辉钼矿-碳酸钙氧化焙烧及熟料在碳酸铵溶液中的浸出
来源期刊:中国有色金属学报(英文版)2017年第7期
论文作者:周秋生 云卫涛 席俊涛 李小斌 齐天贵 刘桂华 彭志宏
文章页码:1618 - 1626
关键词:辉钼矿;碳酸钙;碳酸铵;氧化焙烧;浸出
Key words:molybdenite; limestone; ammonium carbonate; oxidizing roasting; leaching
摘 要:石灰焙烧-硫酸浸出法可大大降低传统氧化焙烧-氨浸工艺处理辉钼矿的含硫烟气污染,但存在设备腐蚀严重、渣量大等缺点。基于石灰焙烧工艺研究,本文作者提出“碳酸钙氧化焙烧-碳酸铵浸出”新工艺来处理辉钼矿。通过热力学计算、热重分析以及焙烧试验等对辉钼矿-碳酸钙氧化焙烧过程进行详细研究。结果表明,MoS2与CaCO3和O2在573~1000 K下反应的主要产物是CaSO4、CaMoO4和CO2;当CaCO3和MoS2的摩尔比为3.6并加入5%矿化剂A在500 °C下焙烧1 h时,钼精矿中的MoS2的分解率达到约99%,固硫率达到约95%。用碳酸铵溶液浸出焙烧后熟料,控制碳酸铵浓度为600 g/L、液固比为10 mL/g,在85 °C下浸出7 h,Mo浸出率可达 98.2%。该研究结果将有助于钼酸铵清洁生产新技术的开发。
Abstract: Oxidizing roasting of molybdenite with lime can significantly reduce SO2 pollution compared with the traditional roasting without lime. However, the calcine is subsequently leached by sulfuric acid, resulting in serious equipment corrosion and abundant non-recyclable CaSO4 slag. In this work, a novel process, in which the molybdenite was roasted with CaCO3 followed by (NH4)2CO3 solution leaching, was proposed to improve the art of lime roasting-sulfuric acid leaching. Oxidizing roasting of molybdenite with CaCO3 was investigated through thermodynamic calculation, thermogravimetric analysis and roasting experiments. The results show that the products of the oxidizing reaction of MoS2 in the presence of CaCO3 and O2 are CaSO4, CaMoO4 and CO2 at 573-1000 K. The MoS2 conversion rate achieves approximately 99% and the sulfur-retained rate attains approximately 95% with a CaCO3-to-MoS2 molar ratio of 3.6 at 500 °C for 1 h by adding 5% mineralizer A (mass fraction). The leaching results show that the leaching rate of Mo reaches 98.2% at 85 °C for 7 h with a (NH4)2CO3 concentration of 600 g/L and a liquid-solid ratio of 10 mL/g. The results presented are potential to develop a novel cleaner technique for ammonium molybdate production.
Trans. Nonferrous Met. Soc. China 27(2017) 1618-1626
Qiu-sheng ZHOU, Wei-tao YUN, Jun-tao XI, Xiao-bin LI, Tian-gui QI, Gui-hua LIU, Zhi-hong PENG
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 21 April 2016; accepted 5 July 2016
Abstract: Oxidizing roasting of molybdenite with lime can significantly reduce SO2 pollution compared with the traditional roasting without lime. However, the calcine is subsequently leached by sulfuric acid, resulting in serious equipment corrosion and abundant non-recyclable CaSO4 slag. In this work, a novel process, in which the molybdenite was roasted with CaCO3 followed by (NH4)2CO3 solution leaching, was proposed to improve the art of lime roasting-sulfuric acid leaching. Oxidizing roasting of molybdenite with CaCO3 was investigated through thermodynamic calculation, thermogravimetric analysis and roasting experiments. The results show that the products of the oxidizing reaction of MoS2 in the presence of CaCO3 and O2 are CaSO4, CaMoO4 and CO2 at 573-1000 K. The MoS2 conversion rate achieves approximately 99% and the sulfur-retained rate attains approximately 95% with a CaCO3-to-MoS2 molar ratio of 3.6 at 500 °C for 1 h by adding 5% mineralizer A (mass fraction). The leaching results show that the leaching rate of Mo reaches 98.2% at 85 °C for 7 h with a (NH4)2CO3 concentration of 600 g/L and a liquid-solid ratio of 10 mL/g. The results presented are potential to develop a novel cleaner technique for ammonium molybdate production.
Key words: molybdenite; limestone; ammonium carbonate; oxidizing roasting; leaching
1 Introduction
China is rich in molybdenum resource and contributes more than 40% of the global molybdenum production. However, molybdenite concentrate is still primarily processed using the traditional route, in which the concentrate is oxidizing roasted followed by ammonium hydroxide leaching [1]. This process presents many drawbacks because of its outdated technology as well as small production scale [2]. Firstly, the traditional technique exhausts a large amount of low concentration sulfur exhaust gases [3] during oxidizing roasting, exacerbating environmental pollution and limiting sustainable development. Secondly, the resultant MoO3 can volatilize at high roasting temperatures. Thirdly, the calcine may block as the molybdenite contains low melting point metal impurities, such as Cu, Pb and Bi [4]. In addition, the overall recovery is not high, and Re in molybdenite is mostly lost with the exhaust gases [5,6], resulting in a large waste of resources. Finally, the traditional technique is not applicable for complex and low grade ores [7-9]. Molybdenum oxidizing–chlorinating roasting [10] can decrease the oxidizing temperature of molybdenite from 550-600 to 450 °C; however, it needs high requirement for the equipment because of generating chlorine gases.
