粉煤灰中莫来石及刚玉高碱溶液分解动力学
来源期刊:中国有色金属学报(英文版)2019年第4期
论文作者:刘春力 马淑花 丁健 罗扬 郑诗礼 张懿
文章页码:868 - 875
关键词:粉煤灰;莫来石;刚玉;分解动力学;氧化铝
Key words:coal fly ash; mullite; corundum; decomposition kinetics; alumina
摘 要:对粉煤灰中莫来石和刚玉在高碱溶液分解动力学进行研究。考察反应温度和反应时间对莫来石和刚玉分解率及氧化铝回收效率的影响。结果表明,升高反应温度及延长反应时间有利于莫来石、刚玉分解和氧化铝回收,且莫来石的分解温度低于刚玉的分解温度。当在220 °C条件下反应90 min后,1 L循环碱液可回收多于100 g氧化铝。莫来石和刚玉的分解过程符合收缩核模型,且反应速率受化学反应控制,二者的反应活化能分别为67.46和161.82 kJ/mol。
Abstract: Decomposition kinetics of mullite and corundum in coal fly ash with highly alkaline solution was studied. The effects of the reaction temperature and reaction time on decomposition rates of mullite and corundum and alumina extraction efficiency were investigated. The results show that increasing reaction temperature and reaction time increases the decomposition rates of mullite and corundum and alumina extraction efficiency, with the decomposition temperature of mullite lower than that of corundum. After 90 min reaction at 220 °C, more than 100 g alumina was extracted when recycling 1 L of alkaline solution. The decomposition processes of mullite and corundum corresponded with the shrinking unreacted core model, and the reaction rate was under chemical reaction control, with the activation energies of mullite and corundum being 67.46 and 161.82 kJ/mol, respectively.
Trans. Nonferrous Met. Soc. China 29(2019) 868-875
Chun-li LIU1,2, Shu-hua MA1, Jian DING1, Yang LUO1,2, Shi-li ZHENG1, Yi ZHANG1
1. CAS Key Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China;
2. University of Chinese Academy of Sciences, Beijing 100049, China
Received 20 June 2018; accepted 7 December 2018
Abstract: Decomposition kinetics of mullite and corundum in coal fly ash with highly alkaline solution was studied. The effects of the reaction temperature and reaction time on decomposition rates of mullite and corundum and alumina extraction efficiency were investigated. The results show that increasing reaction temperature and reaction time increases the decomposition rates of mullite and corundum and alumina extraction efficiency, with the decomposition temperature of mullite lower than that of corundum. After 90 min reaction at 220 °C, more than 100 g alumina was extracted when recycling 1 L of alkaline solution. The decomposition processes of mullite and corundum corresponded with the shrinking unreacted core model, and the reaction rate was under chemical reaction control, with the activation energies of mullite and corundum being 67.46 and 161.82 kJ/mol, respectively.
Key words: coal fly ash; mullite; corundum; decomposition kinetics; alumina
1 Introduction
Coal fly ash (CFA) is an important solid waste produced by coal-fired power plants, with its annual production in China currently exceeding 5.8×108 t [1]. Despite the successful applications of some of the produced CFA in metal recycling and building/functional materials production [2-5], large amounts of CFA are currently in storage, causing soil, ground water, and air pollution [6-8]. The alumina content of CFA in some parts of North-Western China, can reach as high as 40%-50% [9] and is a potential bauxite substitute for producing alumina. The annual production of high-alumina CFA is estimated to be 3×107 t, containing (1.2-1.5)×107 t of alumina [10]. The alumina in CFA is particularly important in light of the scarcity of Chinese bauxite resources, with bauxite import reaching 5.58×106 t in 2015 [11]. Hence, alumina extraction from CFA has become the focus of numerous research projects.
Considerable researches have been performed on alumina extraction from CFA, mainly involving acid leaching [12,13], sintering [14-16], and hydro-chemical extraction [17,18]. However, acid leaching inevitably causes severe equipment corrosion and generates gaseous pollutants [19,20], whereas sintering requires high reaction temperatures (1100-1300 °C), incurring high energy costs [21,22]. In contrast, hydro-chemical extraction is amenable for large-scale production due to its moderate reaction conditions and wet process. The hydro-chemical process can effectively extract alumina from CFA by transforming the silica into insoluble alumina-free NaCaHSiO4 using a mixed alkaline (NaOH + Ca(OH)2) solution. The caustic ratio (Na2O- to-Al2O3 molar ratio) of the alkaline solution must be higher than 10:1. Thus, only 30 g of alumina can be extracted when recycling 1 L of the alkaline solution, which is much lower than the 120 g that can be extracted via the traditional Bayer process [23]. The high silica content (~40%) of CFA will result in large amounts of solid residues, which cannot be used efficiently because of their high alkali content [24]. To date, the industrial extraction alumina from CFA via hydro-chemical processes has not been achieved.
