
Deep removal of copper from cobalt sulfate electrolyte by ion-exchange
WEN Jun-jie(温俊杰), ZHANG Qi-xiu(张启修), ZHANG Gui-qing(张贵清), CAO Zuo-ying(曹左英)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 25 August 2009; accepted 13 January 2010
Abstract: SP-C was applied for the removal of Cu2+ from simulated cobalt sulfate electrolyte containing Co2+ 50 g/L and Cu2+ 0.5-2.0 g/L. Experimental conditions included pH of 2-4, temperature of 20-60 °C and contact time of 10-40 min. The investigation demonstrated that SP-C had recommendable efficiency in adsorbing Cu2+ from the electrolyte with 25- to 100-fold of Co2+. The optimal adsorption conditions of SP-C were pH of 4, contact time of 30 min and ambient temperature. The study also showed that the loaded resin could be effectively eluted with 2.0 mol/L H2SO4 solution at a contact time of 40 min; the peak concentration of Cu2+ in the eluate was about 35 g/L. The sorption characteristics of Cu2+ by SP-C could be described by Langmuir isotherm and the pseudo second-order kinetic equation. Infrared spectra showed that nitrogen atoms in the functional group coordinated with Cu2+ to form coordination bands.
Key words: cobalt sulfate electrolyte; removal of copper; chelating resin; ion exchange
1 Introduction
In cobalt electrorefining, there are many impurities in cobalt anode, among which copper is a major component. Cu2+ easily deposits on the cathode because of its higher positive potential than Co2+, which affects the quality of the cathode cobalt product. Therefore, Cu2+ must be removed from cobalt electrolyte.
Nowadays, the most widely used techniques for removing copper from cobalt electrolyte include sulfide precipitation[1-3], solvent extraction[4] and ion exchange [5-6]. Sulfide precipitation has the advantages of simple operation and rapid reaction rate. However, plenty of precipitates are produced, which increases the difficulty of solid waste disposal. Additionally, the significant amount of cobalt trapped by the precipitate often requires extra measure to recover this valuable metal. Solvent extraction is a relatively established technology, but emulsification, interfacial flocculation and the third phase[7-8] often occur, which inevitably decreases the extraction effect. More important, the volatile organic phase and wastewater containing organic compound have a negative impact on the environment. Compared with the above processes, ion exchange is more promising. The vantage points associated with ion-exchange include simple device, easy operation and high separation efficiency. To boot, the separation process generates no precipitate or organic pollution. As an environment-friendly separation method, ion exchange has received extensive attention in the field of hydrometallurgy[9-13].
In cobalt electrorefining, sulfate, chloride and sulfate-chloride mixed systems are most widely used, but the major available types of ion-exchange resins in removal of copper from cobalt electrolyte are anion resins. The mechanism for their adsorption toward copper is based on the complex anion [CuCl4]2- formed in the chloride solution. Therefore, its application is limited to cobalt chloride system or the mixed system and it is necessary to find a kind of resin that adopts to cobalt sulfate system.
In this research, a novel SP-C resin was investigated for the removal of copper from cobalt sulfate electrolyte. SP-C is a silica-polyamine composite chelating resin. Its synthesis involves the coating of polyamine on the silica gel matrix to form an organic-inorganic composite material polymer, followed by the addition of aminomethyl pyridine functional group to the polymer matrix[14-15]. The results proved that this type of resin had good selectivity for copper and the procedure of its elution and regeneration is comparatively simple. The efficiency of the adsorption of copper onto SP-C was investigated by varying contact time, pH, initial concentration of copper and temperature. The isothermal equilibrium and kinetics of copper adsorbed onto SP-Cwere also studied.
2 Experimental
2.1 Materials
Cobalt sulfate electrolyte containing copper was prepared with 50 g/L Co2+ and 0.5-2.0 g/L Cu2+. This composition is the simulated electrolyte in cobalt electrorefining industry[6]. The pH value of the solutions was adjusted around 2-4.