DAUGHERTY [11] suggested the use of lime-roasting to reduce the exhaust gases and recover Re. Mo and Re in the molybdenite transform to CaMoO4 and Ca(ReO4)2, respectively, whereas sulfur turns to CaSO4 when lime is added in the oxidizing roasting process. Ca(ReO4)2 in the calcine can be leached out immediately by water. CaMoO4 can transform to H2MoO4 and CaSO4 when the calcine is leached by sulfuric acid. Then, Mo can be recovered by ion exchange or solvent extraction. CHEN [12] and ZHOU et al [13] conducted further studies on the lime-roasting process and increased the recovery of Mo to 95% and Re to more than 86%. CHEN et al [14] obtained the sulfur-retained rate up to 91.5% and the leaching rate of molybdenum up to 99.1% through investigating calcium-based roasting of low grade molybdenum concentrates and acid leaching process. However, some deficiencies remain in practical application. This process generates a high amount of non-recyclable residue mainly containing CaSO4 because of the lime addition in the roasting process. Moreover, lime-roasting results in serious equipment corrosion and produces much acid-bearing wastewater because a high amount of non-recyclable sulfuric acid is used to leach the calcine. Ion exchange and solvent extraction for recovering Mo and Re further increase the complexity of the suggested process and discharge a large quantity of wastewater, resulting in a high production cost.
CaCO3 is more frequently used as a sulfur-fixing agent in industrial production [15] as it is more convenient to store, more stable, and more economical compared with lime. Moreover, (NH4)2CO3 can react with CaMoO4, forming CaCO3 and (NH4)2MoO4. Additionally, adding some (NH4)2CO3 in the traditional aqueous ammonia leaching process can increase Mo recovery from 83%-85% to 93%-96% by preventing the generation of CaMoO4 and FeMoO4 and inhibiting the coverage of Fe(OH)2 up to the Mo-bearing ore particles [16]. Thus, if CaCO3 is employed to replace CaO during the oxidizing process of molybdenite, and (NH4)2CO3 solution as leaching agent to replace sulfuric acid in the leaching process, we will obtain leaching residue mainly consisting of CaCO3 which could be conveniently reused in the raw meal preparation for roasting, and the remaining (NH4)2SO4 solution after extracting Mo could be used as chemicals. Under these considerations, a novel cleaner process for extracting Mo from molybdenite concentrate was proposed, where the oxidizing roasting of molybdenite was conducted in the presence of CaCO3 and the resultant calcine was leached by (NH4)2CO3 solution.
In this work, oxidizing roasting process in the presence of CaCO3 and O2 was thoroughly studied by thermodynamic calculation, thermogravimetric analysis (TGA) and roasting experiments, and then the leaching process of the calcine obtained under the optimal roasting conditions was preliminarily tested using (NH4)2CO3 solution as the leaching agent. Finally, we attempted to propose a cleaner schematic technological process for producing ammonium molybdate.
2 Thermodynamic calculation and thermo- gravimetric analysis
2.1 Thermodynamic calculation
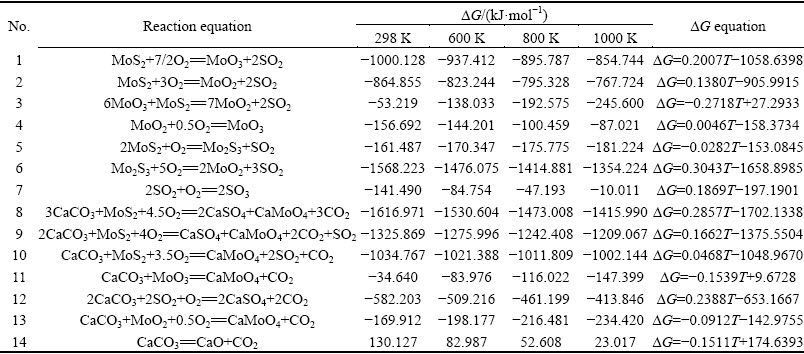
The possible reactions during molybdenite oxidizing roasting in the presence of CaCO3 mainly involve the oxidation of MoS2 [17,18] and the reactions between MoS2 and CaCO3 in the presence of O2. The reactions were conducted at constant temperature. Thus, the changes in standard Gibbs free energies of the reactions can be calculated in accordance with the classical thermodynamic theory [19]. Most of the thermodynamic data required for the calculation are drawn from Refs. [20-22]. The reactions and thermodynamic calculation are shown in Table 1.
Table 1 indicates that the changes in the Gibbs free energies of Reactions (1) - (13) are all negative at 298- 1000 K. This finding suggests that all possible reactions, except for Reaction (14), could occur spontaneously, and that CaCO3 would not decompose below 1000 K. The results also show that changes in the Gibbs free energies of some reactions, i.e., Reactions (1), (2), (4), (6)-(10), and (12) increase with increasing temperature, whereas those of the other reactions decrease. However, the change in the Gibbs free energy of Reaction (8) always presents the lowest value at 298-1000 K, suggesting that Reaction (8) is most likely to occur during molybdenite oxidizing roasting process in the presence of CaCO3 and O2.
