DOI: 10.11817/j.issn.1672-7207.2015.06.003
Fe-Cr-Ni-O体系碳还原产物热力学计算及实验分析
刘洋,张延玲,郭文明,贾昕磊
(北京科技大学 钢铁冶金新技术国家重点实验室,北京,100083)
摘要:对Fe-Cr-Ni-O体系碳还原产物的组成与特性进行探讨。首先对不同温度及碳氧摩尔比条件下Fe-Cr-Ni-O体系碳还原产物进行热力学分析,然后对Fe-Cr-Ni-O体系及模拟不锈钢粉尘进行等温碳还原实验,并对还原产物进行系统分析。研究结果表明:升高温度有利于体系中各金属氧化物的还原及合金相的生成;当碳氧摩尔比(nC:nO)小于1.0时,体系中金属氧化物尤其是Cr2O3的还原不完全;当碳氧摩尔比大于1.0时,还原产物中容易出现金属碳化物。当碳氧摩尔比为1.0时,渣铁分离效果较好。此外,温度主要影响反应后期的失氧速率,而碳氧摩尔比主要影响样品的最终失氧率,但温度与碳氧摩尔比对反应前期的影响均不大;推断前期主要发生NiO和Fe2O3的还原,而后期主要发生Cr2O3的还原。
关键词:Fe-Cr-Ni-O体系;碳;还原产物;合金;金属碳化物
中图分类号:TF644 文献标志码:A 文章编号:1672-7207(2015)06-1989-10
Thermodynamic calculation and experimental analysis of the carbothermal reduction products of Fe-Cr-Ni-O system
LIU Yang, ZHANG Yanling, GUO Wenming, JIA Xinlei
(State Key Laboratory of Advanced Metallurgy, University of Science and Technology Beijing, Beijing 100083, China)
Abstract: The composition and characteristics for the carbothermal reduction products of Fe-Cr-Ni-O system were studied. Thermodynamic analysis of the carbothermal reduction products of Fe-Cr-Ni-O system were carried out firstly. Then the carbothermal reduction products of Fe-Cr-Ni-O system and simulative stainless steel dust were investigated through isothermal reduction experiments. The results show that higher temperature is beneficial to both the reduction of metal oxides and the generation of alloys, and the reduction of metal oxides (Cr2O3 particularly) is not complete at nC:nO (the initial molar ratio of C to O in the sample) <1.0, while the metal carbides are easy to appear at nC:nO>1.0. The separation of alloy and slag in the reduction products is good at nC:nO=1.0. Temperature mainly affects the oxygen loss rate in the later stage and nC:nO mainly affects the final reduction degree, while the effect of temperature and nC:nO ratio are not obvious in the early stage. It is speculated that the reduction of NiO and Fe2O3 happens in the early stage while the reduction of Cr2O3 occurs in the later stage.
Key words: Fe-Cr-Ni-O system; carbon; reduction products; alloy; metal carbides
