超级电池负极材料的电化学行为
陈绪杰,蒋良兴,彭彬,张治安,赖延清,李劼,刘业翔
(中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘要:利用循环伏安、阴极极化等测试手段研究超级电池负极中活性炭(AC)电极在铅酸电池测试环境中的电化学行为,比较AC电极与Pb电极在相同电位区间下的极化特性,考察-0.9~-0.4 V(vs. SCE)电位范围内AC及Pb并联电极的电化学特性,并对循环后并联电极中的AC电极进行电子能谱(EDX)分析。研究结果表明:活性炭在-0.9~-0.4 V电位范围内表现出双电层电容特性;在10 mV/s扫描速度下析氢电流随着硫酸浓度的增大而增大,比容量在硫酸浓度为3 mol/L时最大;在浓度为5 mol/L的硫酸中,活性炭的比容量随着扫描速度的增大而减小;在相同电位下,AC电极的析氢较Pb电极的析氢严重;AC与Pb的并联电极既表现出Pb电极的法拉第电池特性,又表现出AC电极的双电层电容与析氢特性。
关键词:活性炭;铅电极;并联电极;双电层;析氢过电位
中图分类号:O646 文献标志码:A 文章编号:1672-7207(2012)06-2023-06
Electrochemical behavior of ultrabattery negative electrode material
CHEN Xu-jie, JIANG Liang-xing, PENG Bin, ZHANG Zhi-an, LAI Yan-qing, LI Jie, LIU Ye-xiang
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Cyclic voltammetry and cathodic polarization tests were used to investigate the electrochemistry behaviors of activated carbon (AC) for ultrabattery negative electrode in the testing environment of lead acid battery. Comparison between the polarization properties of AC electrodes and Pb electrodes in some potentials was made, and the electrochemical characteristics of parallel electrode from -0.9 V to -0.4 V(vs. SCE) were also investigated. EDX was used to analyze the element components of AC in parallel electrode after three cycles in cyclic voltammetry tests. The results show that AC electrode presents character of double layer capacitance from -0.9 V to -0.4 V, and the hydrogen evolution current increases with the increase of the sulfuric acid concentration at the scan rate of 10 mV/s. The largest specific capacitance is obtained in 3 mol/L sulfuric acid. In 5 mol/L sulfuric acid, the specific capacitance decreases with the increase of the scan rate. At the same potential, the AC electrode is much easier in producing hydrogen in 5 mol/L sulfuric acid than that of the Pb electrode. The parallel electrode has both the effects of faraday battery and double layer capacitor characteristics.