Previous studies indicated that the inert mullite and corundum in CFA could be decomposed in highly alkaline solutions [25,26]. Furthermore, CFA is mainly composed of mullite, corundum, and amorphous silica. Approximately half of the silica in CFA exists in the form of amorphous silica and can easily be removed by the pre-desilication process [27]. Therefore, in the current study, alumina extraction from CFA was performed under highly alkaline conditions (only NaOH). Amorphous silica in the CFA was firstly removed by a pre-desilication process to increase alumina content. The pre-desilicated CFA (PCFA), which mainly contained mullite and corundum, was used as a raw material for the preparation of alumina. The effects of the reaction temperature and time on the alumina extraction efficiency were also determined. To better understand the alumina extraction mechanism, the decomposition processes of mullite and corundum were systematically investigated. In addition, the kinetic behavior of the above decomposition was evaluated and experimental data were fitted with theoretical equations to gain mechanism insights and promote the application of alumina extraction from CFA. Thus, a reference is provided for the utilization of other mullite- or corundum-containing solid wastes (e.g. aluminum dross).
2 Experimental
2.1 Materials
CFA was obtained from a thermal power plant located in Inner Mongolia, China, and dried at 100 °C for 12 h in an oven prior to analysis.
The PCFA was prepared according to the following steps. Raw CFA was milled in a Teflon resin mill chamber using zirconia balls. The milled CFA was then reacted with acid solution, and the slurry was filtered to obtain the acid-treated CFA. Finally, the acid-treated CFA reacted with an alkali solution to obtain PCFA.
All reagents were obtained from Tianjin Kemiou Chemical Reagent Co., Ltd., China, and deionized water was used throughout the experiments.
2.2 Experimental principles
In this work, the alumina extraction from PCFA and the decomposition of mullite and corundum under highly alkaline conditions were investigated. According to previous studies [17,26], this process can be modeled by the following equations:
3Al2O3·2SiO2+10NaOH+7H2O=2Na2SiO3+6NaAl(OH)4 (1)
Al2O3+2NaOH+3H2O=2NaAl(OH)4 (2)
SiO2+2NaOH=Na2SiO3+H2O (3)
6NaAl(OH)4+6Na2SiO3=Na8Al6Si6O24(OH)2(H2O)2+10NaOH+4H2O (4)
Utilizing standard Gibbs energies taken from the thermodynamic data manual [28], we calculated the standard Gibbs energies (ΔrGΘ) of dissolution within a temperature range of 25-325 °C, as summarized in Fig. 1. Importantly, all obtained ΔrGΘ values were negative, indicating that the above reactions are thermo- dynamically feasible. However, the decomposition of corundum was less thermodynamically favorable than that of mullite, and required harsher conditions.
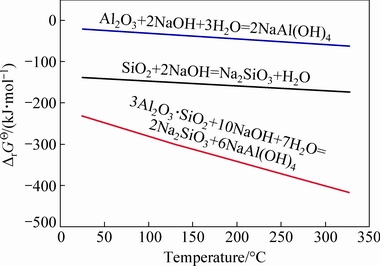
Fig. 1 Standard Gibbs energy change versus temperature for dissolution reactions under highly alkaline conditions
2.3 Experimental methods
Experiments were performed in a 5 L high-pressure autoclave fitted with internal cooling and external heater systems. The autoclave was protected by a pure nickel vessel from corrosion due to the highly alkali solution. An automatic proportional, integral and derivative (PID) control system was used to adjust the heating rate, agitation, and temperature of the autoclave. A sampling pipe was inserted into the bottom of the autoclave to enable real-time sampling during the reaction.
A solution of sodium aluminate (3 L) reacted with PCFA at an initial caustic ratio of 25:1, Na2O concentration of 370 g/L, and liquid-to-solid (L/S) mass ratio of 5:1. The above solution was prepared by mixing and dissolving pure H2O, NaOH, and Al(OH)3. The effects of the reaction temperature and time on the decomposition of mullite and corundum were investigated. The leached slurry was filtered, and the residue was washed three times with deionized water at 80 °C and dried at 100 °C for 24 h. After drying, the liquor and residue were analyzed to determine their chemical and mineralogical compositions.