Table 1 Possible reactions involved in oxidizing roasting process of molybdenite concentrate and changes in Gibbs free energies at different temperatures
2.2 Thermogravimetric analysis (TGA)
Analytically pure MoS2, analytically pure mixture of MoS2 and CaCO3 with a CaCO3 to MoS2 molar ratio of 3.6, and analytically pure CaCO3 were subjected to TGA at a heating rate of 10 K/min from 20 to 1000 °C in an oxidizing atmosphere by using a thermogravimetric analyzer (STA2409PC, NETZSCH Company, Germany).
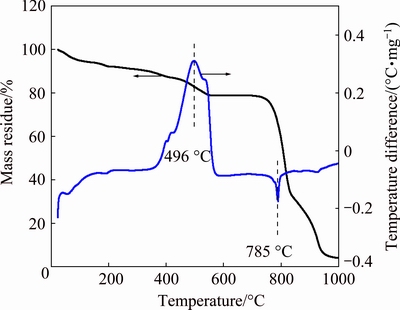
Fig. 1 Thermogravimetric and differential thermal curves of analytically pure MoS2
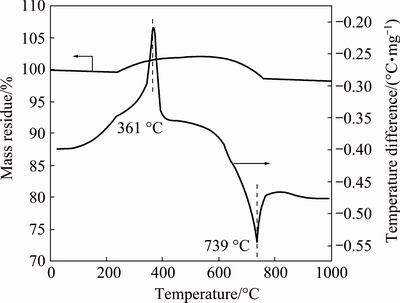
Fig. 2 Thermogravimetric and differential thermal curves of mixture of MoS2 and CaCO3 at n(CaCO3):n(MoS2)=3.6
Figure 1 illustrates the results from the TGA and differential thermal analysis (DTA) of MoS2. An exothermic peak is found in the approximate range from 360 to 580 °C, during which MoS2 is oxidized. The oxidation of MoS2 begins at approximately 360 °C, accelerates at 400 °C and then terminates at approximately 580 °C. Correspondingly, the sample continuously loses mass below 580 °C, generating SO2. Then, the sample does not sharply lose mass until about 780 °C because of the evaporation of the generated MoO3, which is consistent with the endothermic peak at approximately 800 °C in the DTA curve. Figure 2 shows the TGA and DTA curves of the analytically pure mixture of MoS2 and CaCO3 with a CaCO3 to MoS2 molar ratio of 3.6. This result indicates an exothermic peak at 250-400 °C, in which the sample slightly gains mass as the sulfur in the molybdenite is fixed by CaCO3. That is, the oxidizing roasting of molybdenite may be performed as Reaction (8). The sample then slightly loses mass at 600-800 °C, which corresponds to the endothermic peak at approximately 740 °C in the DTA curve. Compared with the TGA and DTA curves of analytically pure CaCO3 in Fig. 3, the slight mass loss at 600-800 °C for the TGA curve in Fig. 2 should be caused by the decomposition of spare CaCO3.

Fig. 3 Thermogravimetric curve of CaCO3
The oxidizing roasting temperature of MoS2 is reduced to 300–500 °C (Fig. 2) from 400–600 °C (Fig. 1) by adding CaCO3, suggesting that adding CaCO3 in the oxidizing process of MoS2 can not only fix the sulfur to reduce SO2 emission but also apparently promote the oxidizing process of MoS2 and consequently reduce the oxidizing roasting temperature. This result agrees with the thermodynamic calculation in Section 2.1.
3 Experimental
3.1 Raw materials
Molybdenite concentrate used in the experiments was provided by a molybdenum refinery in Jiangxi Province, China. The chemical compositions and the X-ray diffraction (XRD) pattern of the concentrate are shown in Table 2 and Fig. 4, respectively, indicating that the MoS2 is the main essential component and the content of Mo is 40.33%. Additionally, CaCO3, Na2CO3, mineralizer and (NH4)2CO3 used in this work are analytical grade.
Table 2 Chemical composition of molybdenite concentrate (mass fraction, %)
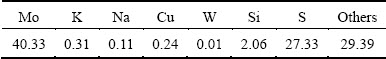

Fig. 4 XRD pattern of molybdenite concentrate
3.2 Experimental procedure
3.2.1 Raw meal preparation
The concentrate was firstly mixed with CaCO3 at a certain ratio. The mixture was then milled in a low-scale vibration mills thrice for 120 s each instance to ensure that the samples were well mixed and to control the particle size of the mixture to 50-70 μm.
3.2.2 Oxidizing roasting
The raw meal prepared was put in a corundum crucible (100 mm × 30 mm × 20 mm) with a meal thickness of 8-10 mm and then roasted under an oxidizing airflow in a muffle furnace at preset temperatures for certain duration. The calcine obtained was subsequently taken out from the furnace and naturally cooled in air to room temperature.