Key words: activated carbon; lead electrode; parallel electrode; double electrode layer; overpotential of hydrogen
铅酸电池主要优点是价格低廉、安全性高[1],目前,铅酸电池逐渐向纯电动汽车(EV)和混合电动汽车(HEV)等领域发展[2-3],其一个突出的问题是电池失效造成的寿命短,失效的主要原因之一是负极的硫酸盐化[4-5]。目前,人们研究较多的是通过掺杂来降低负极的硫酸盐化,但也没能很好地解决这一问题。近年来,铅酸电池与一些新技术的结合使其获得了新的发展,并引起了国内外广泛关注[6-11]。2003年,澳洲科学与工业研究组织(CSIRO)开发出一种超级电池(Ultrabattery)[12],采用C-Pb复合电极或C电极与Pb电极的并联电极为负极,同时保持PbO2为正极,实现混合电容器与铅酸电池内结合。炭电极在高倍率电流充放电时分担一部分电流,起到降低铅酸电池负极电流的作用。超级电池将超级电容器的高比功率、长寿命等优势融合到铅酸电池中,在改善功率和延长电池寿命的同时,简化了外电路,提高了比能量,并降低了总费用。据文献[12],超级电池的循环寿命至少是铅酸电池的4倍,充放电功率高50%;与Ni-MH电池相比,它的循环性能、功率特性与Ni-MH电池的相当,且制造成本降低70%。超级电池可在车辆加速时快速提供电能,刹车时吸收电能,能满足小型及中型混合动力车的需求。超级电池目前仍处于初期研发阶段,其电极过程等基础理论问题有待进一步研究。超级电池负极中的炭电极一般直接采用超级电容器中广泛应用的高比表面积活性炭材料[13-15]。但是,超级电容器中活性炭的工作电压较正(-0.2~0.8 V)。而在超级电池中,活性炭电极工作于铅酸电池负极工作环境中,即高浓度硫酸电解液体系及较负的工作电压(-0.9~ -0.4V)。有关这方面的研究报道很少,为此,本文作者研究活性炭在铅酸电池负极工作环境下的析氢行为及双电层电容特性,比较AC电极与Pb电极在相同电位区间下的极化特性,并考察AC与Pb并联电极的电化学特性,以便为超级电池的开发提供理论基础。
1 实验
1.1 电极的制备
以活性炭-YP17(日本可乐丽)为原料,按活性炭、乙炔黑、聚偏氟乙烯(PVDF)的质量比8:1:1研磨混合均匀,涂覆在钛箔上,于60 ℃时干燥8 h。钛箔在涂覆前,先用砂纸磨光,并在超声波中用丙酮洗掉表面残存的有机物,最后用蒸馏水冲洗、干燥。涂覆活性炭的钛箔取活性炭表观面积为10 mm×10 mm,其余部分用AB胶密封,预留部分连接铜导线。Pb电极加工成长×宽为10 mm×10 mm,铅电极纯度(质量分数)为99.99%。用环氧树脂封于圆形塑料管中,露出下端矩形工作面,以铜线为导线。电极用金相试样预磨机(上海顺辉金相设备厂)打磨,二次蒸馏水洗净。将制备好的活性炭电极和Pb电极用外部铜导线连接得到活性炭与Pb电极面积相同的AC-Pb并联电极。电解液采用H2SO4溶液(由分析纯试剂和二次蒸馏水配置),辅助电极为铂片电极(长×宽为25 mm×20 mm),参比电极为饱和甘汞电极(SCE)。
1.2 电化学性能测试
循环伏安和阴极极化曲线测试均在美国PE公司的电化学综合测试系统PARSTAT 2273 (PerkinElmer instrument. USA)上进行。铅电极阴极极化曲线测试前先在-1.2 V下进行10 min预极化处理,除去表面的氧化物。
1.3 材料表面成分表征
采用GENESIS60S型X线能谱分析仪(EDX)检测AC-Pb并联电极3次循环伏安测试后电极表面的物质组成。
2 结果与讨论
2.1 AC电极的电化学特性
2.1.1 不同硫酸浓度下的电化学特性
图1所示是活性炭在浓度为1,3和5 mol/L硫酸中的循环伏安曲线。理想的超级电容器的循环伏安曲线呈对称的矩形形状。由图1可以看出:循环伏安曲线在-0.9~-0.4 V有明显的双电层电流出现,即表明活性炭在较负的电位下表现出双电层电容特性,但较大程度地偏离了矩形,同时出现了代表氢气析出的还原峰;随着硫酸浓度的增大,析氢电流也明显增大,说明氢离子浓度的增大加剧了析氢现象。
由循环伏安曲线,并根据下式计算活性炭的比容量:
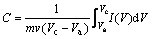 (1)
(1)
其中:m为活性物质的质量;v为扫描速度;Vc为扫描上电位;Va为扫描下电位;I(V)为响应电流;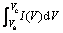 为循环伏安曲线的积分面积。所得比容量如表1所示。
