
J. Cent. South Univ. (2019) 26: 2668-2680
DOI: https://doi.org/10.1007/s11771-019-4204-6
Thermodynamic analysis of Li-Ni-Co-Mn-H2O system and synthesis of LiNi0.5Co0.2Mn0.3O2 composite oxide via aqueous process
LI Yun-jiao(李运姣)1, 2, LI Ling(李玲)1, 2, SU Qian-ye(苏千叶)1, 2, LU Wei-sheng(卢伟胜)2,HAN Qiang(韩强)1, 2, LI Lin(李林)1, CHEN Yong-xiang(陈永祥)1, 2,DENG Shi-yi(邓诗易)1, 2, LEI Tong-xing(雷同兴)1, 2
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Citic Dameng Mining Industries Limited, Nanning 530028, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: The constructed potential-pH diagrams of Li-Ni (Co, Mn)-H2O system indicate that the LiNiO2, LiCoO2 and LiMnO2 are thermodynamically stable in aqueous solution within the temperature range of 25-200 °C and the activity range of 0.01-1.00. A predominant co-region of LiNiO2, LiCoO2 and LiMnO2 oxides (Li-Ni-Co-Mncomposite oxide) is found in the Li-Ni-Co-Mn-H2O potential-pH diagrams, in which the co-precipitation region expands towards lower pH with rising temperature, indicating the enhanced possibility of synthesizing Li-Ni-Co-Mn composite oxide in aqueous solution. The experimental results prove that it is feasible to prepare the LiNi0.5Co0.2Mn0.3O2 cathode materials (NCM523) by an aqueous routine. The as-prepared lithiated precursor and NCM523 both inherit the spherical morphology of the hydroxide precursor and the obtained NCM523 has a hexagonal α-NaFeO2 structure with good crystallinity. It is reasonable to conclude that the aqueous routine for preparing NCM cathode materials is a promising method with the guidance of the reliable potential-pH diagrams to some extent.
Key words: aqueous process; potential-pH diagrams; thermodynamics; LiNi0.5Co0.2Mn0.3O2; cathode materials
Cite this article as: LI Yun-jiao, LI Ling, SU Qian-ye, LU Wei-sheng, HAN Qiang, LI Lin, CHEN Yong-xiang, DENG Shi-yi, LEI Tong-xing. Thermodynamic analysis of Li-Ni-Co-Mn-H2O system and synthesis of LiNi0.5Co0.2Mn0.3O2 composite oxide via aqueous process [J]. Journal of Central South University, 2019, 26(10): 2668-2680. DOI: https://doi.org/10.1007/s11771-019-4204-6.
1 Introduction
The potential-pH diagrams which derive from thermodynamic data represent the predominant domains of various species in a system. Generally, the potential-pH diagrams are helpful in predicting the aqueous reactions and the stability regions of species in aqueous solutions under different equilibrium conditions, which are not only widely used in the fields of corrosion technology, electro- deposition, but also in the area of various composite oxides synthesis [1-3].
In recent years, LiNiO2, LiCoO2 and LiMnO2 cathode materials have attracted enormous interests from scholars and automobile industries. LiNixCoyMn1-x-yO2 (02, LiCoO2 and LiMnO2 materials and are considered the most promising power source for new energy vehicles. Nevertheless, the severe poor electrochemical properties of the LiNixCoyMn1-x-yO2 materials hinder their widely commercial application.
Many strategies, such as new synthetic methods, ion doping, coating and structure design, have been proposed by the researchers to improve the electrochemical performances [4-9]. Among them, synthetic methods including co-precipitation method [10], sol-gel method [11-13], spray process [14-17] and hydrothermal synthesis [11, 18] have been regarded as effective routines for advanced lithium ion battery and many aqueous reactions are involved in these synthetic methods, especially for wet-chemical synthesis. Thus, it is important for the stability regions of species in aqueous solutions to control the aqueous reactions.
Wet-chemical synthesis exhibits huge potential in synthesizing the LiNixCoyMn1-x-yO2 cathode materials and has been studied by many researchers. However, there are few reports on the thermodynamics of the Li-Ni-Co-Mn-H2O systems. The further progress of the LiNixCoyMn1-x-yO2 cathode materials synthesized by wet-chemical method is slow to some extent due to the lack of the aqueous thermodynamics theories. In this paper, the potential-pH diagrams for Li-Ni-H2O, Li-Co-H2O, and Li-Mn-H2O systems as well as Li-Ni-Co- Mn-H2O complex systems from 25 to 200 °C were constructed. And the influences of the activity and temperature were discussed in detail. The LiNi0.5Co0.2Mn0.3O2 cathode material, as an example, was prepared by the hydrothermal synthesis routine followed by heat treatment under the guidance of the obtained potential-pH diagrams. The results show that the as-constructed potential-pH diagrams play a significant role in the synthesis of the Li-Ni (Co, Mn) cathode materials and the high value- added products in nickel and cobalt metallurgical processes via aqueous solution.
2 Construction of potential-pH diagrams
2.1 Thermodynamic calculation
The reactions occurring in the system can be summarized as Eq. (1):
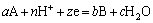 (1)
(1)
So the Gibbs free energy change △rGT for the above reaction at a given temperature can be calculated by Eq. (2):
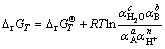 (2)
(2)
where 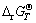 is the standard Gibbs free energy of the reaction at a certain temperature, kJ/mol; R represents the gas constant, 8.314 J/(mol·K); T is the thermodynamic temperature, K; α is the activity for different species in the solution, such as Mn2+, Ni2+ and Co2+.
is the standard Gibbs free energy of the reaction at a certain temperature, kJ/mol; R represents the gas constant, 8.314 J/(mol·K); T is the thermodynamic temperature, K; α is the activity for different species in the solution, such as Mn2+, Ni2+ and Co2+.