3.2.3 Calcine leaching
The cooled calcine was accurately weighed and then leached by 100 mL (NH4)2CO3 solution in a 150 mL sealed rotating steel reactor. The reactor was immersed in a glycerol cell at a preset temperature with temperature precision of 1 °C. Two 15 mm-diameter and two 5 mm- diameter steel balls were put into the steel reactor to strengthen stirring.
3.3 Analysis methods
3.3.1 Phase analysis
Phase analysis was performed for calcines and leaching residue through a Bruker X-ray diffractometer (D8-Advance, Bruker corporation) with Cu Kα radiation.
3.3.2 Mo and S contents
The Mo(VI) contents in the calcine and leaching residue were measured by the gravimetric method [23]. The CaMoO4 in the calcine was leached by Na2CO3 leaching [24] at 90 °C for 2 h with a Na2CO3 concentration of 120 g/L and a liquid-solid ratio of 25 mL/g, which was employed to determine the conversion rate of MoS2. The sulfur contents were determined by a sulfur analyzer (HDS3000, Huade Company, China).
The conversion rate of MoS2, the leaching rate of Mo, and the sulfur-retained rate were calculated through Eqs. (1), (2), and (3), respectively as follows:
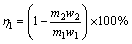 (1)
(1)
where η1 is the conversion rate of MoS2; m1 and m2 are the masses of raw meal and leaching residue by sodium carbonate solution, respectively; w1 and w2 are the Mo mass fractions of raw meal and leaching residue by sodium carbonate solution, respectively.
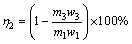 (2)
(2)
where η2 is the leaching rate of Mo; m3 is the mass of leaching residue by ammonium carbonate solution; w3 is the Mo mass fraction of leaching residue by ammonium carbonate solution.
 (3)
(3)
where η3 is the sulfur-retained rate; m4 is the mass of calcine; w4 is the S mass fraction of calcine.
3.3.3 Morphology analysis
The analysis of morphology of the calcine was conducted by scanning electron microscopy (JSM-6360LV, JEOL Company, Japan).
4 Results and discussion
4.1 Oxidizing roasting of molybdenite in the presence of calcium carbonate
4.1.1 Influence of roasting temperature
Temperature is a momentous parameter for both kinetics and thermodynamics. Thus, roasting temperature is the key influencing factor for calcine quality. MoS2 in the molybdenite concentrate is oxidized to MoO3 during 400-600 °C in the traditional oxidizing roasting process [25]. However, based on the thermodynamic and TGA analyses above, MoS2 in molybdenite can be oxidized into CaMoO4 at above 300 °C in the presence of CaCO3. Consequently, subsequent experiments were performed at 400-550 °C to investigate the influence of roasting temperature.
Figure 5 shows the XRD patterns of roasted products at 400, 450, 500 and 550 °C for 2 h with a molar ratio of CaCO3 to MoS2 of 3.6. The characteristic peaks of MoS2 are evidently receding with increasing roasting temperature, and disappear at above 500 °C. By contrast, the characteristic peaks of CaSO4 and CaMoO4 become stronger. Moreover, the molar ratio of CaMoO4 and CaSO4 in the calcine is approximately 1:2, corresponding to Reaction (8). Reaction (8) is also a typical gas–solid and solid–solid reaction [26]. Thus, elevating temperature can increase the conversion rate of MoS2 because it improves mass and heat transfer. This result is consistent with the result in Fig. 6 which shows the conversion rate of MoS2 at different roasting temperatures. The conversion rate of MoS2 is about 95% when the temperature is only 450 °C (Fig. 6), suggesting that adding CaCO3 in the oxidizing process of MoS2 can remarkably reduce the oxidizing roasting temperature compared with the temperature of 600 °C for the traditional oxidizing roasting process. This coincides with the previous thermodynamic and TGA analyses.

Fig. 5 XRD patterns of roasted products at different temperatures, n(CaCO3):n(MoS2)=3.6 and t=2 h
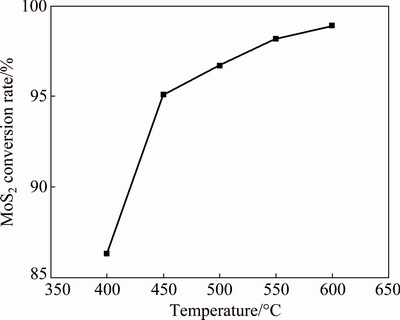
Fig. 6 Influence of roasting temperature on conversion rate of MoS2 at n(CaCO3):n(MoS2)=3.6 and t=2 h
4.1.2 Influence of roasting duration
Duration is another important kinetic factor for chemical reactions. The longer the duration is, the closer the reaction reaches balance. Conceivably, the conversion rate of MoS2 is affected not only by the roasting temperature but also by roasting duration. Thus, the relationship between MoS2 conversion rate and roasting duration was studied by roasting the milled samples with a CaCO3 to MoS2 molar ratio of 3.6 at 500 and 550 °C for different roasting durations, and the results are presented in Fig. 7.
