
Thermodynamic analysis of Ti powder synthesized by
SHS thermitreaction
ZHANG Peng-lin(张鹏林)1, 2, XIA Tian-dong(夏天东)1, 2, ZHANG Guo-dong(张国栋)3,
YAN Li-jing(闫丽静)2, ZHAO Wen-jun(赵文军)1
1. State Key Laboratory of Gansu Advanced Nonferrous Metal Materials,
Lanzhou University of Technology, Lanzhou 730050, China;
2. Key Laboratory of Non-ferrous Metal Alloys, Ministry of Education,
Lanzhou University of Technology, Lanzhou 730050, China;
3. College of Power and Mechanical Engineering, Wuhan University, Wuhan 430072, China
Received 15 July 2007; accepted 10 September 2007
Abstract: According to the thermodynamics theories, the reactive Gibbs free energies, the reactive adiabatic temperature, the melting rate of Ti and the gasification mass of Mg in the Mg-TiO2 and Al-TiO2 systems were theoretically calculated and analyzed respectively. The results show that the reactions of Mg-TiO2 and Al-TiO2 are very easy to take place and the reaction of producing various suboxides of Ti may occur in Mg-TiO2 and Al-TiO2 reaction system; the adiabatic temperature of Mg-TiO2 becomes lower with increasing mass fraction of Mg. The adiabatic temperature is below 1 800 K when the mass fraction of excessive Mg exceeds 25%; The adiabatic temperature of Al-TiO2 also becomes lower with increasing mass fraction of Al, but it becomes higher with the preheat temperature increment. The adiabatic temperature plateau is the result of Ti melting endotherm; owing to the gasification of a great deal of Mg in Mg-TiO2 reaction process, Mg should be properly excessive in order to get Ti.
Key words: self-propagating high-temperature synthesis(SHS); thermodynamic analysis; adiabatic temperature; Mg-TiO2; Al-TiO2
1 Introduction
Self-propagating high-temperature synthesis (SHS) provides an attractive promising alternative to the conventional methods of producing the advanced materials such as borides, carbides, silicides, aluminides, and other intermetallic compounds from powder mixture of metal and nonmetal elements, in respect that SHS superior in process economics and process simplicity[1-5]. The basis of studying the process of SHS lies in the thermodynamic analysis of combustion system, which can gives us the information about reaction direction and extent as well as phase composition and stability at elevated temperature[6-7]. As for the formation of compounds, it has been demonstrated empirically that the reaction will not be self-sustaining unless the adiabatic temperature Tad≥1 800 K[8].
There are several varieties of thermitreactions and the most commonly used involves reduction of the oxide with either Al or Mg[9]. The SHS of metallic titanium powders form Mg-TiO2 initial mixtures was first carried out in ISMAN, Russia[10]. NERSISYAN et al[11] have synthesized nanocrystalline Ti Powder with Mg-TiO2 system using NaCl as an inert diluent. LIANG et al[12-13] have synthesized Ti powder with Al-TiO2 system and put forward the reaction mechanism.
In this study, reactive Gibbs free energies, reactive adiabatic temperature and the melting rate of Ti along with the gasification mass of Mg were calculated. the influence of the excessive Mg upon the adiabatic temperature, and the influence of the excessive Al and heating temperature in advance upon the adiabatic temperature in the Al-TiO2 system were investigated.
2 Description of basis theory
2.1 Gibbs free energy
For any reaction system, the condition for the reaction is[14]
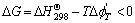 (1)
(1)
where 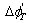 represents the Gibbs free energies function (J/(K?mol)), and thus it can be viewed as the difference of Gibbs free energies of all the resultants and reactants. The more minus
represents the Gibbs free energies function (J/(K?mol)), and thus it can be viewed as the difference of Gibbs free energies of all the resultants and reactants. The more minus 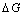 is, the easier the reaction will be.
is, the easier the reaction will be.