It is known that △rGT will be equal to zero when a reaction reaches equilibrium. So combined with the Nernst equation (Eq. (3)), we can get the relationship (Eqs. (4)-(6)) of pH and E to construct the E-pH diagram:
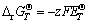 (3)
(3)
1) For reactions that are involved with electrons but are independent of pH, we have
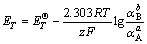 (4)
(4)
2) For reactions that are involved with both electrons and H+, we have
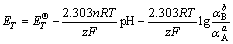 (5)
(5)
3) For reactions that are involved H+ but are independent of E, we have
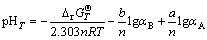 (6)
(6)
where ET denotes the electrode potential of the reaction, V; z is the number of the electrons participating in the reaction; F refers to the Faraday constant, 96485.34 c/mol.
The standard free energy of the reaction at high temperature is calculated by the given equation:
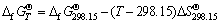 (7)
(7)
where 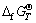 and
and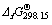 are the standard Gibbs free energy of formation of species at T and 298.15 K, respectively.
are the standard Gibbs free energy of formation of species at T and 298.15 K, respectively. 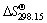 is the value of absolute entropy.
is the value of absolute entropy.
The common species in Li-Ni-H2O, Li-Co-H2O and Li-Mn-H2O systems are chosen from the diagrams reported [4, 19-23], which are cited in the construction of most potential-pH diagrams.
The standard Gibbs free energies and the standard entropies of most species in the Li-Me-H2O (Me=Ni, Co, Mn) systems are taken from Lange's Handbook [24] and Refs. [22, 23], and the others standard entropies are estimated by empirical relations. For example, the entropies of aqueous oxy-anions are estimated by:
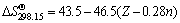 (8)
(8)
where Z is the absolute value of the charge of the ions and n is the number of O atoms excluding those in the hydroxyl groups.
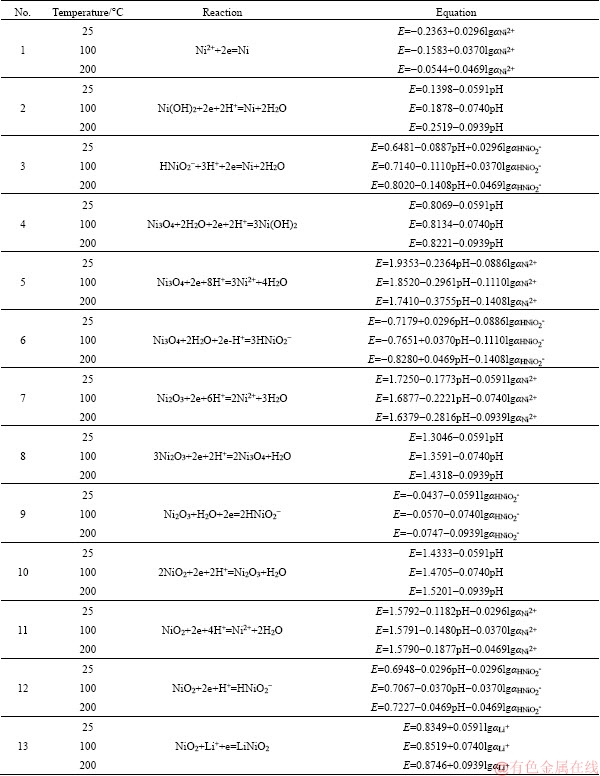
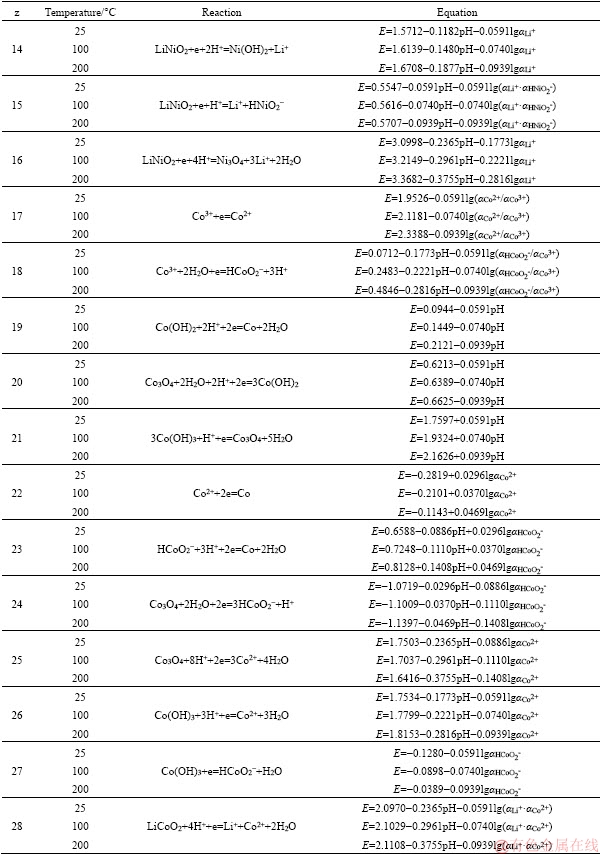
The more details of the methods for estimating the entropy and constructing the potential-pH diagrams are described in our previous publication [2]. The obtained relationships between potential and pH of the reactions considered in the systems at different temperatures of 25, 100 and 200 °C are given in the Appendix.