2.4 Characterization techniques
The chemical compositions of the dried solid residue and liquor were examined by inductively coupled plasma-optical emission spectroscope (ICP-OES, Optimal 5300DV, PerkinElmer Instruments, 1300 W, peristaltic pump flow 1.5 L/min, carrier gas flow 0.08 L/min). The crystalline phases and morphologies were characterized by X-ray diffractometry (XRD, X’Pert ProMPD, Panalytical, Cu Kα radiation, 40 kV, 100 mA) and scanning electron microscope (SEM, JSM7100F), respectively.
The extents of mullite and corundum decomposition were determined by quantitative XRD analysis [29]. The matrix-flushing method was successfully used to determine the extent of crystal phase transformations by quantitative XRD analysis [30] and fitting the experimental data with the following equation:
In/Ii=K(xn/xi) (5)
where I is the intensity of a given peak, xn is the content of mullite or corundum (depending on n) in the PCFA, subscript i refers to the internal standard, and K is a constant that can be calculated from the XRD pattern of the mullite in PCFA and the internal standard using a diffraction peak area with the same order of magnitude, or the mixture of corundum in PCFA and the internal standard using a diffraction peak area with the same order of magnitude.
Zinc oxide was chosen as the internal standard. The strongest peaks of corundum and mullite, located at 2θ values of ~25.55° and ~26.34°, respectively, were chosen for kinetic analyses, with average values of K being 0.084 and 3.027, respectively. The amounts of unreacted mullite and corundum in the residue and the corresponding extents of decomposition were obtained by analyzing the XRD patterns of the homogeneous mixtures of fixed amounts of the zinc oxide and residue.
3 Results and discussion
3.1 Raw material characterization
Table 1 Chemical compositions of CFA and PCFA (wt.%)

Fig. 2 XRD patterns (a, c) and SEM micrographs (b, d) of CFA (a, b) and PCFA (c, d)
The chemical compositions of pristine CFA are listed in Table 1 with major constituents of Al2O3, SiO2, Fe2O3, TiO2, and CaO. Due to the presence of amorphous silica, the mass ratio of Al2O3 to SiO2 of CFA was far lower than the theoretical value of mullite (2.55:1). After pre-desilication process, amorphous silica in the CFA can be efficiently removed, and the Al2O3 content was greatly improved. In addition, the mineralogical analyses of CFA and PCFA are shown in Fig. 2. Figures 2(a) and (b) show that the CFA consists of two major crystalline phases, mullite and corundum, and a considerable amorphous silica phase. The broad diffraction peaks at 2θ=19°-25° indicated that the amorphous silica in the CFA was dominated by a glass phase [31]. Meanwhile, the CFA sample is composed of many inhomogeneous small spherical particles with smooth surfaces. Figures 2(c) and (d) showed that broad diffraction peaks (19°-25°) disappeared after pre-desilication. Furthermore, the smooth surface of the raw CFA particles disappeared entirely and revealed the inner structure of the particles. It demonstrated that the phases in PCFA were mainly mullite and corundum. This necessitated further study of the decomposition mechanism of these minerals in alumina extraction process.
3.2 Phase transformations and chemical composition analysis during alumina extraction
Figure 3 shows the XRD patterns of residues obtained at different reaction temperatures, showing that at 140 °C, these residues were mainly composed of mullite, corundum, and Na8Al6Si6O24(OH)2(H2O)2, a basic sodalite [32]. This basic sodalite is well-known as a major constituent of the Bayer process residue [33,34]. As the reaction temperature was increased to 180 °C, the diffraction peaks of mullite disappeared completely, whereas those of corundum remained unchanged, disappeared only when the reaction temperature reached 220 °C. In addition, Fig. 4 shows the XRD patterns of the residues sampled in different reaction time at 200 °C, showing that at 15 min, the diffraction peaks of mullite disappeared, those of Na8Al6Si6O24(OH)2(H2O)2 appeared, and peaks of corundum were observed at 2θ values of ~25.55° and 57.42°, respectively. The corundum peaks lost intensity with increasing reaction time, and disappeared completely after 120 min. Figures 3 and 4 qualitatively show that the mullite decomposed faster than corundum, allowing the transformation of PCFA to Na8Al6Si6O24(OH)2(H2O)2 to be divided into two parts, i.e. the mullite and corundum decomposition.
