZView2 software (Scribner Associates Inc.). To analyze the experimental data, modified Randles equivalent electrical circuit (Fig. 5) was used. The equivalent circuit consists of solution resistance (Rs), polarization resistance (Rp) (charge transfer process–corrosion process) and CPE (constant phase element). In original Randles equivalent circuit there is an ideal capacitor C but its application is limited only to electrodes with very flat surface without inhomogeneities and irregularities. The constant phase element was used because the surface of the electrode is never ideally flat and in the electrochemical measurements this element replaces the ideal capacitor [47-51]. The impedance of the CPE is described by
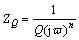 (3)
(3)
where Q is a pre-exponential factor, which is a frequency-independent parameter; n is the exponent, which defines the character of frequency dependence (-1≤n≤1); 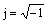 is the imaginary unit;
is the imaginary unit; 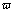 = 2pf[rad·s-1] is angular frequency. With n=1, Q is an ideal capacitance and has the unit of capacitance (F/cm2), in another case when n≠1, Q has the unit of S·sn·cm-2.
= 2pf[rad·s-1] is angular frequency. With n=1, Q is an ideal capacitance and has the unit of capacitance (F/cm2), in another case when n≠1, Q has the unit of S·sn·cm-2.
Simulated spectra are shown together with the experimental results in Figs. 3 and 4. Generally, good agreement is found which is supported by the values of chi-square test which are 10-2-10-3. The parameters describing the equivalent circuit elements are presented and summarized in Tables 4 and 5.
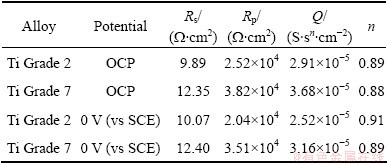
Table 4 Parameters obtained from analysis of impedance spectra (Fig. 3) for metals immersed in PBS with pH 7.4
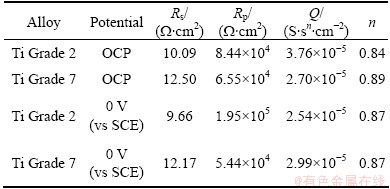
Table 5 Parameters obtained from analysis of impedance spectra (Fig. 4) for metals immersed in PBS (pH 5.2)+H2O2
4 Discussion
The parameters derived from the potentiodynamic measurements which characterize the alloy corrosion resistance are passivation current densities. The passivation current densities for Ti Grade 2 and Ti Grade 7 in PBS solution with pH 7.4 are comparable and their values are 10-6 A/cm2. Average Jpass for Ti-Pd alloy (Ti Grade 7) is equal to 5.76×10-6 A/cm2, and this value is a little bit bigger than that obtained for pure Ti (Ti Grade 2, -4.31×10-6 A/cm2). One can see that there is no difference in Ti and its alloy behaviors when they are immersed in the PBS solution with pH 7.4.
The situation changed when Ti Grade 2 and Ti Grade 7 samples were immersed in the solution simulating the conditions of inflammatory state, when the effect of H2O2 addition on corrosion resistance was studied. Passivation current density increases but this change is large for Ti Grade 2. The value of Jpass for Ti Grade 2 increased to 2.56×10-5 A/cm2 and for Ti Grade 7 the Jpass increased slightly from 5.76×10-6 A/cm2 to 1.31×10-5 A/cm2. These results are presented in Fig. 6, which shows the change of corrosion resistance of both studied metals with the change of pH of the solution.