2.2 Potential-pH diagrams of Li-Ni (Co, Mn)- H2O system
The potential-pH diagrams of Li-Ni(Co, Mn)-H2O system, are constructed based on the Section 2.1 and corresponding Appendix. Figure 1 shows the potential-pH diagrams of Li-Ni-H2O system at activity 1.00 in the temperature range from 25 to 200 °C. Figures 2-4 represent the potential-pH diagrams of Li-Me-H2O system at 200 °C, in which the activities of all the dissolved species are assumed to be 1.00, 0.10 and 0.01, respectively.
As shown in Figure 1, Ni2+ shows up at low pH over a wide potential range, and reacts with hydroxide ions to form Ni(OH)2 with increasing pH. As the pH further increases to higher alkaline level, Ni(OH)2 transforms into LiNiO2. More specifically,the predominant region of LiNiO2 presents in the right, of the middle of the potential-pH diagram. It suggests that LiNiO2 composite oxide is thermodynamically stable in aqueous solution. The stability regions of different species in the potential-pH diagrams are significantly affected by temperature. It is obviously observed that the equilibrium lines of Ni(OH)2/LiNiO2 move towards lower pH and negative potential with rising temperature, resulting in the enlargement of the stability domain of LiNiO2. It is greatly possible to synthesize LiNiO2 at relatively higher temperature.
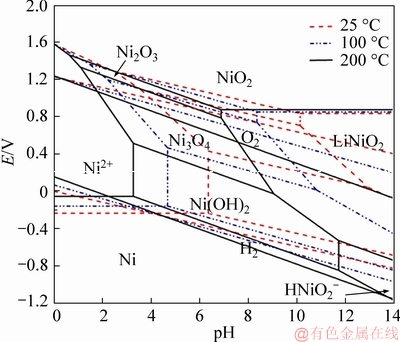
Figure 1 Potential-pH diagrams of Li-Ni-H2O system in temperature range from 25 to 200 °C
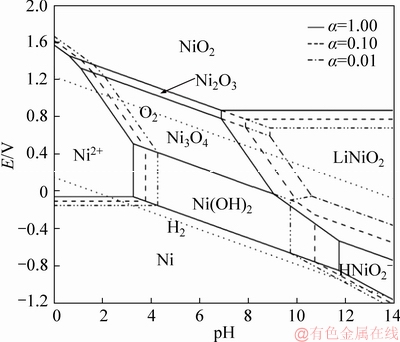
Figure 2 Potential-pH diagrams of Li-Ni-H2O system at 200 °C at different activities
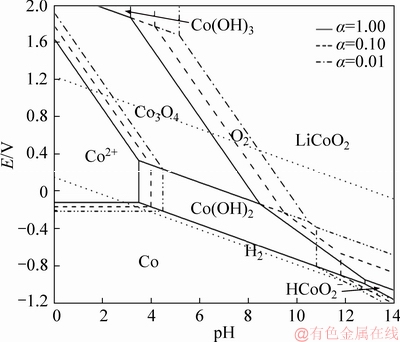
Figure 3 Potential-pH diagrams of Li-Co-H2O system at 200 °C at different activities
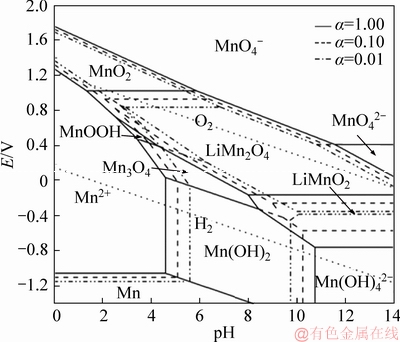
Figure 4 Potential-pH diagrams of Li-Mn-H2O system at 200 °C at different activities
The potential-pH diagram of Li-Ni-H2O system at 200 °C at different activities is illustrated in Figure 2. The predominant regions of various dissolved species are significantly affected by the activities. Apparently, the stability regions of Ni(OH)2 and LiNiO2 enlarge at a high activity, indicating that the higher activities of the dissolved species are beneficial for increasing the stabilities of the Ni(OH)2 and LiNiO2 phases in aqueous systems. Noteworthy, the equilibrium line of Ni2+/Ni(OH)2 moves from pH 4.26 to 3.26 with increasing the activity from 0.01 to 1.00, while that of the Ni(OH)2/HNiO2- shifts in an opposite direction (from pH 9.72 to 11.72). Furthermore, all the equilibrium lines of Ni3O4/LiNiO2, Ni(OH)2/LiNiO2 and HNiO2-/LiNiO2 move towards lower pH and more negative potential. All of those result in the expanding of the LiNiO2 stable region. The higher activities of the species greatly facilitate the synthesis of LiNiO2 composite oxide. GUO et al [23] also confirmed that it is feasible to synthesize the LiNiO2 in the aqueous solution by soft chemistry method. From the potential-pH diagram, it shows that Ni, Ni2+, Ni(OH)2 and Ni3O4 can be chosen as the candidate precursors for the synthesis of LiNiO2 composite oxide in aqueous solution.