All reactions that are probable to take place in the Mg-TiO2 and Al-TiO2 systems are shown as
Mg+3TiO2→Ti3O5+MgO (2)
Mg+2TiO2→Ti2O3+MgO (3)
Mg+TiO2→TiO+MgO (4)
2Mg+TiO2→Ti+2MgO (5)
2Al+9TiO2→3Ti3O5+Al2O3 (6)
2Al+6TiO2→3Ti2O3+ Al2O3 (7)
2Al+3TiO2→3TiO+ Al2O3 (8)
4Al+3TiO2→3Ti+2Al2O3 (9)
2.2 Adiabatic temperature
For any binary reaction system:
A(s)+B(s)→C(s) (10)
When the thermodynamic effect is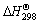 , the Kirchhoff formula can be adopted to calculate the
, the Kirchhoff formula can be adopted to calculate the 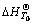 when the temperature is T0, that is
when the temperature is T0, that is
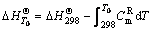 (11)
(11)
where 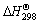 means the difference between the summation of all the standard reaction enthalpy of the resultants and the summation of the standard reaction enthalpy of all reactants, T0 is the initial reactive temperature, and
means the difference between the summation of all the standard reaction enthalpy of the resultants and the summation of the standard reaction enthalpy of all reactants, T0 is the initial reactive temperature, and 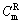 is the mole thermal capacity of the resultants.
is the mole thermal capacity of the resultants.
Supposing that the reaction takes place under the adiabatic condition, and the exothermic heat is used to heat up the resultants, then the highest temperature that the resultants can reach is the adiabatic temperature. The adiabatic temperature can be calculated by
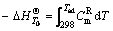 (12)
(12)
where the minus denotes that the reaction is an exothermic one, Tad is the adiabatic temperature.
In the most complicated cases, the reactive resultants experience a process of solid state phase change, melting and gasification. The formula for calculating the adiabatic temperature is
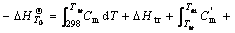
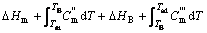 (13)
(13)
where Cm, 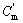 ,
, 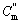 and
and  represent the mole thermal capacities under the low temperature solid state, the high temperature solid state, liquid state and gaseity of the resultant, respectively, Ttr stands for the phase change temperature, and Htr for the phase change heat, Tm for the melting point, and Hm for the melting heat, TB for the boiling point, and HB for the gasification heat. In Eqn.(13), the items on the right can be reduced according to the actual situation. The phase change rate under solid state, the melting rate and evaporation rate can be calculated by
represent the mole thermal capacities under the low temperature solid state, the high temperature solid state, liquid state and gaseity of the resultant, respectively, Ttr stands for the phase change temperature, and Htr for the phase change heat, Tm for the melting point, and Hm for the melting heat, TB for the boiling point, and HB for the gasification heat. In Eqn.(13), the items on the right can be reduced according to the actual situation. The phase change rate under solid state, the melting rate and evaporation rate can be calculated by
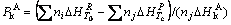 (14)
(14)
In this work, the calculation concerns about the phase change and melting of Ti, melting and gasification of Mg when it is excessive, and the melting when Al is excessive.
3 Design of experiment
Let the mass fractions of Mg, Al (x, y) be global variables, establish the correlation functions to calculate the melting rate of Ti, as well as the gasification rate of Mg and adiabatic temperatures for melting rates and gasification rates. Because most of the TiO2 powder is the mixture of rutile and anatase, and anatase is in the major proportion of the mixture[15], the following calculations include rutile and anatase. The thermodynamic parameters used in the calculation can be found in Ref.[16].
4 Results and discussion
4.1 Change of free energy in synthesis reactions
Fig.1 shows the change of reaction free energy with temperatures for reactions (2)-(9). It is obvious that all reactions can take place spontaneously and at the same time a great deal of heat is released during reaction, which makes SHS reaction easily occur and follow on.