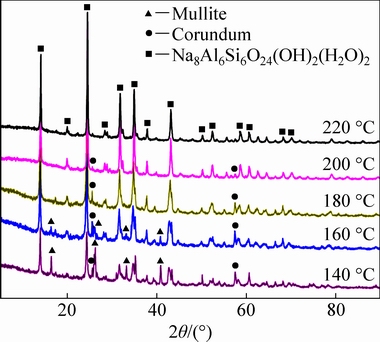
Fig. 3 XRD patterns of residues obtained at different reaction temperatures for 90 min
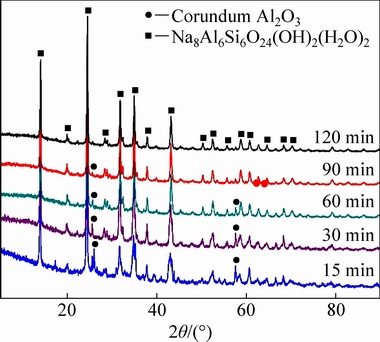
Fig. 4 XRD patterns of residues obtained in different reaction time at 200 °C
To further clarify the decomposition mechanism, the variations of liquor composition and Al2O3/SiO2 mass ratio in leached residue were analyzed and the results are shown in Tables 2 and 3. Table 2 indicates that the Al2O3 content of the liquor exhibited only slight changes at reaction temperatures of 140-180 °C, and rapidly increased above 180 °C. Conversely, the SiO2 and Na2O contents sharply decreased with increasing the reaction temperature from 140 to 180 °C, and showed only slight changes above 180 °C. According to the XRD analysis (Fig. 3), the formation of Na8Al6Si6O24(OH)2(H2O)2 reduced the SiO2 and Na2O contents of the liquor with increasing the reaction temperature from 140 to 180 °C, despite the concomitant dissolution of mullite. In addition, at reaction temperatures above 180 °C, the complete decomposition of mullite and decreased SiO2 content of the liquor inhibited further formation of Na8Al6Si6O24(OH)2(H2O)2, whereas the Al2O3 content of the liquor rapidly increased due to corundum decomposition. These observations confirmed that the mullite decomposed and transformed into Na8Al6Si6O24(OH)2(H2O)2 under the highly alkaline conditions at reaction temperatures below 180 °C, whereas the decomposition of corundum only occurred at higher temperatures.
Table 3 shows that the Al2O3 content of the liquor gradually increased with increasing reaction time from 15 to 90 min, exhibiting only slight changes after 90 min, whereas those of SiO2 and Na2O decreased slightly with increasing reaction time. These results indicated that mullite decomposed rapidly and increasing reaction time allowed for the decomposition of corundum at 200 °C. The SiO2 content in the leached liquor was very low, which is beneficial to the precipitation of sodium aluminate, similar to the Bayer process for alumina preparation.
Table 2 Main liquor components and Al2O3/SiO2 mass ratios of leached residue at different reaction temperatures for 90 min
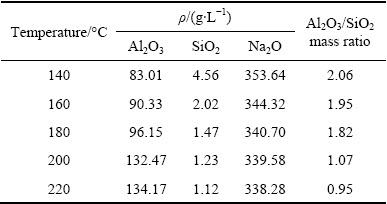
Table 3 Main liquor components and Al2O3/SiO2 mass ratios of leached residue at 200 °C for different reaction time
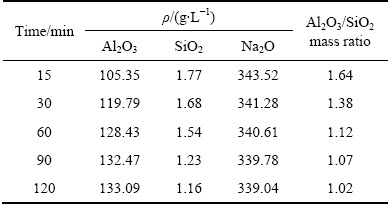
Table 4 and Fig. 5 show the chemical composition and SEM image, respectively, of the leached residue obtained after reaction at 220 °C for 90 min. The residue is mainly composed of SiO2, Al2O3, and Na2O. This result agrees well with the XRD analysis. The residue exhibited a uniform size distribution and well-developed polyhedral crystals, and a similar morphology was observed in previous studies [35,36]. Furthermore, the extracted alumina was more than 100 g when recycling 1 L of the alkaline solution.