The SP-C resin was provided by Luoyang Water Treatment Ltd. Its moisture content is 40%-45% (mass fraction). The effective particle size of SP-C is from 0.1 to 0.35 mm by particle size analysis. Specific surface area and mean pore size of SP-C are 50.1 m2/g and 123.5 nm, respectively. The resin was first soaked in de-ionized water for 24 h followed by being immersed in 1.0 mol/L H2SO4 solution for 2 h, and the above step was repeated for three times. Finally, the resin particles were rinsed with de-ionized water until the pH value of the wash solution reached 3-4 before test. All the other chemicals employed in this study were of analytical reagent grade and were used without further purification.
2.2 Methods
2.2.1 Methods of column experiments for adsorption and elution
The experiments of adsorption and elution were carried out in a glass column of covered jacket d 20 mm×300 mm. 40 mL of resin particles as treated in section 2.1 were wet-packed into the glass column. The adsorption and elution processes were conducted in the same continuous mode; the feed solution was introduced into the column through the inlet on the top of the column at a certain flow rate and the sample effused from the outlet at the bottom of the column. Samples as effluent from the column were collected at certain time intervals and analyzed to determine copper content by flame atomic absorption spectrophotometer with three duplicate injections. The loaded resin was eluted using 2.0 mol/L H2SO4 solution. Thermostatic circulating water equipment was employed to adjust the experimental temperature; the pH value of the cobalt sulfate electrolyte was regulated with CoCO3 or H2SO4; the concentration of copper in initial solution was adjusted with CuSO4. Symbols used in column experiments were as follow: c0 is the initial concentration of copper (mg/L); c is the copper concentration of effluent (mg/L); V is effluent volume (mL); V0 is resin volume (mL); V/V0 the volume ratio of effluent to resin; τ is contact time (min); t is experimental temperature (°C). In the adsorption column experiment, the breakthrough point was set to c/c0=0.05 and the saturation point was set to c/c0=0.95.
2.2.2 Isothermal equilibrium experiment
Isothermal equilibrium experiments were performed by shaking the mixture of resin and 100 mL stock solution with desired concentration using a temperature- controlled water-bath shaker (HZS-H). Four batches of pre-treated wet resins were weighed and put into 250 mL conical flasks. Each batch included six samples with quantity of 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 g, respectively; then, each flask was filled with 100 mL of stock solution with 0.5 g/L copper at pH value of 4. Whereafter, the batch samples were put into the shaker set at temperatures of 15, 30, 45 and 60 °C, respectively. The shaking process at a constant speed of 130 r/min lasted for 24 h, which was proved to be enough for the adsorption to reach equilibrium. After equilibration, the concentration of copper in the liquid phase was measured also by the flame atomic absorption spectrophotometer with three duplicate injections.
Finally, the amount of copper exchanged onto the resin was calculated by the mass balance relation represented by[16]:
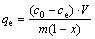 (1)
(1)
where qe is the copper amount absorbed onto the resin at equilibrium (mg/g); c0 is the initial concentration of copper (mg/L); ce is the equilibrium concentration of copper (mg/L); V is the solution volume (L); m is the quality of resin (g); x is the moisture content of resin (%).
2.2.3 Kinetics experiment
The kinetics experiments were carried out in a 1.0 L three-necked bottle. The 1.0 L stock solution of pH 4 and containing 0.5 g/L copper was poured into a three- necked bottle. Subsequently, 5 g of the pre-treated resin was incorporated into the solution. In order to explore the reaction rate as a function of temperature, the three- necked bottle was put into a thermostatic water bath (temperature deviation ±1 °C) to maintain the solution temperature at 15, 30, 45 and 60 °C, respectively. The mixtures were agitated at a constant stirring rate of 300 r/min. During the kinetic experiments, solution samples were taken from the reactor at desired time intervals to determine the copper concentration. The volume of the samples sucked out for testing is negligible compared with the bulky solutions.