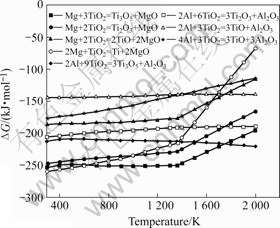
Fig.1 Relationship between reaction free energy and temperature for possible reaction systems of sorts
For Mg-TiO2 system, the reaction between Mg and TiO2 occurs at 298-600 K, and the possibility of forming Ti is the highest. With rising of temperature and declining reduction of Ti, the main phase of the reactant is Ti3O5 between 600 and 1 000 K and the secondary reactant is Ti. When the temperature is above 1 000 K, the possibility of the appearance of Ti is lower than those for Ti3O5 and Ti2O3. When the temperature reaches 1 363 K, the ΔG—T curve of every possible reaction is changed, which results from the gasification of Mg at 1 363 K. When the temperature is above 1 600 K, the possibility of the appearance of Ti is lower than any other suboxide of TiO2. Therefore, in order to obtain Ti, it is important to control the temperature in the reaction process.
For Al-TiO2 system, ΔG of the reaction Al+TiO2→Ti+Al2O3 rise perpendicularly with the increase of temperature and ΔG of the other suboxide shows a tendency of rising at the beginning and then declining. And this change is not obvious with the temperature variation. When the temperature is between 298 and 1 400 K, the main phase of the reactant is Ti3O5, and then Ti2O3, Ti and TiO. When the temperature is higher than 1 400 K, the possibility of the appearance of Ti is lower than that of TiO. So it is difficult for the reaction to take place. Like the Mg-TiO2 system, in order to get Ti easily, the temperature in the reaction should be controlled properly.
Over all, the reducibility of Mg is stronger than that of Al, hence the reaction between Mg and TiO2 takes place more easily than Al and TiO2 at a low temperature. The change of ΔG between Mg and TiO2 is obvious with the temperature variation. When the temperature reaches 1 363 K, ΔG changes greatly with the rising of temperature. When the temperature reaches 1 750 K, the possibility of reaction Mg+TiO2→Ti+MgO is lower than that of the reaction Al+TiO2→Ti+Al2O3 due to the gasification of Mg.
4.2 Adiabatic temperature of Mg-TiO2 reaction
According to formula (5), supposing that the reaction takes place at 298 K (T0=298 K) and neglecting the gasification of Mg, the adiabatic temperature curve can be figured out by the Matlab program, as shown in Fig.2.
Fig.2 shows that the adiabatic temperature falls with increasing mole number of Mg(n(Mg)). When Mg is excessive w(Mg)<20%, the adiabatic temperatures of the reaction of both rutile and anatase are higher than 1 800 K, which matches the empirical criterion put forward by MERZHANOV. Only when the adiabatic temperature meets the condition Tad≥1 800 K, the SHS reaction can continue self-sustaining, otherwise, the outside energy is needed. When w(Mg)=25%, the adiabatic temperatures of the reaction between Mg and rutile or anatase are both lower than 1 800 K. Therefore, under the condition of no preheating, the mass fraction of excessive Mg may not be more than 25% in order to maintain the SHS reaction. Moreover, the synthetic temperature of Mg-TiO2 reaction is much higher than that of the gasification of Mg, and in the process of the SHS reaction, the loss of Mg caused by gasification will lead to building up process and the structure of final resultants. As a result, in order to control the gasification of Mg, it is necessary to control the temperature in the building up process.
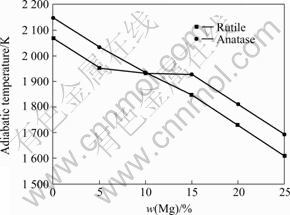
Fig.2 Effect of excessive mass fraction of Mg on adiabatic temperature
In Fig.2, it can also be found that when 5%<w(Mg)<10% (rutile) or 10%<w(Mg)<15% (anatase), a plateau appears in the curve of the adiabatic temperature, which is related with the melting of Ti. The melting rate of Ti can be calculated by formula (14). If 5.5%≤w(Mg)≤10.5% (rutile) or 8.5%≤w(Mg)≤14% (anatase), 0≤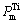 ≤1. So when 5.5%≤w(Mg)≤10.5% (rutile) or 8.5%≤w(Mg)≤14% (anatase), the heat released during the reaction is just used for maintaining the melting of Ti and the temperature of reaction does not rise obviously.
≤1. So when 5.5%≤w(Mg)≤10.5% (rutile) or 8.5%≤w(Mg)≤14% (anatase), the heat released during the reaction is just used for maintaining the melting of Ti and the temperature of reaction does not rise obviously.