The potential-pH diagrams of the Li-Co-H2O (Figure 3) and Li-Mn-H2O (Figure 4) systems are similar to that of the Li-Ni-H2O system. LiCoO2 and LiMnO2 composite oxides are also thermodynamically stable in aqueous solution. It is observed that the LiCoO2 and LiMnO2 can be obtained from various Co and Mn compounds at certain pH and potential, respectively. From Figure 3, it shows a large LiCoO2 stability field, in wide pH and potential ranges. Compared with the stable areas of LiNiO2 and LiCoO2, the stability area of LiMnO2 is a small zone and it locates in the right bottom corner of the diagram (Figure 4). The activities of the dissolved species also play positive roles in the synthesis of LiCoO2 and LiMnO2. Above all, the synthesis of LiNiO2, LiCoO2 and LiMnO2 under a low-alkaline environment is thermodynamically possible by enhancing temperature and activities, which are consistent with the previous reports [4, 25-27].
2.3 Potential-pH diagrams of Li-Ni-Co-Mn-H2O system
Figures 5 and 6 represent the potential-pH diagrams of Li-Ni-Co-Mn-H2O system at 100 and 200 °C, respectively. The activities of all dissolved species are taken at 1.00.
The predominant co-region of Li-Ni-Co-Mn composite oxide is expressed by the shaded areas. It is specially noted that the co-region of Li-Ni-Co-Mn oxide is determined by the stable areas of LiNiO2, LiCoO2, and LiMnO2 in the given temperature range, especially for the LiNiO2 and LiMnO2. Besides, there are also the co-regions of metal ion and metal hydroxide (metal=Ni, Co and Mn). The metal ions (Ni2+, Co2+ and Mn2+) are hydrolyzed to form complex Ni-Co-Mn hydroxide with an increase in pH, which has been confirmed in Ref. [19]. Furthermore, the Ni-Co-Mn hydroxide converts into the Li-Ni-Co-Mn composite oxide with an increase in pH and potential. The predominant co-regions of the Li-Ni-Co-Mn composite oxide (Figures 5 and 6) show a significant expansion as rising temperature, indicating that the synthesis of the Li-Ni-Co-Mn composite oxide is greatly affected by temperature. The higher temperature is obviously favorable to the synthesis of the Li-Ni-Co-Mn composite oxide. Carefully, the predominant co-region of the Li-Ni-Co-Mn composite oxide is a small triangle at 100 °C, which is located at pH above 12.27 and the corresponding potential changes from -0.45 to 0.20 as shown in Figure 5. Interestingly, the predominant co-region enlarges from a triangle to an irregular quadrangle when the temperature increases to 200 °C and the corresponding lowest pH limit also decreases to 9.73 as well as the potential goes down in the range of -0.74-0.19 V. It is suggested that high temperature is much beneficial to synthesizing the Li-Ni-Co-Mn composite oxide.
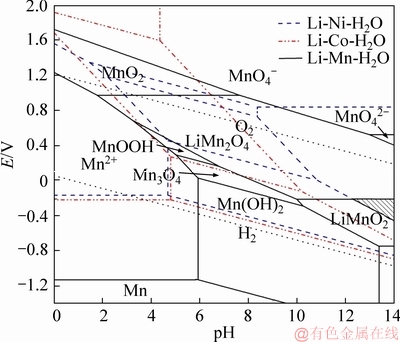
Figure 5 Potential-pH diagrams of Li-Ni-Co-Mn-H2O system at 100 °C
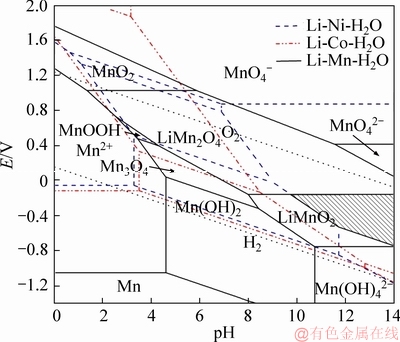
Figure 6 Potential-pH diagrams of Li-Ni-Co-Mn-H2O system at 200 °C
Above all, Li-Ni-Co-Mn compound oxide is thermodynamically stable at a certain pH and potential range in an aqueous solution (i.e., pH>12.27 and potential -0.45 to 0.20 V at 100 °C). Therefore, it is speculated that an aqueous method may be adopted to synthesize the composite oxide LiNixCoyMn1-x-yO2 under an alkaline aqueous environment.
3 Experimental confirmation
The predominant region of the Li-Ni-Co-Mn composite oxide is successfully predicted by the constructed potential-pH diagrams in aqueous solution. In order to verify the results, the LiNi0.5Co0.2Mn0.3O2 cathode material, as an example, has been obtained in aqueous solution by a hydrothermal process followed by heat treatment.
3.1 Sample preparation
The Ni0.5Co0.2Mn0.3(OH)2 (NCM hydroxide, Xiamen Tungsten Co., Ltd, China) and LiOH·H2O (analytical grade, Tianjin chemical reagent Co., Ltd, China) were used as the raw materials. 200 g of the (Ni0.5Co0.2Mn0.3)(OH)2 (NCM hydroxide) and 93.4 g of LiOH·H2O were mixed and added into an autoclave (Parr 4568 reactor, USA) together with the required amount of deionized water. The autoclave was sealed and heated up to 250 °C and then maintained for 4 h. Then the slurry was discharged and subjected to solid-liquid separation. The solid was dried at 90 °C overnight to obtain the lithiated precursor (LNCM precursor). The precursor was heat-treated at 500 °C for 6 h and then at 900 °C for 10 h in air to obtain the LiNi0.5Co0.2Mn0.3O2 (NCM523 cathode material).