Table 4 Chemical composition of leached residue obtained after reaction at 220 °C for 90 min (wt.%)
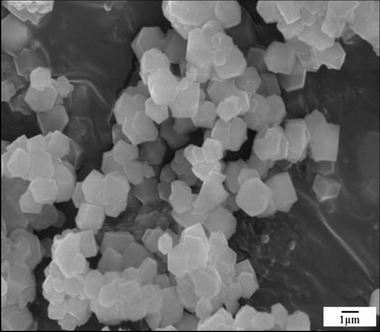
Fig. 5 SEM image of residue after reaction at 220 °C for 90 min
3.3 Decomposition kinetics of mullite and corundum
Since the alumina extraction efficiency is closely related to the decomposition of mullite and corundum, the decomposition kinetics of these minerals was investigated. Figure 3 shows that the diffraction peaks of mullite and corundum disappeared after reaction at 180 and 220 °C for 90 min, respectively. Therefore, the decomposition kinetics of mullite was investigated at 120-180 °C in 20 °C interval and the decomposition kinetics of corundum was evaluated at 190-220 °C in 10 °C interval.
Figure 6 shows that the extent of mullite decomposition increased with increasing reaction temperature and time, with 49.87% of the mullite dissolved after reaction at 120 °C for 90 min, whereas the extent of decomposition increased to 76.13% after reaction at 160 °C for 45 min, and almost complete decomposition (95.38%) was observed after reaction at 180 °C for 30 min. Therefore, high reaction temperatures favored mullite decomposition. Similarly, Fig. 7 shows that the extent of corundum decomposition increased with increasing reaction temperature and time, reaching 95.61% after reaction at 220 °C for only 15 min, demonstrating that the reaction temperature significantly affected the decomposition of the PCFA constituents.
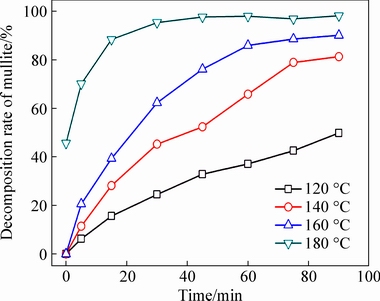
Fig. 6 Effect of reaction time and temperature on extent of mullite decomposition
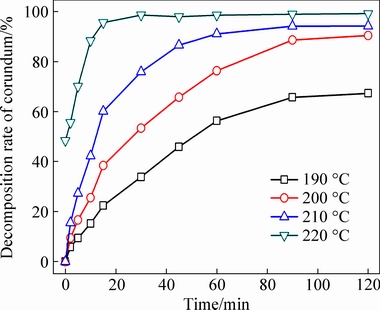
Fig. 7 Effect of reaction time and temperature on extent of corundum decomposition
To determine the kinetic parameters and rate controlling step of the decomposition of mullite and corundum in the highly alkaline solution, the shrinking unreacted core model was applied to experimental data. According to this model, the rate of reaction between the solid particle and leaching reagent is controlled by one of the following steps: external diffusion, product layer diffusion, or chemical reaction. Furthermore, the reaction rate equations for the shrinking unreacted core model were previously determined [37].
When the reaction is under external diffusion control, we have
x=kt (6)
When the reaction is under product layer diffusion control, we have
1-3(1-x)2/3+2(1-x)=kt (7)
When the reaction is under chemical reaction control, we have
1-(1-x)1/3=kt (8)
where x is the extent of reaction, k is the apparent reaction rate constant, and t is the reaction time. The fitting coefficients (R2) for the mullite and corundum decomposition are listed in Tables 5 and 6, respectively. Through the comparison and analysis, the decomposition of mullite and corundum was best fit using the chemical reaction control model. Additionally, the linear relationships between 1-(1-x)1/3 and reaction time for the decomposition of mullite and corundum at different reaction temperatures are plotted in Figs. 8 and 9, respectively. The results indicated that the kinetics of the decomposition reaction of mullite and corundum agreed well with the shrinking unreacted core model.