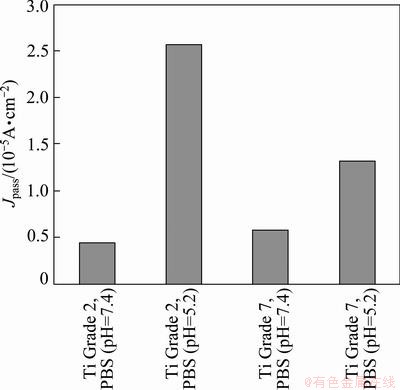
Fig. 6 Comparison of passivation current densities for studied titanium alloys in PBS solution
On the basis of these results one can conclude that in more acidic solution (pH 5.2) with the addition of H2O2, Ti Grade 2 is more susceptible to corrosion than Ti-Pd alloy. This situation changes in neutral solution (pH 7.4), in which Ti Grade 2 is slightly more corrosion resistant than Ti-Pd alloy. It can be speculated that this phenomenon is connected with the change in the structure and composition of the passive oxide layer. It was found that in the Ti-Pd-O system ternary oxides Ti3PdO, PdTiO3, Ti4Pd2O exist [52,53]. It cannot be excluded that one of them may be a part of the oxide layer, which certainly may change conductivity of the passive layer. Another possibility, which also may influence the structure and conductivity of the layer, is the reaction of the oxide with OH- ions which results in the formation of hydroxide layer with the porous structure. The existence of more complicated oxide layer in the solution with pH 7.4 can be also justified by the EIS measurements.
No experimental evidence about the structure of the surface oxide layer the simplest equivalent circuit shown in Fig. 5 was adopted. Such a circuit was also used recently [9,54,55]. Comparison of calculated spectra with experimental data showed that this model circuit gives satisfactory results except for measurements done in the PBS solution with pH 7.4. For these samples small deviation between measured and calculated values can be seen at low frequencies. These deviations can be connected either with the diffusion process or with the complicated passive oxide structure. However, since kinetic parameters are obtained at high and medium frequencies, observed deviation should have no large influence on determined polarization resistance.
Table 4 can affirm that the values of polarization resistance for Ti Grade 2 and Ti Grade 7 immersed in PBS solution with pH 7.4 are 105 W·cm2. For Ti Grade 7, the value is a little bit lower and is around 6×104 W·cm2. This suggests worse corrosion resistance of this alloy in PBS solution simulating the conditions of healthy human body in comparison with commercially pure Ti. On the basis of Table 5 the values of polarization resistance for both metals immersed in PBS solution with pH 5.2 and addition of H2O2 are 104 W·cm2 and are lower in comparison with values of Rp obtained for studied alloys in PBS solution without H2O2. One can see that the addition of hydrogen peroxide increases the corrosion rate of both materials, but the change in corrosion rate is larger in the case of pure titanium. It can be suggested that under the influence of hydrogen peroxide, Ti Grade 7 is more corrosion resistant than Ti Grade 2, which is also confirmed by the results of potentiodynamic measurements. The values of the parameter n for constant phase element (CPE) shown in Tables 4 and 5 are not equal to 1 but vary from 0.84 to 0.91. This means that the passive layer formed on the surface of the studied metals does not behave as an ideal capacitor during the immersion in both solutions. These values suggest that the reaction at metal-solution interface is under mixed control, between pure charging (n=1) and pure diffusion process (n=0.5).
5 Conclusions
1) Ti Grade 7 alloy immersed in PBS with pH 7.4 has a little bit smaller corrosion resistance (higher values of passivation current density) than Ti Grade 2 immersed in the same solution.
2) Situation changed with the change of the solution of PBS with pH 5.2 and with the addition of hydrogen peroxide. The corrosion resistance of both alloys is decreased, but in comparison of passivation current densities one can conclude that Ti Grade 7 (Ti-Pd alloy) is more corrosion resistant in this environment. Better performance of Ti Grade 7 in PBS at pH 5.2 is caused by cathodic modification connected with the Pd addition which apparently does not work in neutral solution.
3) It can be suggested that Ti Grade 7 can be a good candidate as a material for orthopedic implant application.
Acknowledgements
This work was supported by the Polish Ministry of Science and Higher Education under grant No. N507 190 32/2954.
References
[1] SOLAR R J. Corrosion resistance of titanium surgical implant alloys: A review. [C]//SYRETT B C, ACHARYA A. Corrosion and Degradation of Implant Materials. Baltimore: ASTM, 1979: 259-273.
[2] YU S Y, SCULLY J R. Corrosion and passivity of Ti-13%Nb- 13%Zr in comparison to other biomedical implant alloys [J]. Corrosion, 1997, 53(12): 965-976.
[3] GONZALEZ J E G, MIRZA-ROSCA J C. Study of the corrosion behavior of titanium and some of its alloys for biomedical and dental implant applications [J]. Journal of Electroanalytical Chemistry, 1999, 471: 109-115.