2.2.4 Analysis of adsorption mechanism
A Fourier transform IR spectrophotometer (PE-2000) was employed to identify the changes of functional groups for the resin before and after metal ions were adsorbed. The resin samples were dried at 80 °C for 24 h; subsequently, the samples were powdered with KBr and pressed to form pellets.
3 Results and discussion
3.1 Column experiments
3.1.1 Effect of contact time on adsorption
The effect of contact time on copper adsorption onto SP-C was investigated, and the results are shown in Fig.1.
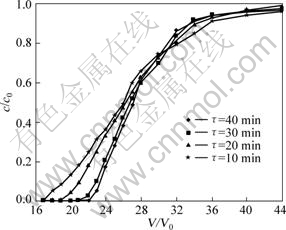
Fig.1 Effect of contact time on adsorption (copper initial concentration: 1.0 g/L; pH=4; t=20 ?C)
It could be seen from Fig.1 that the breakthrough volume of copper increased when the contact time varied from 10 to 40 min and the V/V0 increased from 16 to 21. Accordingly, the breakthrough exchange capacity was 25.670-33.740 mg/g (dry resin) and the saturated capacity was 42.063- 43.271 mg/g (dry resin). These findings indicated that copper was adsorbed more efficiently with longer contact time. Correspondingly, the utilization efficiency of resin was greater and the exchange capacity of resin increased. It was also observed that when the contact time extended from 30 to 40 min, the value of V/V0 did not change remarkably, almost remained 21 BV. This showed that the adsorption velocity of resin was rapid and a contact time of 30 min was enough for adsorption saturation.
3.1.2 Effect of pH on adsorption
The pH value of industrial cobalt electrolyte solution is usually around 2.5-3.5. Therefore, stock solutions with pH=2, 3 and 4 were used to investigate the effect of pH on the adsorption of copper at ambient temperature. All the copper concentrations were 1.0 g/L. The results are shown in Fig.2.
Fig.2 revealed that the pH value of the stock solutions had a significant influence on the adsorption of copper. With the pH value increasing from 2 up to 4, the breakthrough volume of copper increased from 16 to 20 BV; the breakthrough capacity and the saturated capacity
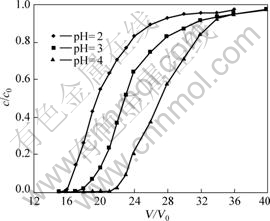
Fig.2 Effect of pH value on adsorption (copper initial concentration: 1.0 g/L, 30min, 20 ?C)
copper were calculated to be 23.319-32.088 mg/g (dry resin) and 31.516-43.334 mg/g (dry resin), respectively. It was obvious that higher pH value was advantageous for copper adsorption onto resin. Nevertheless, it is very likely for the copper to form hydroxide precipitate when the pH value of the stock solution is above 4. So, the optimal pH value for copper adsorbed onto the resin was 4.
3.1.3 Effect of initial concentration of copper on adsorption
Stock solutions containing copper of 0.5, 1.0 and 2.0 g/L with pH value of 4 were prepared to investigate the effect of copper initial concentration on its adsorption at ambient temperature. The results are shown in Fig.3. The copper concentration of industrial solution is usually lower than 1.0 g/L; the reason for adopting copper with a concentration of 2.0 g/L is to explore the stability of the resin when the feed copper concentration has a high fluctuation in practical operation. The contact time was

Fig.3 Effect of copper initial concentration on adsorption (pH=4, 30 min, 20 °C)
30 min for all tests.
It could be seen from Fig.3 that when the copper initial concentration varied from 0.5 to 2.0 g/L, V/V0 changed from 40 to 10; the breakthrough capacity and the saturated capacity of copper were calculated to be 31.198-32.088 mg/g (dry resin) and 43.340-44.097 mg/g (dry resin), respectively. There was no evident change of the copper exchange capacity with the change of copper initial concentrations in solution. But, higher copper initial concentration led to faster saturation of resin and earlier effusion of the copper.