Table 5 Fitting coefficients R2 of mullite decomposition data at 120-180 °C
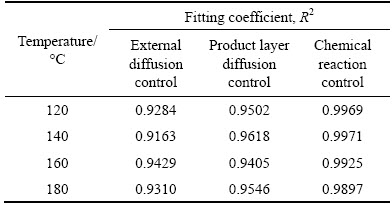
Table 6 Fitting coefficients R2 of corundum decomposition data at 190-220 °C
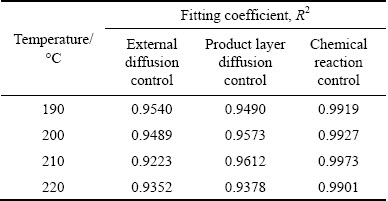
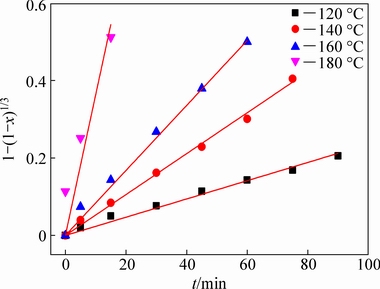
Fig. 8 Plots of 1-(1-x)1/3 as function of reaction time at different reaction temperatures for mullite decomposition in highly alkaline solution
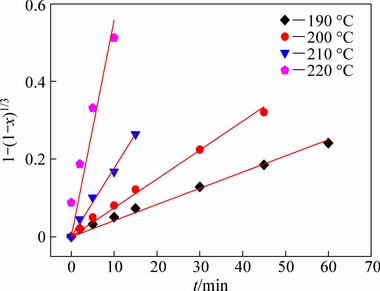
Fig. 9 Plots of 1-(1-x)1/3 as function of reaction time at different reaction temperatures for corundum decomposition in highly alkaline solution
Using the data in Tables 5 and 6, the apparent activation energies of mullite and corundum were calculated using the Arrhenius equation, and the kinetics data are plotted in Figs. 10 and 11 using the natural logarithm of the reaction rate as a function of 1/T. The apparent activation energy of mullite, which is equal to the slope of the straight line, was determined to be 67.46 kJ/mol, whereas the apparent activation energy of corundum was 161.82 kJ/mol. This indicates that corundum is more difficult to decompose than mullite under identical reaction conditions. In addition, the activation energy values (>40 kJ/mol) of mullite and corundum demonstrated that the decomposition process was controlled by chemical reactions [37].
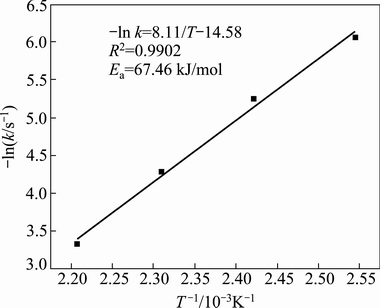
Fig. 10 Arrhenius plot of mullite decomposition data at 120-180 °C
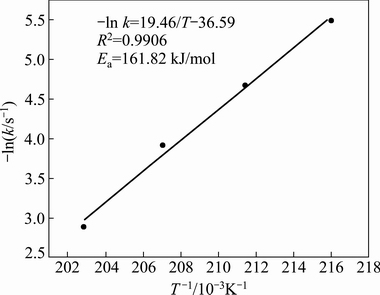
Fig. 11 Arrhenius plot of corundum decomposition data at 190-220 °C
4 Conclusions
(1) The alumina extraction efficiency and decomposition of mullite and corundum in CFA in a highly alkaline medium were investigated. The mullite decomposition rate reached 95.38% after reaction at 180 °C for 30 min, and that of corundum decomposition reached 95.61% after reaction at 220 °C for 15 min.
(2) The decomposition temperature of mullite was significantly lower than that of corundum. When recycling 1 L of the alkaline solution, more than 100 g of alumina was extracted after reaction at 220 °C for 90 min.
(3) The decomposition of mullite and corundum was best fit with the shrinking unreacted core model and the reaction rate was under chemical reaction control, with activation energies of dissolution determined to be 67.46 and 161.82 kJ/mol for the mullite and corundum, respectively.
References
[1] LIU Jing, DONG Ying-chao, DONG Xin-fa, HAMPSHIRE S, ZHU Li, ZHU Zhi-wen, LI Ling-ling. Feasible recycling of industrial waste coal fly ash for preparation of anorthite-cordierite based porous ceramic membrane supports with addition of dolomite [J]. Journal of the European Ceramic Society, 2016, 36: 1059-1071.
[2] CHENG Wei, BIAN Zheng-fu, DONG Ji-hong, LEI Shao-gang. Soil properties in reclaimed farmland by filling subsidence basin due to underground coal mining with mineral wastes in China [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 2627-2635.
[3] Huang K, Katsutoshi I, Hiroyuki H, Hidetaka K, Keisuke O. Leaching behavior of heavy metals with hydrochloric acid from fly ash generated in municipal waste incineration plants [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 1422-1427.
[4] ZHANG Yi-xin, DONG Ji-xiang, GUO Fan-hui, SHAO Zhong-ye, WU Jian-jun. Zeolite synthesized from coal fly ash produced by a gasification process for Ni2+ removal from water [J]. Minerals, 2018, 8: 1-14.