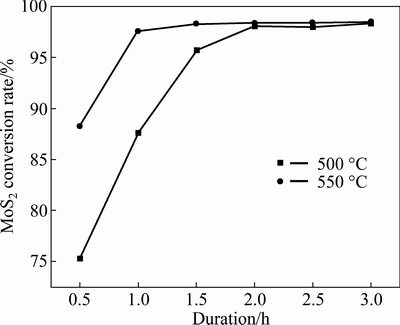
Fig. 7 Influence of roasting duration on conversion rate of MoS2 at n(CaCO3):n(MoS2)=3.6
As shown in Fig. 7, prolonging the roasting duration obviously results in higher conversion rate of MoS2 and the MoS2 conversion rate approaches the maximum after roasting for a period of time. This shows that extending the roasting time facilitates the conversion of MoS2 to CaMoO4 and CaSO4. On the other hand, the MoS2 conversion rate is up to 98% when roasting at 550 °C for 1 h while at 500 °C for 2 h, illustrating that the duration acquired for reaching the maximum is shorter at elevated temperature than that at low temperature. This suggests that roasting temperature is the decisive factor for the phase transformation rate.
4.1.3 Influence of mineralizer
In view of the problems such as elevated roasting temperature and long roasting duration, mineralizer A, always being used as an additive in roasting process to decrease the roasting temperature [27,28], was added into the oxidizing roasting process of molybdenite to reduce the roasting temperature or shorten the duration.
The XRD patterns of samples roasted at 500 °C for 1 h with 5% (mass fraction) mineralizer A and without mineralizer A are shown in Fig. 8. The characteristic peaks of MoS2 for the sample without mineralizer A still exist whereas those for the sample with 5% mineralizer A disappear. Furthermore, stronger characteristic peaks of CaMoO4 and CaSO4 for the sample with 5% mineralizer A are found compared with those for the sample without mineralizer A. This indicates that the MoS2 can convert into CaMoO4 and CaSO4 more completely by adding 5% mineralizer A. The reason may be that the incorporation of F- in the O2- lattice sites intensifies the mass transfer or heat transfer of the roasting reaction. In addition, characteristic peaks of mineralizer A still exist in the XRD patterns of samples roasted with 5% mineralizer A, implying that mineralizer A may be reused.

Fig. 8 XRD patterns of samples roasted without (a) and with (b) 5% mineralizer A at n(CaCO3):n(MoS2)=3.6, T=500 °C and t=1 h
4.1.4 Influence of CaCO3 dosage
Based on the study mentioned above, we can make a conclusion that the oxidizing roasting of molybdenite is mainly performed as Reaction (8) when the CaCO3 is plenty. As CaCO3 dosage can affect both the MoS2 conversion rate and the sulfur-retained rate, further experiments were carried out to examine the influence of CaCO3 dosage by roasting molybdenite concentrate mixed with different dosages of CaCO3 and 5% mineralizer A at 500 °C for 1 h. The results are listed in Table 3.
Table 3 Influence of CaCO3 dosage on MoS2 conversion rate and sulfur-retained rate
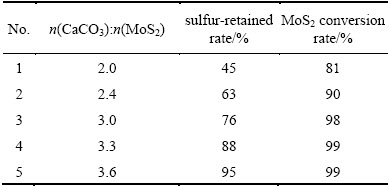
From Table 3, we can see that both the MoS2 conversion rate and sulfur-retained rate increase with the increase of CaCO3 dosage, and the conversion rate of MoS2 can achieve 98% when roasting with a theoretical amount of CaCO3, almost being in agreement with Reaction (8). This validates the thermodynamic result that adding CaCO3 can reduce the roasting temperature and intensify the roasting reaction. In addition, the sulfur-retained rate can reach 95% when roasting with 1.2 times that of the theoretical amount of CaCO3, disclosing that CaCO3 is a good sulfur-retained agent and that oxidizing roasting of molybdenite in the presence of limestone can significantly reduce SO2 pollution.
4.2 Preliminary leaching results of calcine in (NH4)2CO3 solution
CaMoO4 is ready to decompose with higher CO32- concentration through thermodynamics analysis and practical work on Ca-Mo-CO3-H2O system [29]. The leaching reaction of calcine by the (NH4)2CO3 solution is a typical liquid–solid reaction. Thus, leaching conditions, such as solution concentration, liquid–solid ratio, temperature, duration, stirring intensity, and particle size, are all important factors of leaching efficiency.

Fig. 9 SEM images of calcine under different magnification
Table 4 Preliminary leaching experimental results of calcine in (NH4)2CO3 solution

From the SEM images of the calcine as shown in Fig. 9, we can see that the calcine particles are less than 80 μm and take on spheroid in shape with loose surface, facilitating gas diffusion and thus improving the roasting effect. It is also beneficial to diffusion transport of the reactants and products in the leaching process. The preliminary experiments of the calcine leached by (NH4)2CO3 solution were conducted and the results are shown in Table 4.
It is indicated in Table 4 that elevating leaching temperature, extending leaching time, increasing liquid–solid ratio and the concentration of (NH4)2CO3 solution all can intensify the leaching process. The leaching rate of Mo in the calcine can achieve 98.2% at a leaching temperature of 85 °C for 7 h with a (NH4)2CO3 concentration of 600 g/L and a liquid–solid ratio of 10 mL/g.