The crystal structure of the powders was characterized by the Rigaku D/max-2500 X-ray powder diffraction (XRD) using Kα radiation. The morphology of the particles was examined with the scanning electron microscope (SEM) using JEOL JSM-6360LV operated at 20 kV.
3.2 Results and discussion
XRD patterns of the NCM hydroxide, the LNCM precursor and the as-prepared NCM523 cathode material are depicted in Figure 7. The XRD patterns of the NCM hydroxide (Figure 7(a)) is indexed with the typical fingerprint of Me(OH)2 (Me=Ni, Co, Mn) with a hexagonal α-NaFeO2 structure and the same as that reported by ZHU [27] and LEE et al [10]. It is evidently observed that the peaks of the LNCM precursor (Figure 7(b)) is quite different from that of the NCM hydroxide, but similar to that of the as-prepared NCM523 cathode material. In addition, there are no LiOH peaks detected. So it is reasonable to deduce that the NCM hydroxide precursor has reacted with LiOH·H2O in the aqueous solution and transformed into the LNCM composite oxide precursor with a hexagonal α-NaFeO2 structure. The LNCM oxide precursor shows a broader and low-intensity diffraction peaks, which demonstrates that the material has a low crystallinity and an imperfect crystal structure. Compared to the LNCM oxide precursor (Figure 7(b)), the diffraction peaks of the NCM523 cathode material become sharper and higher, indicating a good crystallinity with a hexagonal α-NaFeO2 structure. Furthermore, no diffraction peaks of impurity or other phases are detected, suggesting a pure phase structure. In conclusion, the LNCM oxide precursor with α-NaFeO2 hexagonal structure but a low crystallinity has been synthesized via an aqueous process with the guidance of the constructed potential-pH diagrams, indicating the reliability of the constructed potential-pH diagrams.
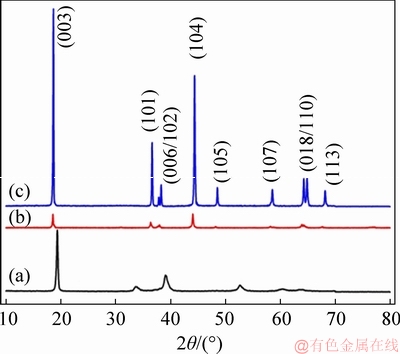
Figure 7 XRD patterns of NCM hydroxide precursor (a), LNCM precursor (b) and NCM523 cathode materials (c)
Figure 8 represents the SEM images of the NCM hydroxide, the LNCM precursor and the as-prepared NCM523 cathode material. The NCM hydroxide precursor (Figure 8(a)) is composed of the spherical powders with a smooth and clean surface. The LNCM oxide precursor (Figure 8(b)) inherits the primary morphology of the NCM hydroxide at treatment. The obtained LiNi0.5Co0.2Mn0.3O2 cathode material (Figure 8(c)) exhibits a much rougher surface and the second particles are clearly observed, demonstrating the good crystallinity of the NCM523 cathode material. Above all, the constructed potential-pH diagrams of Li-Ni (Co, Mn)-H2O systems provide a good interpretation for the occurrence of the Li-Ni (Co, Mn) oxides.
4 Conclusions
The potential-pH diagrams of Li-Ni (Co, Mn)-H2O systems at different activities and temperatures are constructed. The Li-Ni-Co-Mn composite oxides are thermodynamically stable in an aqueous solution, which indicates the great possibility of preparing the composite oxides via an aqueous process. The constructed potential-pH diagrams demonstrate that temperature and activity have significant influences on the stable fields of the composite oxides. As a result of the movement of the equilibrium lines in the potential-pH diagrams, the stability regions of the composite oxides expand towards lower pH with an increase in temperature and activity. Under the guidance of the potential-pH diagrams, the LiNi0.5Co0.2Mn0.3O2 cathode material has been successfully prepared via the hydrothermal process followed by heat treatment. The good crystallinity and well layered structure as well as perfect morphology of the as-prepared material have confirmed the reliability of the potential-pH diagrams. It implies a most promising future to develop an aqueous routine for the preparation of the NCM cathode materials for lithium ion batteries.