[5] HOU Li-jun, LIU Tao-yong, LU An-xian. Red mud and fly ash-based ceramic foams using starch and manganese dioxide as foaming agent [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 591-598.
[6] Cho J H, Eom Y J, Park J M, Lee S B, Hong J H, Lee T G. Mercury leaching characteristics of waste treatment residues generated from various sources in Korea [J]. Waste Management, 2013, 33: 1675-1681.
[7] MartIn J A R, Niko N. Soil as an archive of coal-fired power plant mercury deposition [J]. Journal of Hazardous Materials, 2016, 308: 131-138.
[8] POYKIO R, MAKELA M, WATKINS G, NURMESNIEMI H, DAHL O. Heavy metals leaching in bottom ash and fly ash fractions from industrial-scale BFB-boiler for environmental risks assessment [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 256-264.
[9] DAI Shi-feng, ZHAO Lei, PENG Su-ping, CHOU Chen-lin, WANG Xi-bo, ZHANG Yong, LI Dan, SUN Ying-ying. Abundances and distribution of minerals and elements in high-alumina coal fly ash from the Jungar Power Plant, Inner Mongolia, China [J]. International Journal of Coal Geology, 2010, 81: 320-332.
[10] YUAN Guo-jun, ZHANG Jian-bin, ZHANG Yong-feng, YAN Yi-nan, JU Xin-xin, SUN Jun-min. Characterization of high-alumina coal fly ash based silicate material and its adsorption performance on volatile organic compound elimination [J]. Korean Journal of Chemical Engineering, 2015, 32: 436-445.
[11] National Bureau of Statistics of China. China statistical year book [M]. Beijing: China Statistics Press, 2015. (in Chinese)
[12] BAI Guang-hui, QIAO Yun-hai, SHEN Bo, CHEN Shuang-li. Thermal decomposition of coal fly ash by concentrated sulfuric acid and alumina extraction process based on it [J]. Fuel Processing Technology, 2011, 92: 1213-1219.
[13] SANGITA S, NAYAK N, PANDA C R. Extraction of aluminium as aluminium sulphate from thermal power plant fly ashes [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 2082-2089.
[14] GUO Chun-bin, ZOU Jing-jing, JIANG Yin-shan, HUANG Tian-ping, CHENG Yan, WEI Cun-di. Thermal activation of coal fly ash by sodium hydrogen sulfate for alumina extraction [J]. Journal of Materials Science, 2014, 49: 4315-4322.
[15] WU Yu-sheng, XU Ping, CHEN Jiao, LI Lai-shi, LI Ming-chun. Effect of temperature on phase and alumina extraction efficiency of the product from sintering coal fly ash with ammonium sulfate [J]. Chinese Journal of Chemical Engineering, 2014, 22: 1363-1367.
[16] XIAO Yong-feng, SUN Qi, WANG Bao-dong, LIU Xiao-tong, WANG Xiao-huan, YU Geng-zhi. A study on sintering process optimization of alumina attraction from fly ash [J]. Light Metals, 2014, 2014: 117-119.
[17] YANG Quan-cheng, MA Shu-hua, ZHENG Shi-li, ZHANG Ran. Recovery of alumina from circulating fluidized bed combustion Al-rich fly ash using mild hydrochemical process [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 1187-1195.
[18] LI Hui-quan, HUI Jun-bo, WANG Chen-ye, BAO Wei-jun, SUN Zhen-hua. Extraction of alumina from coal fly ash by mixed-alkaline hydrothermal method [J]. Hydrometallurgy, 2014, 147: 183-187.
[19] WU Cheng-you, YU Hong-fa, ZHANG Hui-fang. Extraction of aluminum by pressure acid-leaching method from coal fly ash [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 2282-2288.
[20] Tripathy A K, Sarangia C K, Tripathya B C, Sanjaya K, Bhattacharyaa I N, Mahapatraa B K, Beherab P K, Satpathy B K. Aluminium recovery from NALCO fly ash by acid digestion in the presence of fluoride ion [J]. International Journal of Mineral Processing, 2015, 138: 44-48.
[21] MATJIE R H, BUNT J R, van HEERDEN J H P. Extraction of alumina from coal fly ash generated from a selected low rank bituminous South African coal [J]. Minerals Engineering, 2005, 18: 299-310.
[22] LI Lai-shi, LIAO Xin-qin, WU Yu-sheng, LIU Ying-ying. Extracting alumina from coal fly ash with ammonium sulfate sintering process [J]. Light Metals, 2012, 2012: 215-217.