The XRD pattern of the leaching residue by the (NH4)2CO3 solution is shown in Fig. 10, indicating that the strong diffraction peaks of the residue are the diffraction lines of CaCO3 and unreacted SiO2. Moreover, some weak diffraction peaks of CaWO4 and mineralizer A are observed. This implies that most of the Mo in the calcine enters the leaching solution, and the SiO2 in the MoS2 concentrate seems not to participate in reactions in the oxidizing roasting or leaching process by (NH4)2CO3 solution.

Fig. 10 XRD pattern of leaching residue
4.3 Schematic technological process for producing ammonium molybdenate
Based on the results mentioned above, a novel technique for preparing ammonium paramolybdate from molybdenite concentrate was proposed in this work and the schematic technological process is presented in Fig. 11. In this process, molybdenite is roasted in the presence of oxygen and CaCO3, converting Mo and Re in the concentrate to CaMoO4 and Ca(ReO4)2 respectively and sulfur to CaSO4. The resultant calcine is leached by water to recover Re and then leached by (NH4)2CO3 solution to form (NH4)2MoO4, (NH4)2SO4, and CaCO3. Thus, the lixivium consists of (NH4)2SO4, (NH4)2MoO4, and excessive (NH4)2CO3. (NH4)2CO3 is ready to decompose at above 70 °C. Thus, this compound can be isolated from the lixivium by evaporation [30] and recycled conveniently. The remaining (NH4)2SO4 and (NH4)2MoO4 in the lixivium can be separated by the traditional precipitation of Mo by adding acid, and the (NH4)2MoO4 precipitates can be transformed to (NH4)6Mo7O24 by dissolution and evaporative crystallization. Mo and Re in the lixivium can also be collected directly by ion exchange or solvent extraction [31-33]. The (NH4)2SO4 solution left in the spent liquor may be used as a chemical fertilizer after evaporative crystallization. In addition, the leaching residue, mainly containing CaCO3, can be reused in the raw meal preparation.
Compared with the process of lime-roasting followed by sulfuric acid leaching [11-14], substituting lime with CaCO3 in the oxidizing roasting process of molybdenite concentrate is cost effective and allows recycling. Employing (NH4)2CO3 solution as a leaching agent not only directly produces (NH4)2MoO4 but also eliminates equipment corrosion caused by sulfuric acid leaching of calcine. Furthermore, (NH4)2CO3 and CaCO3 are both recyclable, and the sulfur in the molybdenite is finally converted to (NH4)2SO4, which is used as a chemical fertilizer [34]. Thus, further improvement of the process of limestone-roasting followed by (NH4)2CO3 leaching is potential to establish a recyclable, efficient, and clean process for treating molybdenite concentrate.
5 Conclusions
Thermodynamic calculation and experimental results show that MoS2 is converted to CaSO4 and CaMoO4 in the oxidizing roasting process with addition of CaCO3. The oxidizing roasting reaction can occur at relatively low temperatures of 300-500 °C. The reaction rate is mainly influenced by mass transfer and heat transfer. Increasing temperature, extending duration, and adding mineralizer A are all beneficial to the improvement of the conversion rate. The sulfur-retained rate in the roasting process increases with the increase of CaCO3 dosage and can achieve approximately 95% with a CaCO3 to MoS2 mole ratio of 3.6. CaCO3 and unreacted SiO2 are the main phases in the residue obtained by leaching the calcine with (NH4)2CO3 solution. The leaching rate of Mo in the calcine can reach 98.2% at 85 °C for 7 h with a (NH4)2CO3 concentration of 600 g/L and a liquid–solid ratio of 10 mL/g.
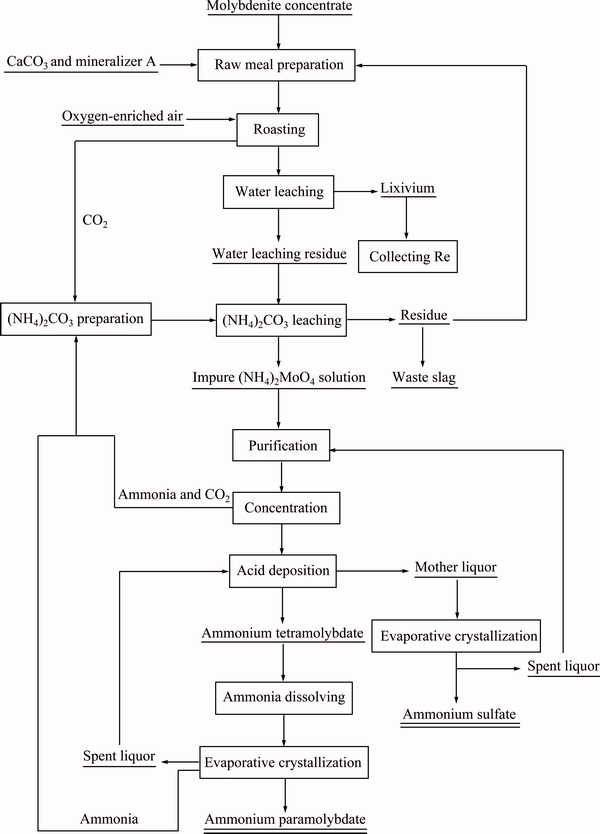
Fig. 11 Schematic technological process for preparing ammonium paramolybdate from molybdenite concentrate
References
[1] FENG Han-kun, CAI Zong-ying, LI Yun-gang, QI Yan-fei. Domestic and overseas research status on molybdenum resources and its use [J]. Advanced Materials Research, 2014, 835-836(5): 401-406.