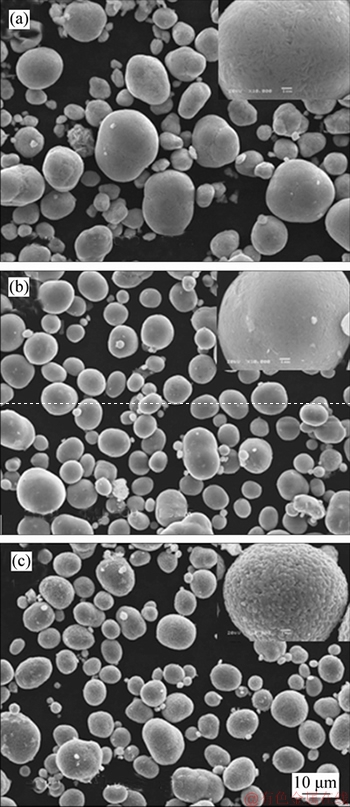
Figure 8 SEM images of NCM hydroxide (a), LNCM precursor (b) and as-prepared NCM523 cathode materials (c)
Appendix
Reactions in the Li-Me-H2O systems (Me=Ni, Co, Mn) and relationships between potential and pH of these reactions at different temperatures
to be continued
Continued
to be continued
Continued
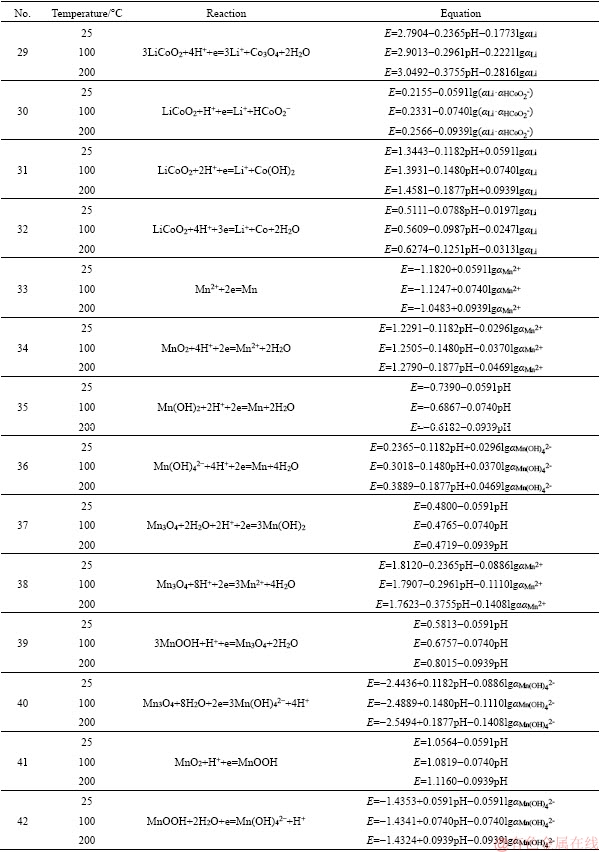
to be continued
Continued
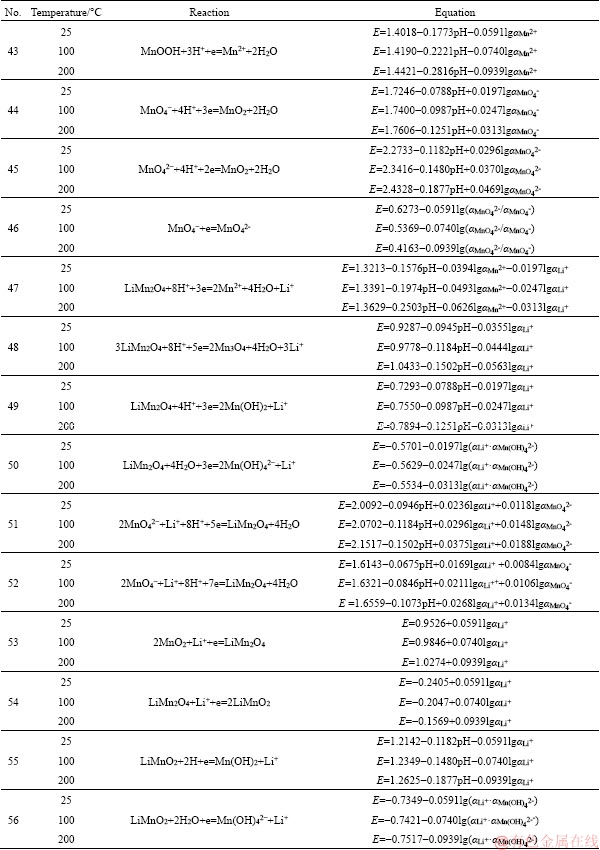
to be continued
Continued
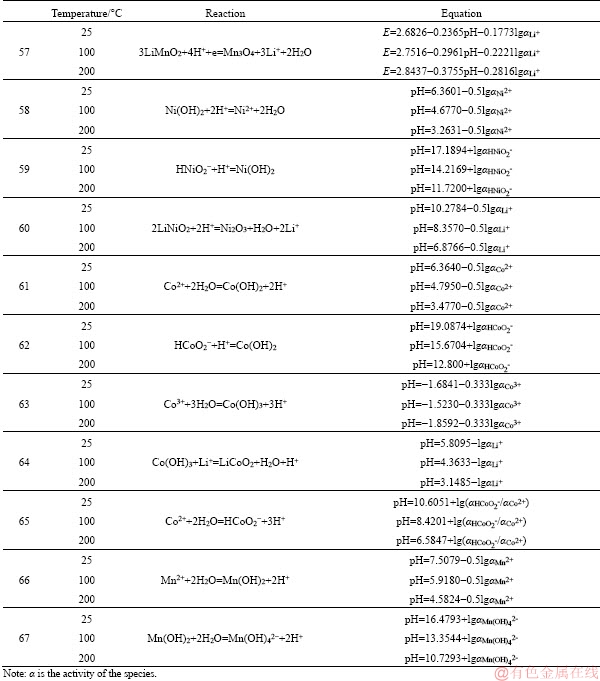
References
[1] PORTHAULT H, LE CRAS F, BADDOUR-HADJEAN R, PEREIRA-RAMOS J P, FRANGER S. One step synthesis of lamellar R-3m LiCoO2 thin films by an electrochemical– hydrothermal method [J]. Electrochimica Acta, 2011, 56(22): 7580-7585. DOI: 10.1016/j.electacta.2011.06.083.