3.1.4 Effect of temperature on adsorption
The temperature of cobalt electrolysis is usually controlled at 50-65 °C, so the studied temperature on the adsorption of copper was set at 20-60 °C. The other operation conditions were kept constant, namely, the stock solution containing 1.0 g/L copper, pH=4 and contact time of 30 min. The results are shown in Fig.4.
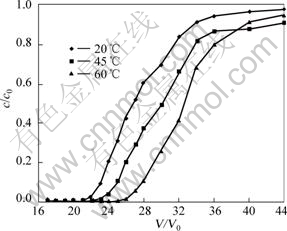
Fig.4 Effect of temperature on adsorption (copper initial concentration: 1.0 g/L, pH=4, 30 min)
Fig.4 showed that when temperature changed from 20 to 60 °C, V/V0 changed from 19 to 25; the breakthrough capacity and the saturated capacity of copper were calculated to be 32.088-37.298 mg/g (dry resin) and 43.334-48.799 mg/g, respectively. The exchange capacity was raised with increasing temperature, which indicated that higher temperature benefited the adsorption of copper. However, higher temperature had no significant influence the adsorption of copper, so ambient temperature was selected.
3.1.5 Elution of loaded resin
The loaded resin was eluted using 2.0 mol/L H2SO4 solution at a contact time of 40 min. The results are shown in Fig.5.
It can be seen from Fig.5 that the loaded resin could be completely eluted using 2.0 mol/L H2SO4 solution. A
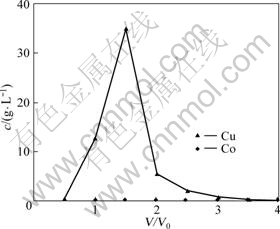
Fig.5 Elution curves of loaded resin (eluent: 2.0 mol/L H2SO4, 20 °C, 40 min)
peak concentration of copper in the eluate of 35 g/L was achieved for the sample collected at V/V0 of 1.5. In contrast, the cobalt concentration was constantly low (usually <20 mg/L). The highest concentration ratio of Cu/Co in the eluate at V/V0 of 1.5 was 3 806. It could be concluded that SP-C is an excellent resin to be utilized in the removal of copper in cobalt sulfate electrolyte.
3.2 Adsorption isothermal equilibrium
Langmuir and Freundlich isotherm equations have been most widely used for describing the behavior of metal ions adsorption onto resin. Langmuir and Freundlich isotherm equations can be expressed as the following linear forms, respectively[17-18]:
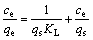 (2)
(2)
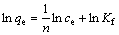 (3)
(3)
where qe is the amount of copper adsorbed onto the resin at equilibrium (mg/g); ce is the equilibrium concentration of copper in solution (mg/L); qs is the maximum amount of copper adsorbed on the resin (mg/g); KL is Langmuir isotherm constant (L/mg); Kf and n are constants indicative of adsorption capacity and adsorption intensity, respectively.
The experimental data were fitted into the above equations to infer the function parameters. The linear relationship between ce/qe and ce at various temperatures is plotted in Fig.6, while the one between lnqe and lnce at various temperatures is given in Fig.7. The correlated parameters of Langmuir and Freundlich isotherm equations derived from the slope and intercept of the linear plots are listed in Table 1.