[2] OVTSYN D V, KOSTYUNIN V V, POTAPOV V N, KOZHEVNIKOV G N. Method of burning the molybdenite concentrates [J]. Russian Journal of Non-ferrous Metals, 2009, 50(3): 206-208.
[3] EBRAHIMI-KAHRIZSANGI R, ABBASI M H, SAIDI A. Molybdenite alkali fusion and leaching: Reactions and mechanism [J]. International Journal of Minerals, Metallurgy and Materials, 2010, 17(2): 127-131.
[4] HUANG Hui, CHEN Fu-liang, JIANG Yan, RONG Hui, DUO Yun-feng, YANG Zhi-hong. The current situation of domestic molybdenum resources and molybdenum smelting analysis [J]. Yunnan Metallurgy, 2014(2): 66-70. (in Chinese)
[5] JOO S H, KIM Y U, KANG J G, KUMAR J R, YOON H S, PARHI P K, SHIN S M. Recovery of rhenium and molybdenum from molybdenite roasting dust leaching solution by ion exchange resins [J]. Materials Transactions, 2012, 53(11): 2034-2037.
[6] KIM H S, PARK J S, SEO S Y, TRAN T, KIM M J. Recovery of rhenium from a molybdenite roaster fume as high purity ammonium perrhenate [J]. Hydrometallurgy, 2015, 156: 158-164.
[7] WANG Ming-yu, WANG Xue-wen, LIU Wan-li. A novel technology of molybdenum extraction from low grade Ni-Mo Ore [J]. Hydrometallurgy, 2009, 97(1-2): 126-130.
[8] KIM B S, LEE H I, CHOI Y Y, KIM S. Kinetics of the oxidative roasting of low grade Mongolian molybdenite concentrate [J]. Materials Transactions, 2009, 50(11): 2669-2674.
[9] GAN Min, FAN Xiao-hui, ZHANG Lin, JIANG Tao, QIU Guan-zhou, WANG Yong, DENG Qiong, CHEN Xu-ling. Reaction behavior of low grade molybdenum concentrates in oxidation roasting process [J]. The Chinese Journal of Nonferrous Metals, 2014, 24(12): 3115-3122. (in Chinese)
[10] ALEKSANDROV P V, MEDVEDEV A S, KADIROV A A, IMIDEEV V A. Processing molybdenum concentrates using low- temperature oxidizing-chlorinating roasting [J]. Russian Journal of Non-ferrous Metals, 2014, 55(2): 114-119.
[11] DAUGHERTY E. Process for the recovery of rhenium and molybdenum values from molybdenite concentrate: US 3739057 [P]. 1973-07-11.
[12] CHEN Ting-zhang. Extraction of Mo and Re from molybdenite by lime-roasting [J]. Hunan Metallurgy, 1979(3): 19-22. (in Chinese)
[13] ZHOU Zhen-qiu, ZHOU Qin-jian. Recovery of molybdenum and rhenium from molybdenite concentrate in Dexing Copper Ore by lime roasting-N235 extraction method [J]. Mining and Metallurgical Engineering, 2002(1): 79-84. (in Chinese)
[14] CHEN Xu-ling, WANG Hai-bo, GAN Min, FAN Xiao-hui, ZHANG Lin, DENG Qiong, WANNG Yong, ZENG Jin-lin. Extraction of molybdenum from low grade molybdenum concentrates by calcium-based roasting and acid leaching process [J]. The Chinese Journal of Nonferrous Metals, 2015, 25: 2913-2920. (in Chinese)
[15] CAI Chao-jun, HUA Yi-xin, LIANG Duo-qiang. Non-isothermal kinetics of roasting of copper sulfide concentrate in the presence of calcium carbonate [J]. Non-ferrous Metal (Metallurgy Part), 2004(3): 2-12. (in Chinese)
[16] XIANG Tie-gen, YANG Bo-hua. Metallurgy of molybdenum [M]. Changsha: Central South University Press, 2009: 87-88. (in Chinese)
[17] UTIGARD T. Oxidation mechanism of molybdenite concentrate [J]. Metallurgical & Materials Transactions B, 2009, 40(4): 490-496.
[18] WANG Lu, ZHANG Guo-hua, DANG Jie, ZHOU Guo-zhi. Oxidation roasting of molybdenite concentrate [J]. Transaction of Nonferrous Metals Society of China, 2015, 25(12): 4167-4174.
[19] YE Da-lun, HU Jian-hua. Applicable thermodynamic handbook of inorganic substances [M]. Beijing: Metallurgical Industry Press, 2002: 6-20. (in Chinese)
[20] BARIN I. Thermodynamic data of pure substances [M]. Weinheim: VCH Verlagsgesellschaft, 1995: 563-580.
[21] KNACKE O, KUBASCHEWSKI O, HESSELMANN K. Thermochemical properties of inorganic substance Ⅱ [M]. Berlin: Springer Verlag, 1991: 1160-1172.