为循环伏安曲线的积分面积。所得比容量如表1所示。

图1 活性炭在不同浓度硫酸中的循环伏安曲线
Fig.1 CV curves of AC in different sulfuric acid solutions
表1 不同硫酸浓度下活性炭的比容量及最大析氢值
Table 1 Specific capacity and maximal hydrogen-evolution value of AC in different sulfuric acid solutions

由表1可以看出:在-0.9~-0.4 V电位范围内,硫酸浓度对电容器比容量的影响较大;活性炭的比容量随着硫酸浓度的增大先增大后减小,当硫酸浓度为3 mol/L时活性炭的比容量最高。这是由于浓度为3 mol/L的硫酸既能满足电解液对离子浓度的要求,又能满足双电层形成的要求。硫酸浓度为1 mol/L及5 mol/L时的比容量都较小,说明在-0.9~-0.4 V电位范围内,过高及过低的硫酸浓度都不利于活性炭比容量的提高。因此,活性炭在铅酸电池高浓度硫酸体系中的比容量提高幅度受限。
图2所示为活性炭在1,3和5 mol/L硫酸中的阴极极化曲线。从图2可以看出:在相同的极化电位下,硫酸浓度越大,单位质量活性炭的极化电流也越大,且开始明显析氢的电位也越大。这说明氢离子浓度的增大使氢气的析出更容易。因此,当活性炭作为铅酸电池负极的一部分时,析氢是需要解决的问题之一。
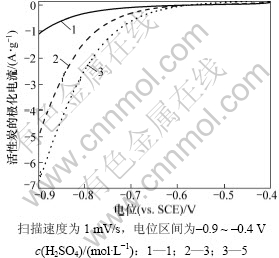
图2 活性炭在不同浓度硫酸中的阴极极化曲线
Fig.2 Cathodic polarization curves of AC in different sulfuric acid solutions
2.1.2 电位区间对活性炭比容量的影响
为研究电位区间对活性炭比容量的影响,实验采用浓度为5 mol/L的硫酸,分别在电位区间-0.9~-0.4,-0.5~0,-0.4~0.1和0~0.5 V条件下测试活性炭电极的循环伏安曲线,根据式(1)计算比容量。
图3所示为活性炭在不同电位区间的循环伏安曲线。图3(b),(c)和(d)中的曲线形状比较接近矩形,0.4 V左右出现了由活性炭官能团引起的微弱的氧化峰,并产生了部分法拉第电流。从图3(a)可以看出:活性炭开始出现明显的析氢反应的电位在-0.6 V以下。
根据式(1),由图3的循环伏安曲线计算活性炭在不同电位区间的比容量如表2所示。
表2 活性炭在不同电位区间的比容量
Table 2 Specific capacity of AC in different potential ranges

从表2可以看出:活性炭的比容量随着电位区间的正移逐渐增大,说明活性炭的比容量在电位较正时较大。根据离子吸附双电层理论,当体系中的电位较正时,电极表面的剩余电荷也为正,能吸附溶液中的负离子;当体系的电位为负时,电极表面的剩余电荷为负,吸附溶液中的正离子。由于正离子的溶剂化作用,致使双电层紧密层正离子电荷中心到电极表面的距离较远,因此,比容量减小,这也是活性炭做负极比做正极时比容量低的原因。从图3还可以看出:活性炭容量的发挥主要在较正电位区间,-0.6 V以下主要是析氢反应,几乎没有双电层电流。因此,活性炭电极在铅酸电池负极工作环境中的容量发挥程度比在超级电容器工作环境中的容量发挥程度小。
2.1.3 扫描速度对活性炭比容量的影响
实验采用浓度为5 mol/L的硫酸,在三电极体系分别用1,5,10和20 mV/s的扫描速度测试活性炭电极在-0.9~-0.4 V电位范围内的循环伏安曲线,结果如图4所示。
由图4可以看出:随着扫描速度的增大,响应电流也不同程度地增大,但同时析氢也越严重。根据式(1)计算不同扫描速度下活性炭的比容量如表3所示。

图3 不同电位区间下活性炭在5 mol/L硫酸中的循环伏安曲线
Fig.3 CV curves of AC in different potential ranges with 5 mol/L sulfuric acid solution
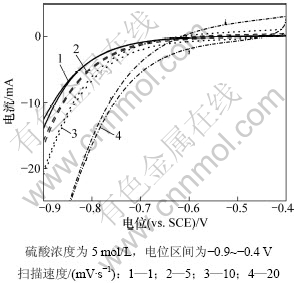
图4 不同扫描速度下活性炭的循环伏安曲线
Fig.4 CV curves of AC at different scan rates
由表3可以看出:不同的扫描速度对活性炭比容量的影响较大;随着扫描速度的增大,活性炭的比容量明显降低;当扫描速度达20 mV/s时比容量只有5.0 F/g。这是由于在较大的扫描速度下,活性炭的双电层形成不完全,活性炭材料的利用率较低。