Table 1 Correlated parameters of Langmuir and Freundlich isotherm equations
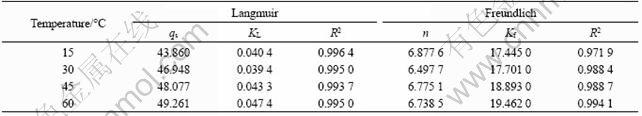
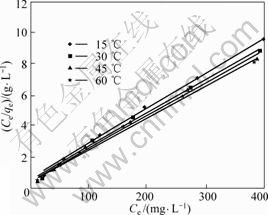
Fig.6 Langmuir isotherms of copper adsorbed onto resin (copper initial concentration: 0.5 g/L, pH=4, 24 h)
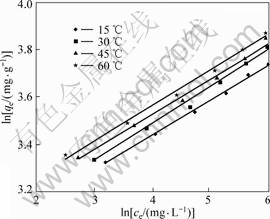
Fig.7 Freundlich isotherms of copper adsorbed onto resin (copper initial concentration: 0.5 g/L, pH=4, 24 h)
The above results showed that the experimental data fitted Langmuir isotherm equation very well. All the R-square values at different temperatures were greater than 0.99. The values of qs shown in Table 1 increased from 43.86 to 49.261 mg/g(dry resin) when the temperatures went up from 15 to 60 °C. These results were consistent with those of column experiment. This increasing trend of qs indicated that the adsorption process was endothermic in nature and higher temperature favored adsorption. The process of adsorption copper onto SP-C obeyed the Freundlich isothermal model as well, all R-square values were greater than 0.97.
3.3 Adsorption kinetics
The kinetics of the process of copper being adsorbed onto SP-C were investigated using pseudo-first-order and pseudo-second-order kinetic expressions, which are generally described by the following equations[19-20], respectively:
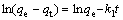 (4)
(4)
 (5)
(5)
where qe is the amount of copper adsorbed onto the resin at equilibrium (mg/g); qt is the amount of copper adsorbed on the resin at time t (mg/g);t is adsorption time (min); k1 is the rate constant of pseudo-first-order model (min-1); k2 is the rate constant of pseudo-second- order model (g?mg-1?min-1).
If the experimental data were visualized by the pseudo-first-order model, a linear relationship between ln(qe-qt) and t should be given. If the experimental data fitted with pseudo-second-order model, a linear relationship between t/q and t is resulted. The experimental data and the fitted trend lines are displayed in Figs.8 and 9 for the first and second models, respectively. The correlated kinetic parameters determined from the slope and the intercept of straight lines are listed in Table 2.
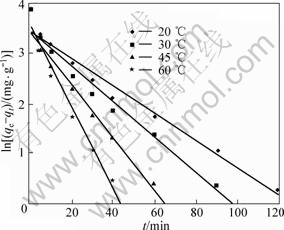
Fig.8 Relationship between ln(qe-qt) and t (copper initial concentration: 0.5 g/L, pH=4)
Table 2 Correlated parameters of pseudo-first-order and pseudo-second-order kinetic equations
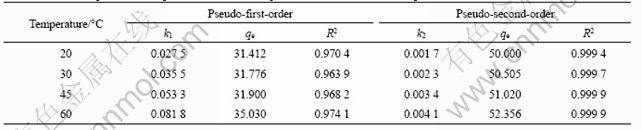
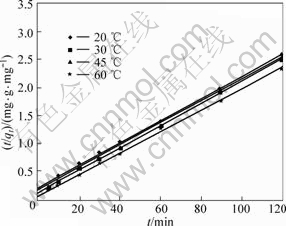
Fig.9 Relationship between t/qt and t (copper initial concentration: 0.5 g/L, pH=4)
As shown above, the pseudo-second-order model has a higher fitting degree to the experimental data compared with the pseudo-first-order model. The former has very high regression correlation coefficients with R-square values all greater than 0.999 at different temperatures. The theoretical values of qe calculated from linear regression equations of the pseudo- second-order model were around 50 mg/g, which is quite close to the above column experimental results. In contrast, the qe values derived from pseudo-first-order were about 31 mg/g, which deviated seriously from the column experimental findings. These dissimilarities indicated that the mode of copper in the cobalt sulfate electrolyte adsorbed onto SP-C approximates more than the pseudo-second-order kinetic model. It was also observed that the rate constant increased with increasing temperature, which implied the acceleration of the adsorption rate at higher temperature.