[2] LI Lin, LI Yun-jiao, XU Cang, PAPANGELAKIS V G, CHU Guang, LI Gui-liang, WANG Xuan-yu, KONG Long. E-pH diagrams from 333.15 to 453.15 K for lithium-titanium composite oxides and their synthesis in aqueous solution [J]. Hydrometallurgy, 2014, 142: 131-136. DOI: 10.1016/j. hydromet.2013.11.010.
[3] HOU Pei-yu, ZHANG Hong-zhou, DENG Xiao-long, XU Xi-jin, ZHANG Lian-qi. Stabilizing the electrode/electrolyte interface of LiNi0.8Co0.15Al0.05O2 through tailoring aluminum distribution in microspheres as long-life, high-rate, and safe cathode for lithium-ion batteries [J]. ACS Applied Materials & Interfaces, 2017, 9(35): 29643-29653. DOI: 10.1021/ acsami.7b05986.
[4] SONG S W, HAN K S, YOSHIMURA M. Effect of 20-200 °C fabrication temperature on microstructure of hydrothermally prepared LiCoO2 films [J]. Journal of the American Ceramic Society, 2004, 83(11): 2839-2844.
[5] COWAN R L, STAEHLE R W. The thermodynamics and electrode kinetic behavior of nickel in acid solution in the temperature range 25 to 300 °C [J]. Journal of the Electrochemical Society, 1971, 118(4): 557. DOI: 10.1149/1.2408111.
[6] HOU Pei-yu, LI Feng, SUN Yan-yun, LI Hui-qiao, XU Xi-jin, ZHAI Tian-you. Multishell precursors facilitated synthesis of concentration-gradient nickel-rich cathodes for long-life and high-rate lithium-ion batteries [J]. ACS Applied Materials & Interfaces, 2018, 10(29): 24508-24515. DOI: 10.1021/acsami. 8b06286.
[7] LI Feng, KONG Ling-long, SUN Yan-yun, JIN Yong-cheng, HOU Pei-yu. Micron-sized monocrystalline LiNi1/3Co1/3Mn1/3O2 as high-volumetric-energy-density cathode for lithium-ion batteries [J]. Journal of Materials Chemistry A, 2018, 6(26): 12344-12352. DOI: 10.1039/ c8ta03363c.
[8] HOU Pei-yu, YIN Jiang-mei, DING Meng, HUANG Jin-zhao, XU Xi-jin. Surface/interfacial structure and chemistry of high-energy nickel-rich layered oxide cathodes: Advances and perspectives [J]. Small, 2017, 13(45): 1701802. DOI: 10.1002/smll.201701802.
[9] HOU Pei-yu, ZHANG Hong-zhou, ZI Zhong-yue, ZHANG Lian-qi, XU Xi-jin. Core–shell and concentration-gradient cathodes prepared via co-precipitation reaction for advanced lithium-ion batteries [J]. Journal of Materials Chemistry A, 2017, 5(9): 4254-4279. DOI: 10.1039/c6ta10297b.
[10] LEE M H, KANG Y J, MYUNG S T, SUN Y K. Synthetic optimization of Li[Ni1/3Co1/3Mn1/3]O2 via co-precipitation [J]. Electrochimica Acta, 2004, 50(4): 939-948. DOI: 10.1016/ j.electacta.2004.07.038.
[11] LU Hua-quan, ZHOU Hai-tao, SVENSSON A M, FOSSDAL A, SHERIDAN E, LU Shi-gang, VULLUM- BRUER F. High capacity Li[Ni0.8Co0.1Mn0.1]O2 synthesized by sol–gel and co-precipitation methods as cathode materials for lithium-ion batteries [J]. Solid State Ionics, 2013, 249-250: 105-111. DOI: 10.1016/j.ssi.2013. 07.023.
[12] LIN Yu-kai, LU Chung-hsin. Preparation and electrochemical properties of layer-structured LiNi1/3Co1/3Mn1/3-yAlyO2 [J]. Journal of Power Sources, 2009, 189(1): 353-358. DOI: 10.1016/j.jpowsour.2008.08.072.
[13] WANG Zhi-xing, FANG Hai-sheng, YIN Zhou-lan, LI Xin-hai, GUO Hua-jun, PENG Wen-jie. Synthesis and characterization of high-voltage cathode material LiNi0.5Mn1.5O4 by one-step solid-state reaction [J]. Journal of Central South University of Technology, 2005, 12(1): 54-58. DOI: 10.1007/s11771-005-0371-8.
[14] LI De-cheng, MUTA T, ZHANG Lian-qi, YOSHIO M, NOGUCHI H. Effect of synthesis method on the electrochemical performance of LiNi1/3Mn1/3Co1/3O2 [J]. Journal of Power Sources, 2004, 132(1, 2): 150-155. DOI: 10.1016/j.jpowsour. 2004.01.016.
[15] LIN Bin, WEN Zhao-yin, GU Zhong-hua, HUANG Sha-hua. Morphology and electrochemical performance of Li[Ni1/3Co1/3Mn1/3]O2 cathode material by a slurry spray drying method [J]. Journal of Power Sources, 2008, 175(1): 564-569. DOI: 10.1016/j.jpowsour.2007.09.055.
[16] SHUI Miao, GAO Shan, SHU Jie, ZHENG Wei-dong, XU Dan, CHEN Liang-liang, FENG Lin, REN Yuan-long. LiNi1/3Co1/3Mn1/3O2 cathode materials for LIB prepared by spray pyrolysis. II. Li+ diffusion kinetics [J]. Ionics, 2013, 19(1): 47-52. DOI: 10.1007/s11581-012-0723-y.