4.3 Adiabatic temperature of Al-TiO2 reaction and melting rate of Ti
Fig.3 shows the effect of excessive mass fraction of Al and preheating temperature on adiabatic temperature; Fig.4 shows the effect of excessive mass fraction of Al and preheat temperature on the melting rate of Ti.
It can be seen that for the same system, with the rising of preheating temperature, the adiabatic temperature and melting rate of Ti increase. The reason is that the initial temperature of the reaction rises when the total heat of the reaction increases, so the adiabatic temperature rises. For TiO2 (rutile), when the preheating temperature reaches 500 K, the melting of Ti occurs. When the preheating temperature reaches 800 K, the melting of Ti occurs completely under the condition of 0≤n(Al)≤1. When TiO2 (anatase) is lower than 100 K, which is related to the standard reaction, heat of formation of TiO2 (rutile) is higher than that of TiO2 (anatase). No matter how changeable the temperature is, with the increase of Al content, both the adiabatic temperature and melting rate of Ti fall because excessive Al plays a role of dilution, making the raw material in unit volume reduced, the release of energy decreasing in unit time and unit volume, and causing the falling of the adiabatic temperature. Like the Mg-TiO2 system, the plateau in the curve of the adiabatic temperature is resulted from the melting rate of Ti between 0 and 100%.
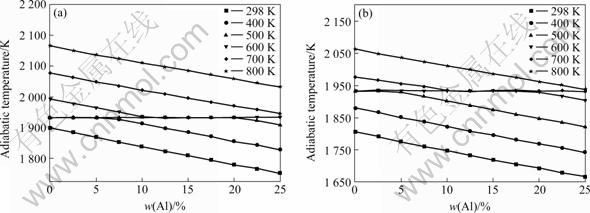
Fig.3 Effects of excessive mass fraction of Al and preheat temperature on adiabatic temperature of anatase (a) and rutile (b)
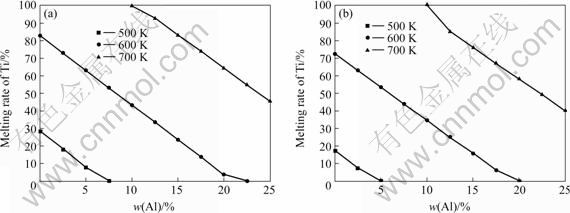
Fig.4 Effects of excessive mass fraction of Al and preheat temperature on melting rate of Ti of anatase (a) and rutile (b)
4.4 Gasification mass of Mg
Table 1 lists the gasification mass traction of Mg at various excessive mass fractions of Mg.
It can be seen clearly from Table 1 that a great deal of Mg is gasified in the process of reaction. In order to make the reaction take place completely to get Ti, theoretically the mass fraction of Mg should reach 46.5% or 51.0%. But, because of the suboxide produced in the reaction and the secondary reaction, the actual gasification mass of Mg may be smaller than that in Table 1. The data in Table 1 also show that with the increase of the mass fraction of Mg, the gasification mass of Mg correspondingly decreases. The reason is that excessive Mg as dilution in the reaction decreases the reaction temperature, so the gasification mass of Mg also declines. Hence during the reaction process, the properly excessive Mg can not only make for the loss caused by the gasification of Mg for the high adiabatic temperature, but also lower the adiabatic temperature of the reaction to reduce the gasification of Mg. In order to reduce the gasification of Mg, it is necessary to control the synthetic temperature in the reaction process.
Table 1 Gasification mass mole of Mg at various excessive molar fractions of Mg
5 Conclusions
1) By calculating the reactive Gibbs free energies of systems Mg-TiO2 and Al-TiO2, it is found that the SHS reaction can take place easily in these two systems. And the reaction can produce various suboxides of Ti, such as Ti3O5, Ti2O3 and TiO. With the rising of temperature, the possibility of forming Ti decreases.