3.4 Analysis of adsorption mechanism
Chelating resins are typically characterized by functional groups containing O, N, S, and P donor atoms which can coordinate to different metal ions. SP-C is a silica-polyamine based composite chelating resin with a function group of aminomethyl pyridine, in which there are two nitrogen atoms, and they can chelated with Cu(Ⅱ). In order to confirm this, the IR spectra of loaded
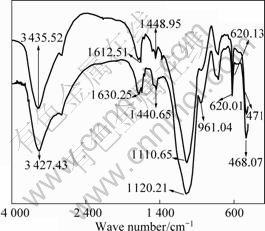
Fig.10 IR spectra of loaded resin (a) unloaded resin (b)
resin and unloaded resin are compared in Fig.10.
Seen from Fig.10, when the Cu(Ⅱ) is adsorbed, the adsorption bands of C=N and N—H at 1 630.25 and 3 427.43 cm-1 shift to lower frequency and higher frequency, respectively; especially, the change of C=N band is the largest. This result is attributed to formation coordination bonds between Cu(Ⅱ) and nitrogen atoms of functional groups. Influenced by the coordination, the absorption bands of C—H and Si—O—Si at 1 440.65 and 1 120.21 cm-1 also shift.
Because aminomethyl pyridine is a weak alkali, it could be protonized in an acidic medium. So, the process of copper adsorbed onto SP-C can be considered as two steps which are illustrated below:
The process of resin protonized by H2SO4:
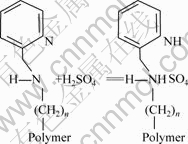 (step 1)
(step 1)
The process of resin chelated with Cu(Ⅱ):
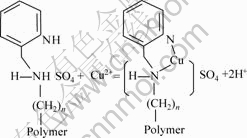 (step 2)
(step 2)
Because the reaction of adsorbed copper can release protons, the adsorption can be affected by the pH value of feed solution.
4 Conclusions
1) It was observed that the adsorption speed of SP-C for copper was fast, and relatively high pH and temperature are advantageous to the adsorption. The changes of copper concentration in solution had no obvious effect on the adsorption capacity. The optimal operation condition for the removal of copper from cobalt sulfate electrolyte with SP-C were pH=4, contact time of 30 min and ambient temperature. The loaded resin can be eluted efficiently by 2.0 mol/L H2SO4 at contact time of 40 min. The peak concentration of copper in eluate was about 35 g/L, in which the concentration ratio of copper to cobalt is as high as 3 806, and a cupric sulphate enriched solution can be obtained.
2) Isothermal equilibrium studies showed that the experimental data fitted Langmuir isotherm equation very well. The R-square values were all greater than 0.99 at different temperatures.
3) The pseudo-first-order and pseudo-second-order models were used in the study of adsorption kinetics. The correlated results indicated that the process of copper being adsorbed onto SP-C in cobalt sulfate electrolyte fitted the pseudo-second-order equation with all the R-square values greater than 0.999 at different temperatures.
4) Infrared spectra show that the nitrogen atoms of the functional group coordinate with Cu(Ⅱ) form the coordination bands.