[17] PENG Qi-ling, ZHOU Hai-hui, HUANG Zhen-hua, CHEN Jin-hua, KUANG Ya-fei. Catalytic graphitization of polyacrylonitrile-based carbon fibers coated with Prussian blue [J]. Journal of Central South University of Technology, 2010, 17(4): 683-687. DOI: 10.1007/s1171-010-0540-2.
[18] MYUNG S T, LEE M H, KOMABA S, KUMAGAI N, SUN Y K. Hydrothermal synthesis of layered Li[Ni1/3Co1/3Mn1/3]O2 as positive electrode material for lithium secondary battery [J]. Electrochimica Acta, 2005, 50(24): 4800-4806. DOI: 10.1016/j.electacta.2005.02.034.
[19] ZHAO Zhong-wei, HUO Guang-sheng. Thermodynamic and kinetic research of Li2O-H2O system [J]. The Chinese Journal of Nonferrous Metals, 2004(8): 2149-2152. DOI: 10.13182/fst85-a24601. (in Chinese)
[20] MAKIMURA Y, OHZUKU T. Lithium insertion material of LiNi1/2Mn1/2O2 for advanced lithium-ion batteries [J]. Journal of Power Sources, 2003, 119-121: 156-160. DOI: 10.1016/ S0378-7753(03)00170-8.
[21] LI Xue-liang, HE Wen-xiang, CHEN Li, GUO Wei, CHEN Jie-jie, XIAO Zheng-hui. Hydrothermal synthesis and electrochemical performance studies of Al2O3-coated LiNi1/3Co1/3Mn1/3O2 for lithium-ion batteries [J]. Ionics, 2014, 20(6): 833-840. DOI: 10.1007/s11581-013-1041-8.
[22] WEN Shi-mei, ZHAO Zhong-wei, HUO Guang-sheng. Thermodynamic analysis and potential-pH diagrams of Li-Co-H2O system [J]. Chinese Journal of Power Source, 2005, 29(7): 423-426. (in Chinese)
[23] GUO Chi-hao, ZHAO Zhong-wei. Thermodynamic analysis on Li-Ni-H2O system [J]. Chinese Journal of Power Source, 2005, 29(6): 7-10. DOI: 10.3969/j.issn.1002-087X.2005.06. 010.(in Chinese)
[24] DEAN J A. Lang’s handbook of chemistry [M]. 3rd ed. Singapore: McGRAW-Hill, 1987.
[25] SONG S, HAN K, SASAGAWA I, WATANABE T, YOSHIMURA M. Effect of LiOH concentration change on simultaneous preparation of LiCoO2 film and powder by hydrothermal method [J]. Solid State Ionics, 2000, 135(1-4): 277-281. DOI: 10.1016/S0167-2738(00)00446-X.
[26] CHEN Yong-xiang, LI Yun-jiao, LI Wei, CAO Guo-lin, TANG Shu-yun, SU Qian-ye, DENG Shi-yi, GUO Jia. High-voltage electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material via the synergetic modification of the Zr/Ti elements [J]. Electrochimica Acta, 2018, 281: 48-59. DOI: 10.1016 /j.electacta.2018.05.154.
[27] ZHU Jie, LI Yun-jiao, XUE Long-long, CHEN Yong-xiang, LEI Tong-xing, DENG Shi-yi, CAO Guo-lin. Enhanced electrochemical performance of Li3PO4 modified Li[Ni0.8Co0.1Mn0.1]O2 cathode material via lithium- reactive coating [J]. Journal of Alloys and Compounds, 2019, 773: 112-120. DOI: 10.1016/j.jallcom.2018.09.237.
(Edited by ZHENG Yu-tong)
中文导读
Li-Ni-Co-Mn-H2O系热力学分析及LiNi0.5 Co0.2Mn0.3O2复合氧化物的湿法合成
摘要:本文构建的Li-Ni(Co, Mn)-H2O系电位-pH图从热力学角度表明,在25~250 °C温度范围和0.01~1.00活度范围内,LiNiO2, LiCoO2和LiMnO2可以稳定存在于水溶液中。在Li-Ni-Co-Mn-H2O系电位-pH图中,同时出现了LiNiO2、LiCoO2和LiMnO2氧化物(即Li-Ni-Co-Mn复合氧化物)的优势区,并且该共沉淀优势区随温度的升高向低pH方向扩大,表明在水溶液中合成Li-Ni-Co-Mn复合氧化物是可能的。在此基础上,本文通过实验采用湿法过程合成了LiNi0.5 Co0.2Mn0.3O2 (NCM523)正极材料,证明了上述理论的可行性。合成的锂化前驱体和NCM523材料均继承了氢氧化物前驱体的球形形貌,同时所得NCM523材料具有良好的六方α-NaFeO2型晶体结构。在电位-pH图指导下,湿法合成工艺将一定程度上成为三元正极材料极具前景的制备方法之一。
关键词:溶液过程;电位-pH图;热力学;LiNi0.5 Co0.2Mn0.3O2;正极材料
Foundation item: Project(FA2019015) supported by the Government of Chongzuo, Guangxi Zhuang Autonomous Region, China; Project(AD18281073) supported by Science and Technology Department of Guangxi Zhuang Autonomous Region, China
Received date: 2018-01-08; Accepted date: 2019-04-01
Corresponding author: LI Yun-jiao, PhD, Professor; Tel: +86-731-88830872; E-mail: yunjiao_li@csu.edu.cn; ORCID: 0000-0001- 8882-7747