[23] Bi Shi-wen. Alumina production process [M]. Beijing: Chemical Industry Press, 2006. (in Chinese)
[24] WANG Ze-hua, MA Shu-hua, TANG Zhen-hua, WANG Xiao-hui, ZHENG Shi-li. Effects of particle size and coating on decomposition of alumina-extracted residue from high-alumina fly ash [J]. Journal of Hazardous Materials, 2016, 308: 253-263.
[25] SU Shuang-qing, YANG Jing, MA Hong-wen, JIANG Fan, LIU Yu-qin, LI Ge. Preparation of ultrafine aluminum hydroxide from coal fly ash by alkali dissolution process [J]. Integrated Ferroelectrics, 2011, 128: 155-162.
[26] DING Jian, MA Shu-hua, ZHENG Shi-li, ZHANG Yi, XIE Zong-li, SHEN S, LIU Zhong-kai. Study of extracting alumina from high-alumina PC fly ash by a hydro-chemical process [J]. Hydrometallurgy, 2016, 161: 58-64.
[27] ZHU Guo-rui, TAN Wei, SUN Jun-min, GONG Yan-bing, ZHANG Sheng, ZHANG Zhan-jun, LIU Li-yan. Effects and mechanism research of the desilication pretreatment for high-aluminum fly ash[J]. Energy Fuels, 2013, 27: 6948-6954.
[28] Barin I I. Thermochemical Data of Pure Substances [M]. 3rd ed. New York: VCH Publishers Inc, 1995.
[29] Murray J, Kirwan L, Loan M, Hodnett B K. In-situ synchrotron diffraction study of the hydrothermal transformation of goethite to hematite in sodium aluminate solutions [J]. Hydrometallurgy, 2009, 95: 239-246.
[30] Chung F H. Quantitative interpretation of X-ray diffraction patterns of mixtures. I: Matrix-flushing method for quantitative multicomponent analysis [J]. Journal of Applied Crystallography, 1974, 7: 519-525.
[31] Gieré R G, Carleton L E, Lumpkin G R. Micro- and nano-chemistry of fly ash from a coal-fired power plant [J]. American Mineralogist, 2003, 88: 1853-1865.
[32] Armstrong J A, Dann S E. Investigation of zeolite scales formed in the Bayer process [J]. Microporous and Mesoporous Materials, 2000, 41: 89-97.
[33] Gerson A R, Zheng K L. Bayer process plant scale: Transformation of sodalite to cancrinite [J]. Journal of Crystal Growth, 1997, 171: 209-218.
[34] Radomirovic T, Smith P, Southam D, Tashi S, Jones F. Crystallization of sodalite particles under Bayer-type conditions [J]. Hydrometallurgy, 2013, 137: 84-91.
[35] Moloy E, Liu Q Y, Navrotsky A. Formation and hydration enthalpies of the hydrosodalite family of materials [J]. Microporous and Mesoporous Materials, 2006, 88: 283-292.
[36] Kendrick E, Dann S. Synthesis, properties and structure of ion exchanged hydrosodalite[J]. Journal of Solid State Chemistry, 2004, 177: 1513-1519.
[37] Levenspiel O. Chemical reaction engineering [M]. 3rd ed. New York: Wiley, 1972.
刘春力1,2,马淑花1,丁 健1,罗 扬1,2,郑诗礼1,张 懿1
1. 中国科学院 过程工程研究所 绿色过程与工程重点实验室,北京 100190;
2. 中国科学院大学,北京 100049
摘 要:对粉煤灰中莫来石和刚玉在高碱溶液分解动力学进行研究。考察反应温度和反应时间对莫来石和刚玉分解率及氧化铝回收效率的影响。结果表明,升高反应温度及延长反应时间有利于莫来石、刚玉分解和氧化铝回收,且莫来石的分解温度低于刚玉的分解温度。当在220 °C条件下反应90 min后,1 L循环碱液可回收多于100 g氧化铝。莫来石和刚玉的分解过程符合收缩核模型,且反应速率受化学反应控制,二者的反应活化能分别为67.46和161.82 kJ/mol。
关键词:粉煤灰;莫来石;刚玉;分解动力学;氧化铝
(Edited by Wei-ping CHEN)
Foundation item: Project (2013CB632601) supported by the National Basic Research Program of China
Corresponding author: Shu-hua MA; Tel: +86-10-82544856; E-mail: shma@ipe.ac.cn
DOI: 10.1016/S1003-6326(19)64997-6