[4] AZIZ-KERRZO M, CONROY K G, FENELON A M, FARRELL S T, BRESLIN C B. Electrochemical studies on the stability and corrosion resistance of titanium-based implant materials [J]. Biomaterials, 2001, 22: 1531-1539.
[5] PAN J, THIERRY D, LEYGRAF C. Electrochemical impedance spectroscopy study of the passive oxide film on titanium for implant application [J]. Electrochimica Acta, 1996, 41: 1143-1153.
[6] MARINO C E B, MACARO L H. EIS characterization of a Ti-dental implant in artificial saliva media: dissolution process of the oxide barrier [J]. Journal of Electroanalytical Chemistry, 2004, 568: 115-120.
[7] IBRIS N, MIRZA-ROSCA J C. EIS study of Ti and its alloys in biological media [J]. Journal of Electroanalytical Chemistry, 2002, 526: 53-62.
[8] RECLARU L, MEYER J M. Effects of fluorides on titanium and other dental alloys in dentistry [J]. Biomaterials, 1998, 19: 85-92.
[9] METIKOS-HUKOVIC M, KWOKAL A, PILJAC J. The influence of niobium and vanadium on passivity of titanium-based implants in physiological solution [J]. Biomaterials, 2003, 24: 3765-3775.
[10] OKAZAKI Y, ITO Y, ITO A, TATEISHI T. Effect of alloying elements on mechanical properties of titanium alloys for medical implants [J]. Materials Transaction JIM, 1993, 34(12): 1217-1222.
[11] LONG M, RACK H J. Titanium alloys in total joint replacement—A materials science perspective [J]. Biomaterials. 1998, 19: 1621-1639.
[12] CHOE H, SAJI V S, KO Y. Mechanical properties and corrosion resistance of low rigidity quaternary titanium alloy for biomedical applications [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 862-865.
[13] TOMASHOV N D, CHERNOVA G P, RUSCOL Y S, AYUYAN G A. The passivation of alloys on titanium bases [J]. Electrochimica Acta, 1974, 19: 159-172.
[14] OKAZAKI Y, ITO A, TATEISHI T, ITO Y. Effect of alloying elements on anodic polarization properties of titanium alloys in acid solutions [J]. Materials Transaction JIM, 1994, 35: 58-66.
[15] TOMASHOV N D. Passivity and corrosion resistance of metal systems [J]. Corrosion Science, 1964, 4: 315-334.
[16] STERN M, WISSENBERG H. The influence of noble metal alloy additions on the electrochemical and corrosion behavior of titanium [J]. Journal of the Electrochemical Society, 1959, 106(9): 759-764.
[17] TOMASHOV N D, ALTOVSKY R M, CHERNOVA G P. Passivity and corrosion resistance of titanium and its alloys [J]. Journal of the Electrochemical Society, 1961, 108(2): 113-119.
[18] COTTON J B. The role of palladium in enhancing corrosion resistance of titanium [J]. Platinum Metals Review, 1967, 11(2): 50-52.
[19] SCHUTZ R W. Platinum group metal additions to titanium: A highly effective strategy for enhancing corrosion resistance [J]. Corrosion, 2003, 59(12): 1043-1057.
[20] SATOH H, SHIMOGURI K, KAMIKUBO F. The crevice corrosion resistance of some titanium materials [J]. Platinum Metals Review, 1987, 31(3): 115-121.
[21] BROSSIA C S, CRAGNOLINO G A. Effects of environmental and metallurgical conditions on the passive and localized dissolution of Ti-0.15%Pd [J]. Corrosion, 2001, 57(9): 768-776.
[22] BROSSIA C S, CRAGNOLINO G A. Effect of palladium on the corrosion behavior of titanium [J]. Corrosion Science, 2004, 46: 1693-1711.
[23] HUA F, MON K, PASUPATHI P, GORDON G, SHOESMITH D. A review of corrosion of titanium Grade 7 and other titanium alloys in nuclear waste repository environments [J]. Corrosion, 2005, 61(10): 987-1003.
[24] VAUGHAN J, ALFANTAZI A. Corrosion of titanium and its alloys in sulfuric acid in the presence of chlorides [J]. Journal of the Electrochemical Society, 2006, 153(1): B6-B12.