表3 不同扫描速度下活性炭的比容量
Table 3 Specific capacity of AC at different scan rates

2.2 Pb电极的电化学行为
图5所示为Pb电极的循环伏安曲线。由图5可以看出:在-0.9~-0.4 V电位范围内主要对应Pb电极的氧化还原反应:-0.7 V以下对应析氢反应;-0.47 V左右的Pb转变为PbSO4的氧化反应;-0.57 V左右的PbSO4转变为Pb的还原反应。由图5可以看出:氧化峰的响应电流比还原峰的响应电流大很多,这说明Pb转变为PbSO4比PbSO4转变为Pb的效率更高,因此随着循环的进行,铅负极存在PbSO4的积累从而也导致负极硫酸盐化的发生。
图6所示为Pb电极与活性炭电极阴极极化曲线的对比结果。从图6可以看出:在-0.6~-0.4 V的电位范围内,相同电位下活性炭电极上的极化电流大于Pb电极上的极化电流。在实验过程中也发现在活性炭电极表面有更明显的气泡析出,表明在活性炭电极表面更容易析氢。这是由于:一方面活性炭比表面积大,电极反应的活化能比Pb电极表面的活化能低,因此,析氢反应更容易进行;另一方面,活性炭较大的比表面积相当于降低了电流密度,当电流密度降低时,析氢过电位必然降低,析氢反应更容易发生。
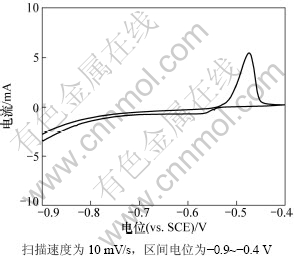
图5 Pb电极在5 mol/L硫酸中的循环曲线
Fig.5 CV curves of Pb in 5 mol/L sulfuric acid solution
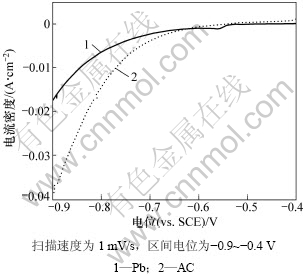
图6 Pb和AC电极在5 mol/L H2SO4中的阴极极化曲线对比图
Fig.6 Comparison of cathodic polarization curves between AC and Pb in 5 mol/L sulfuric acid solution
2.3 AC-Pb并联电极的电化学行为
图7所示为AC,Pb与AC-Pb 3类电极在-0.9~-0.4 V电位范围内循环伏安曲线的对比结果。由图7可以看出:活性炭和Pb并联的循环伏安曲线几乎是两者单独工作时的叠加;电极既表现出Pb电极的氧化还原电池特性,又表现出AC电极的双电层电容特性,响应电流由法拉第电流和非法拉第电流组成。这说明当活性炭和铅电极并联用于超级电池负极时,活性炭电极可以吸收或者释放电荷,在车辆制动及加速时起到缓冲作用。但由图7也可以看出并联以后电极的析氢更严重,这一方面是由于活性炭电极的析氢过电位比Pb电极的析氢过电位低,另一方面是并联电极的面积增大降低了电流密度。

图7 3类电极的循环伏安曲线对比图
Fig.7 Comparison of CV curves of three electrodes
图8所示为AC-Pb并联电极在-0.9~-0.4 V电位范围内循环1,10,20和30次的循环伏安曲线。从图8可以看出:活性炭始终发挥了双电层电容作用,并且在-0.5~0.4 V区间的曲线形状变化不大;但随着循环次数的增加,析氢略严重。此外,随着循环次数的增加,氧化峰明显增大,但还原峰变化不明显。
对并联电极三次循环伏安测试后的AC电极进行EDX元素分析。能谱分析结果表明:电极表面除了主要的C元素外,还含有少量Pb(摩尔数分数为2.28%)和S(摩尔数分数为4.32%),且主要存在活性炭颗粒之间,推测其赋存形态为PbSO4。这说明Pb在氧化还原反应过程中的产物PbSO4部分被活性炭电极吸附。

图8 并联电极的循环伏安曲线
Fig.8 CV curves of parallel electrode
3 结论
(1) 活性炭在-0.9~-0.4 V电位范围内有双电层电容特性和析氢行为,明显析氢的电位为-0.6 V,硫酸浓度对活性炭的电化学性能影响较大。随着硫酸浓度增大,析氢越严重,比容量则在浓度为3 mol/L时 最大。
(2) 随着扫描速度的增大,活性炭的比容量减小,析氢也越严重。活性炭在不同电位区间内表现出不同的电化学行为,随着电位区间负移,比容量降低,析氢增大。
(3) 在5 mol/L硫酸中,在相同的极化电位下活性炭电极的析氢电流比铅电极的更大,因此,开发抑制炭负极析氢的添加剂是提高超级电池寿命及循环性能的关键之一。
(4) 活性炭与铅并联电极的循环伏安曲线既包括Pb电极的法拉第电流,又包括活性炭的非法拉第电流。这说明活性炭可以吸收和释放电荷,活性炭电极能在超级电池中起缓冲作用。
参考文献:
[1] 陈红雨. 国外铅蓄电池研究进展[J]. 蓄电池. 2000(3): 28-30.