References
[1] PENG Ji-shi, SHUAI Gou-shu, WANG Cheng-gang, LI Qin-xian, FAN Xing-yong. A method of removing copper from cobalt acidic solution or nickel acidic solution [P]. CN1115338A, 1996-1-24. (in Chinese)
[2] CHANG Quan-zhong, MAO Xi-kang, MA Yan, ZHAO Cai-xia. A method of removing copper from cobalt electrolyte [P]. CN1598013A, 2005-3-23. (in Chinese)
[3] GAO Zu-de. A method of removing copper from nickel electrolyte and cobalt electrolyte [P]. CN98111806.2, 1998-9-23. (in Chinese)
[4] WANG Sheng-dong, JIANG Xun-xiong, YIN Cai-qiao. Selective solvent extraction of copper from the acid leaching solution of ocean cobalt-rich crust with LIX84 [J]. Nonferrous Metal (Extractive Metallurgy), 2002(2): 6-9. (in Chinese)
[5] LE Song-guang, XIA Zhong-rang, LU Zheng-hua. Cobalt metallurgy [M]. Beijing: Metallurgy Industry Press, 1987, 214-265. (in Chinese)
[6] PAN Yun-cong, JIANG Ji-mu. Design handbook of heavy nonferrous metal (Part copper and nickel) [M]. Beijing: Metallurgy Industry Press, 1996, 667-668. (in Chinese)
[7] LIU Xiao-rong, QIU Guan-zhou, HU Yue-hua, XU Jing. Mechanism of limited coalescence of interfacial emulsion in copper solvent extraction [J]. The Chinese Journal of Nonferrous Metal, 2002, 12(3): 583-586. (in Chinese)
[8] ZHOU Gui-ying, RUAN Ren-man, WEN Jian-kang. Analysis of interfacial emulsification in solvent extraction of Copper [J]. Chinese Journal of Rare Metals, 2006, 30(6): 757-760. (in Chinese)
[9] ZAINOL Z, NICOL M J. Comparative study of chelating ion exchange resins for the recovery of nickel and cobalt from laterite leach tailings [J]. Hydrometallurgy, 2009, 96(4): 283-287
[10] CHEN Ai-liang, QIU Guan-zhou, ZHAO Zhong-wei. Removal of copper from nickel anode electrolyte through ion exchange [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(11): 253-258.
[11] DINIZA C V, CIMINELLI V S, DOYLE F M. The use of the chelating resin Dowex M-4195 in the adsorption of selected heavy metal ions from manganese solutions [J]. Hydrometallurgy, 2005 78(3/4): 147-155.
[12] XIAO L S, ZHANG Q X, GONG B F ,HUANG S Y. Separation of molybdenum from tungstate solution by a combination of moving packed bed and fluid bed ion-exchange techniques [J]. International Journal of Refractory Metals & Hard Materials, 2001, 19(3): 145-148.
[13] KESAVA C S, SUBRAMANIAN M S. A novel solid phase extraction method for separation of actinides and lanthanides from high acidic streams [J]. Separation and Purification Technology, 2007, 55(1): 16-22.
[14] EDWARD R, ROBERT J F. Materials for the separation of copper ions and ferric iron in liquid solution [P]. US6576590B2, 2003-06-10.
[15] BEATTY S T, FISCHER R J, ROSENBERG E. Comparison of novel and patented silica-polyamine composite materials as aqueous heavy metal ion recovery materials[J]. Separation Science and Technology, 1999, 34(14): 2723-2739.
[16] XIONG Chun-hua, YAO Cai-ping. Adsorption behavior of gel-type weak acid resin (110-H) for Pb2+ [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(5): 1290-1294.
[17] SPRYNSKYY M, BUSZEWSKI B, TERZYK A P, NAMIESNIK J. Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+ and Cd2+) adsorption on clinoptilolite [J]. Journal of Colloid and Interface Science, 2006, 304(1): 21-28.
[18] LEE I H, KUAN Y C, CHERN J M. Equilibrium and kinetics of heavy metal ion exchange [J]. Journal of the Chinese Institute of Chemical Engineers, 2007, 38(1): 71-84.
[19] WANG X S, HUANG J, HU H Q, WANG J, QIN Y. Determination of kinetic and equilibrium parameters of the batch adsorption of Ni(II) from aqueous solutions by Na-mordenite [J]. Journal of Hazardous Materials, 2007, 142(1/2): 468-476.
[20] SELVARAJ R, YOUNGHUN KIM, CHEOL K J, JONGHEOP Y. Removal of copper from aqueous solution by aminated and protonated mesoporous aluminas: kinetics and equilibrium[J]. Journal of Colloid and Interface Science, 2004, 273(1): 14-21.
(Edited by FANG Jing-hua)
Corresponding author: WEN Jun-jie; Tel: +86-731-88830143; E-mail: nee-sw@163.com
DOI: 10.1016/S1003-6326(09)60334-4