[25] RAMIRES I, GUASTALDI A C. Electrochemical study of the corrosion of Ti-Pd and Ti-6Al-4V electrodes in sodium chloride solutions [J]. Biomecanica, 2001, 9(1): 61-65.
[26] NAKAGAWA M, MATONO Y, MATSUYA S, UDOH K, ISHIKAWA K. The effect of Pt and Pd alloying additions on the corrosion behavior of titanium in fluoride-containing environments [J]. Biomaterials, 2005, 26: 2239-2246.
[27] YOKOYAMA K, OGAWA T, ASAOKA K, SAKAI J. Fracture of sustained tensile-loaded Ti-0.2%Pd alloy in acid and neutral fluoride solutions [J]. Materials Science and Engineering A, 2006, 419: 122-130.
[28] HANAWA T, OTA M. Calcium phosphate naturally formed on titanium in electrolyte solution [J]. Biomaterials, 1991, 12(8): 767-774.
[29] HANAWA T, OTA M. Characterization of surface film formed on titanium in electrolyte using XPS [J]. Applied Surface Science, 1992, 55(4): 269-276.
[30] MCQUEEN D, SUNDGREN J E, IVARSSON B, LUNDSTROM I, SVENSSON A, BRANEMARK P I, ALBREKTSSON T. Auger electron spectroscopic studies of titanium implants [C]//LEE A J C, ALBREKTSSON T, BRANEMARK P I. Clinical Applications of Biomaterials. New York: John Wiley & Sons; 1982: 179-185.
[31] HODGSON A W E, MUELLER Y, FORSTER D, VIRTANEN S. Electrochemical characterisation of passive films on Ti alloys under simulated biological conditions [J]. Electrochimica Acta, 2002, 47(12): 1913-1923.
[32] TENGVALL P, ELWING H, SJOKVIST L, BJURSTEN L M, LUNDSTROM I. Interaction between hydrogen peroxide and titanium: A possible role in the biocompatibility of titanium [J]. Biomaterials, 1989, 10: 118-120.
[33] TENGVALL P, LUNDSTROM I, SJOKVIST L, ELWING H, BJURSTEN L M. Titanium-hydrogen peroxide interaction: Model studies of the influence of the inflammatory response on titanium implants [J]. Biomaterials, 1989, 10: 166-175.
[34] PARK J B, BRONZINO J D. Biomaterials: Principles and applications [M]. Boca Raton: CRC Press, 2003.
[35] FREEMAN B A, CRAPO J D. Biology of disease: free radicals and tissue injury [J]. Laboratory Investigation, 1982, 47: 412-426.
[36] PAN J, THIERRY D, LEYGRAF C. Electrochemical and XPS studies of titanium for biomaterial applications with respect to the effect of hydrogen peroxide [J]. Journal of Biomedical Materials Research, 1994, 28: 113-122.
[37] PAN J, THIERRY D, LEYGRAF C. Hydrogen peroxide toward enhanced oxide growth on titanium in PBS solution: Blue coloration and clinical relevance [J]. Journal of Biomedical Materials Research, 1996, 30: 393-402.
[38] FONSECA C, BARBOSA M A. Corrosion behaviour of titanium in biofluids containing H2O2 studied by electrochemical impedance spectroscopy [J]. Corrosion Science, 2001, 43: 547-559.
[39] MABILLEAU G, BOURDON S, JOLY-GUILLOU M L, FILMON R, BASLE M F, CHAPPARD D. Influence of fluoride, hydrogen peroxide and lactic acid on the corrosion resistance of commercially pure titanium [J]. Acta Biomaterialia, 2006, 2(1): 121-129.
[40] ASHWORTH M A, DAVENPORT A J, WARD R M, HAMILTON H G C. Microstructure and corrosion of Pd-modified Ti alloys produced by powder metallurgy [J]. Corrosion Science, 2010, 52(7): 2413-2421.
[41] CHENG X, ROSCOE S G. Corrosion behavior of titanium in the presence of calcium phosphate and serum proteins [J]. Biomaterials, 2005, 26: 7350-7356.