2) The adiabatic temperature and the melting rate of Ti of the reactions with various quantity of Mg (rutile or anatase) are calculated theoretically. The adiabatic temperature of the Mg-TiO2 reaction falls with the increase of the mass fraction of Mg; when the mass fraction of excessive Mg arrives at 25%, the adiabatic temperature is below 1 800 K. The adiabatic temperature of the Al-TiO2 reaction falls with the increase of the mass fraction of Al in the same way, but the adiabatic temperature rises with the increase of preheating temperature. For both Mg-TiO2 and Al-TiO2 reactions, the plateau in the curve of the adiabatic temperature is resulted from the melting of Ti.
3) The calculation of the gasification mass of Mg for various quantity shows that a great deal of Mg is gasified in the reaction process. In order to make the Mg-TiO2 reaction take place completely to get Ti, Mg should be excessive properly.
References
[1] MUNIR Z A, ANSELMI-TAMNURINI U. Self-propagating exothermic reactions: The synthesis of high-temperature materials by combustion[J]. Mater Sci Rep, 1989, 3: 277-365.
[2] VARMA A, LEBRAT J P. Combustion synthesis of advanced materials[J]. Chem Eng Sci, 1992, 47: 2179-2194.
[3] MERZHANOV A G. Solid flames: Discoveries, concepts and horizons of cognition[J]. Combust Sci Technol, 1994, 98: 307-336.
[4] MOORE J J, FENG H J. Combustion synthesis of advanced materials: partⅠ. Reaction parameters[J]. Prog Mater Sci, 1995, 39: 243-273.
[5] MOSSINO P. Some aspects in self-propagating high-temperature synthesis[J]. Ceramics International, 2004, 30: 311-332.
[6] YIN Sheng. Combustion synthesis[M]. Beijing: Metallurgical Industry Press, 2002: 50-57.(in Chinese)
[7] WANG L L, MUNIR Z A, MAXIMOV Y M. Thermite reaction: Their utilization in the synthesis and processing of materials[J]. Journal of Materials Science, 1993 28: 3693-3708.
[8] MUNIR Z A, HOLT J B. The combustion synthesis of refractory nitrides partⅠ. Theoretical analysis[J]. Journal of Materials Science, 1987, 22: 710-714.
[9] MOORE J J, FENG H J. Combustion synthesis of advanced materials: part Ⅱ. Classification, applications and modelling[J]. Prog Mater Sci, 1995, 39: 275-316.
[10] MERZHANOV A G. Self-propagating high temperature synthesis: Twenty years of search and finding[M]. New York: VCH Publishers, 1990: 1-53.
[11] NERSISYAN H H, LEE J H, WON C W. Combustion of TiO2-Mg and TiO2-Mg-C systems in the presence of NaCl to synthesize nanocrystalline Ti and TiC powders[J]. Materials Research Bulletin, 2003, 38: 1135-1146.
[12] LIANG Shu-quan, ZHENG Zi-qiao, TAN Cheng-yu, YIN Den-feng, HU Wen-bin. The microstructure analysis of the combustion wave in Al-TiO2 system by self-propagating high-temperature synthesis (SHS)[J]. Rare Metal materials and Engineering, 1993, 22(4): 42-45. (in Chinese)
[13] LIANG Shu-quan, ZHENG Zi-qiao, TAN Cheng-yu, YIN Den-feng, HU Wen-bin. The structural macrokinetics of the Al-TiO2 self-propagating high-temperature syntheses[J]. Material Science and Technology, 1993, 1(1): 34-39. (in Chinese)
[14] JIN Yun-xue, ZhANG Er-lin. Self-propagating high-temperature synthesis technology and in suit composite materials[M]. Harbin: Harbin Industry Press, 2002: 8-9. (in Chinese)
[15] SATTERFIELD C N. Heterogeneous catalysis in industrial practice[M]. New York: McGraw-Hill, 1980: 87-97.
[16] YE Da-lun, HU Jian-hua. Practical data handbook of inorganic thermodynamics[M]. Beijing: Metallurgical Industry Press, 2002. (in Chinese)
Corresponding author: ZHANG Peng-lin; Tel: +86-931-2976642; E-mail: penglin_zh@126.com
(Edited by CHEN Wei-ping)