[22] LIANG Ying-jiao, CHE Yin-chang. Thermodynamic handbook of inorganic substances [M]. Shenyang: Northeastern University Press, 1993: 150-155. (in Chinese)
[23] ZHOU Yu, TAN Yan-shan, ZHU Li-ya, NIU Chun-lin, LUO Chun-hua, LI Yu-xian, WAN Zhong-jian, WANG Qian-hui. Determination of molybdenum in molybdenum concentrate and molybdenum calcine by sodium peroxide fusion—Ammonium thiocyanate differential spectrophotometry [J]. Metallurgical Analysis, 2012, 32(9): 68-72. (in Chinese)
[24] SONG Jian-zheng, ZHANG Yong-qiang. Experimental study on leaching calcium molybdate with sodium carbonate solution [J]. Hebei Chemical Engineering and Industry, 2008, 31(10): 14-16. (in Chinese)
[25] BROCCHI E A, QUEIROZ C A R, VIDAL A C, de DESOUZA R F M. Roasting of a molybdenite concentrate [C]//Proceedings of European Metallurgical Conference. Düsseldorf, 2011: 209-220.
[26] EBRAHIMI-KAHRIZSANGI R, ABBASI M H, SAIDI A. Mechanochemical effects on the molybdenite roasting kinetics [J]. Chemical Engineering Journal, 2006, 121(2-3): 65-71.
[27] MASLENNIKOVA G N, KONESHOVA T I. Effect of mineralizers on the sintering of porcelain bodies: A review [J]. Glass and Cermics, 1987, 44: 152-156.
[28] KACIMI L, SIMON-MASSERON A, GHOMARI A, DERRICHE Z. Influence of NaF, KF and CaF2 addition on the clinker burning temperature and its properties [J]. Comptes Rendus Chimie, 2006, 9(1): 154-163.
[29] XIA Wen-tang, ZHAO Zhong-wei, LI Hong-gui. Thermodynamic analysis on sodium carbonate decomposition of calcium molybdenum [J]. Transactions of Nonferrous Metals Society of China, 2007, 17: 622-625.
[30] QIN Feng, WANG Shu-juan, KIM I, SVENDSEN H F, CHEN Chang-he. Heat of absorption of CO2 in aqueous ammonia and ammonium carbonate/carbamate solutions [J]. International Journal of Greenhouse Gas Control, 2011, 5(3): 405-412.
[31] KHOSHNEVIAN A, YOOZBASHIZADEH H, MOHAMMADI M, ABAZARPOOR A, MAAREFVAND M. Separation of rhenium and molybdenum from molybdenite leach liquor by the solvent extraction method [J]. Minerals and Metallurgical Processing, 2013, 30(1): 53-58.
[32] CAO Zhan-fang, ZHONG Hong, JIANG Tao, LIU Guang-yi, WANG Shuai, XIA Liu-yin. Separation of rhenium from electric-oxidation leaching solution of molybdenite [J]. Journal of Central South University, 2013, 20: 2103-2108.
[33] LOU Zhen-ning, XIONG Ying, SONG Jun-jun, SHAN Wei-jun, HAN Guang-xi, XING Zhi-qiang, KONG Yu-xia. Kinetics and mechanism of Re(VII) extraction and separation from Mo(VI) with trialkyl amine [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 10-14.
[34] HAYASHI K, KOGA N, YANAI Y. Effects of field-applied composted cattle manure and chemical fertilizer on ammonia and particulate ammonium exchanges at an upland field [J]. Atmospheric Environment, 2009, 43(35): 5702-5707.
周秋生,云卫涛,席俊涛,李小斌,齐天贵,刘桂华,彭志宏
中南大学 冶金与环境学院,长沙 410083
摘 要:石灰焙烧-硫酸浸出法可大大降低传统氧化焙烧-氨浸工艺处理辉钼矿的含硫烟气污染,但存在设备腐蚀严重、渣量大等缺点。基于石灰焙烧工艺研究,本文作者提出“碳酸钙氧化焙烧-碳酸铵浸出”新工艺来处理辉钼矿。通过热力学计算、热重分析以及焙烧试验等对辉钼矿-碳酸钙氧化焙烧过程进行详细研究。结果表明,MoS2与CaCO3和O2在573~1000 K下反应的主要产物是CaSO4、CaMoO4和CO2;当CaCO3和MoS2的摩尔比为3.6并加入5%矿化剂A在500 °C下焙烧1 h时,钼精矿中的MoS2的分解率达到约99%,固硫率达到约95%。用碳酸铵溶液浸出焙烧后熟料,控制碳酸铵浓度为600 g/L、液固比为10 mL/g,在85 °C下浸出7 h,Mo浸出率可达98.2%。该研究结果将有助于钼酸铵清洁生产新技术的开发。
关键词:辉钼矿;碳酸钙;碳酸铵;氧化焙烧;浸出
(Edited by Wei-ping CHEN)
Foundation item: Project (51274243) supported by the National Natural Science Foundation of China; Project (2015CX001) supported by the Innovation- driven Plan in Central South University, China
Corresponding author: Qiu-sheng ZHOU; Tel: +86-731-88830453; E-mail: qszhou@csu.edu.cn
DOI: 10.1016/S1003-6326(17)60184-5