CHEN Hong-yu. Current state of research in lead-acid batteries at abroad[J]. Chinese Labat Man, 2000(3): 28-30.
[2] Lam L T, Newnham R H, Ozgun H, et al. Advanced design of valve-regulated lead-acid battery for hybrid electric vehicles[J]. J Power Sources, 2000, 88(1): 92-97.
[3] Cooper A. Development of a lead-acid battery for a hybrid electric vehicle[J]. J Power Sources, 2004, 133(1): 116-125.
[4] Atlung S, Zachau-Christiansen B. Failure mode of the negative plate in recombinant lead/acid batteries[J]. J Power Sources, 1994, 52(2): 201-209.
[5] Lam L T, Haigh N P, Phyland C G., et al.Failure mode of valve-regulated lead-acid batteries under high-rate partial-state-of-charge operation[J]. J Power Sources, 2004, 133(1): 126-134.
[6] 李中奇. 一种超级蓄电池用双性极板: 中国, CN101123139[P]. 2008-02-13.
LI Zhong-qi. A bipolar plate for ultrabattery: China, CN101123139[P]. 2008-02-13.
[7] 柳颖, 韩良军, 张娟, 等. 复合负极板密封铅酸电池:中国, CN101414692[P]. 2009-04-22.
LIU Ying, HAN Liang-jun, ZHANG Juan, et al. Composite negative plate for sealed lead acid battery:China, CN101414692[P]. 2009-04-22.
[8] Lam L T, Louey R. Development of ultra-battery for hybrid-electric vehicle applications[J]. J Power Sources, 2006, 158(2): 1140-1148.
[9] Cooper A, Furakawa J, Lam L, et al. The ultrabattery: A new battery design for a new beginning in hybrid electric vehicle energy storage[J]. J Power Sources, 2009, 188(2): 642-649.
[10] Furukawa J, Takada T, Monma D, et al. Further demonstration of the VRLA-type ultrabattery under medium-HEV duty and development of the flooded-type ultrabattery for micro-HEV applications[J]. J Power Sources, 2010, 195(4): 1241-1245.
[11] Lam L T, Louey R, Haigh N P, et al. VRLA ultrabattery for high-rate partial-state-of-charge operation[J]. J Power Sources, 2007, 174(1): 16-29.
[12] Lam L T. High performance energy storage devices: AU, PCT/AU2004/001262[P]. 2003-09-18.
[13] 江奇, 瞿美臻, 张伯兰, 等. 电化学超级电容器电极材料的研究进展[J]. 无机材料学报, 2002, 17(4): 649-656.
JIANG Qi, QU Mei-zhen, ZHANG Bo-lan, et al. Progress of research on electrode materials for electrochemical supercapacitors[J]. Journal of Inorganic Materials, 2002, 17(4): 649-656.
[14] Emmenegger C, Mauron P, Sudan P, et al. Investigation of electrochemical double-layer (ECDL) capacitors electrodes based on carbon nanotubes and activated carbon materials[J]. J Power Sources, 2003, 124(1): 321-329.
[15] Momma T, LIU Xing-jiang, Osaka T, et al. Electrochemical modification of active carbon fiber electrode and its application to double-layer capacitor[J]. J Power Sources, 1996, 60(2): 249-253.
(编辑 陈灿华)
收稿日期:2011-07-15;修回日期:2011-09-09
基金项目:国家科技支撑计划项目(2007BAE12B01);国家自然科学基金资助项目(20803095)
通信作者:张治安(1975-),男,河南洛阳人,博士,副教授,从事超级电池研究;电话:0731-88830649;E-mail:zza75@163.com