[42] HANAWA T. In vivo metallic biomaterials and surface modification [J]. Materials Science and Engineering A, 1999, 267: 260-266.
[43] TADDEI E B, HENRIQUES V A R, SILVA C R M, CAIRO C A A. Production of new titanium alloy for orthopedic implants [J]. Materials Science and Engineering C, 2004, 24: 683-687.
[44] HANDZLIK P, FITZNER K. Electronic properties of anodic oxide films on titanium in phosphate buffered saline solution and artificial saliva determined by EIS methods [J]. Archives of Metallurgy and Materials, 2007, 52(4): 543-553.
[45] HANDZLIK P, FITZNER K. Semiconducting properties of anodic oxide films grown on titanium in Ringer and PBS solutions [J]. Archives of Metallurgy and Materials, 2010, 55(2): 521-532.
[46] MCCAFFERTY E, HUBLER G K. Electrochemical behavior of palladium-implanted titanium [J]. Journal of the Electrochemical Society, 1978, 125(11): 1892-1893.
[47] BARSOUKOV E, MACDONALD J R. Impedance spectroscopy—Theory, experiment and applications [M]. Chichester: Wiley-Interscience, 2005.
[48] ORAZEM M E, TRIBOLLET B. Electrochemical impedance spectroscopy [M]. Hoboken, New Jersey: John Wiley & Sons, 2008.
[49] WESTING E P M, FERRARI G M, WIT J H W D. The determination of coating performance with impedance measurements – I: Coating polymer properties [J]. Corrosion Science, 1993, 34(9): 1511-1530.
[50] GNEDENKOV S V, SINEBRYUKHOV S L, SERGIENKO V I.Electrochemical impedance simulation of a metal oxide heterostructure/electrolyte interface: A review [J]. Russian Journal of Electrochemistry, 2006, 42(3): 197-211.
[51] ORAZEM M E, PEBERE N, TRIBOLLET B. Enhanced graphical representation of electrochemical impedance data [J]. Journal of the Electrochemical Society B, 2006, 153(4): 129-136.
[52] SHAPLYGIN I S, LAZAREV V B. Novyi Dvoynyi Oksid Palladya(II) PdTiO3—New binary palladium(II) oxide PdTiO3 [J]. Zhurnal Neorganicheskoi Khimii, 1988, 33: 34-37.
[53] LEONARD S R, SNYDER B S, BREWER L, STACY A M. Structure determinations of two new ternary oxides: Ti3PdO and Ti4Pd2O [J]. Journal of Solid State Chemistry, 1991, 92: 39-50.
[54] ZHANG B B, WANG B L, LI L, ZHENG Y F. Corrosion behavior of newly developed Ti–Ag–Fe dental alloys in neutral saline solution [J]. Materials and Corrosion, 2011, 62(8): 766-770.
[55] KARIMI S, NICKCHI T, ALFANTAZI A. Effects of bovine serum albumin on the corrosion behaviour of AISI 316L, Co-28Cr-6Mo, and Ti-6Al-4V alloys in phosphate buffered saline solutions [J]. Corrosion Science, 2011, 53(10): 3262-3272.
P. HANDZLIK, K. FITZNER
Faculty of Non-Ferrous Metals, AGH University of Science and Technology, 30 Mickiewicza Ave., 30-059 Cracow, Poland
摘 要:采用动电位和电化学阻抗谱技术研究了纯Ti(2级)和Ti-Pd合金(7级)的耐腐蚀性能。实验温度为36.6 °C,实验溶液包括模拟的健康人体条件的pH 7.4 的PBS溶液和添加了H2O2 (0.015 mol/L)的pH 5.2的炎症状态的PBS溶液。Ti-Pd合金(7级Ti),在含H2O2的PBS溶液中,其耐腐蚀性能比纯Ti的好(较低的腐蚀电流密度),表明其是一种很好的骨科植入材料。
关键词:Ti;Ti-Pd合金;生物材料;腐蚀;炎症;电化学阻抗谱
(Edited by Xiang-qun LI)
Corresponding author: P. HANDZLIK; Tel: +48126174125; E-mail: phandzli@agh.edu.pl
DOI: 10.1016/S1003-6326(13)62541-8