Fe-Cr-Ni-O体系化合物广泛存在于不锈钢冶炼所产生的粉尘,渣和酸洗污泥等副产品中。随着近年来中国不锈钢产量的快速增加,这些副产品产量也大量增加,其中冶炼1 t不锈钢能产生18~33 kg粉尘[1-2]。一方面,由于这些粉尘中含有有毒物质如Cr和Pb等,故一般被归为有害物质[3-4];另一方面,这些粉尘中的有价金属元素,如Fe,Cr和Ni的质量分数分别为40%~60%,8%~15%,3%~9%[1-2, 5],且大部分以金属氧化物的形式存在[3, 6-7]。若能有效地对其进行回收利用,不仅节约资源,而且对生态环境也能起到一定的保护作用:因此,研究一种合理的方法从不锈钢粉尘中提取Fe,Cr和Ni等有价金属元素并获得可利用的产物具有重要的经济效益与社会意义。从目前查阅的文献资料看,国内外许多学者主要对含Fe,Cr和Ni体系氧化物的还原过程进行了系统研究。魏文洁等[8]和Abdel-Halim等[9]分别利用H2和C作为还原剂,研究了Fe-Ni-O体系中Fe/Ni的还原行为,发现还原是分步进行:首先是Ni氧化物的还原,然后是Fe氧化物的还原,且产物主要为Fe-Ni合金。Mori等[10]研究Cr氧化物的等温碳热还原过程,发现升高温度和合适的载气量以及石墨颗粒尺寸的减小都可以提高还原速率。Khedr[11]研究了H2还原Fe2O3和Cr2O3混合物的等温还原动力学,发现温度和体系中Cr2O3质量分数对Fe2O3的还原都有很大影响。还原过程分为2个阶段:前期由界面化学反应控速,后期由固态扩散和界面化学反应混合控速。Gornerup等[12]利用碳还原电弧炉渣中Fe和Cr的氧化物实验,得出Fe-C熔体的生成促进了Cr2O3的还原,且高温下过量的C则会促进Cr3C2和Cr7C3等碳化物的生成。Ma等[13]对Fe2O3-NiO-Cr2O3体系碳还原过程进行研究,得出了还原是分步进行的,中间氧化物FeCr2O4的形成促进了Cr2O3还原。林万明等[14]采用真空电阻炉选择性还原回收其中的Ni和Cr金属,明确指出在还原后的样品中发现了Fe-Cr-Ni合金,并且在合适的温度和还原剂加入量条件下,产物中Ni质量分数大于8%,Cr质量分数大于13%,金属回收率大于90%。以上研究均重点探讨的是Fe,Cr和Ni这3种元素的还原过程与基本反应机理等,而对于还原后所获得最终产物的组成和特性鲜有报道。这些是决定不锈钢粉尘等2次资源经处理后的产物能否作为合金化原料进入炼钢工序和真正实现良性循环的关键因素。实际上,这也是目前在2次资源利用领域存在的共性问题,前人关注更多的是如何有效地分离回收某种有价资源及其还原分离的过程,而对于所获得产物的特点缺乏系统研究,进而难以实现2次资源的真正循环利用。为此,本文作者首先对不同温度及碳氧摩尔比(nC:nO)条件下Fe2O3-Cr2O3-NiO体系碳还原产物进行热力学分析,然后对温度为1 350~1 550 ℃,碳氧摩尔比nC:nO为0.5~1.5的Fe2O3-Cr2O3-NiO体系样品进行等温还原实验,并对还原的产物组成及特性进行系统分析,最后探讨碳氧摩尔比对模拟不锈钢粉尘碳还原产物渣铁分离情况的影响。
1 热力学计算
Fe-Cr-Ni-O体系碳还原产物的热力学分析采用在热力学领域内广泛应用的数据库软件—FactSage,计算过程中主要用到其中的Equilib模块,其基本计算原理为吉布斯自由能最小原则。计算时,选择FactPS和FSstel数据库,温度设置为900~1 550 ℃,体系压强为101 325 Pa。
1.1 Fe-Cr-Ni-O体系碳还原平衡图分析
图1所示为Fe2O3-Cr2O3-NiO体系(三者摩尔比即n(Fe2O3):n(Cr2O3):n(NiO)= 3:2:2)碳还原产物的质量分数,体系中碳含量按碳氧摩尔比(nC:nO)设置为1.0。在纯Ar气氛下,经FactSage软件中的Equilib模块计算后,得到:1) 气相主要为CO;2) 液相中Fe,C,Cr和Ni等元素质量分数;3) 固相中FeCr2O4和Cr2O3以及Fe3C7和Cr3C7等碳化物的质量分数。
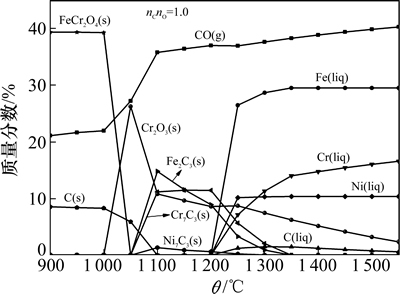
图1 碳氧摩尔比为1.0时不同温度θ下
Fe2O3-Cr2O3-NiO体系碳还原所得产物的质量分数
Fig. 1 Mass fraction of each product for carbothermal reduction of Fe2O3-Cr2O3-NiO at different temperatures when reactions reaches balanced at nC:nO=1.0
从图1可知:当温度低于1 000 ℃时,体系中存在最多的是FeCr2O4;当温度高于1 000 ℃时,FeCr2O4与C发生反应释放出Cr2O3,且当温度为1 050 ℃时,FeCr2O4完全消耗,同时金属碳化物(Fe7C3,Cr7C3和少量Ni7C3)开始出现;当温度高于1 200 ℃时,体系中出现液相且逐渐增多,而金属碳化物的质量分数逐渐减少。可见随着温度的升高,还原产物中首先出现的是金属碳化物,当温度达到一定值后,才会出现液相。由于Cr和Ni均在铁液中有一定溶解度,所以,产物中出现的液相实际上是Fe-Cr-Ni-C多元合金相(以下简称合金相)。前期研究[15]表明:该合金相与低温时出现的金属碳化物相比,最大特点是携带C元素的质量分数(饱和溶解度)偏低,且熔点低、流动性好。因此,若产物中合金相所占的比例高,表明所得产物中的C等杂质元素含量低,渣铁分离效果好,Cr和Ni等金属元素分离回收率高以及脉石质量分数低,进而有可能作为合格的合金化原料进入炼钢(如特殊钢)生产工艺;而若产物中碳化物所占比例高(通常为高熔点,且携带大量的C元素),则势必会带来相反的结果。
1.2 温度和碳氧摩尔比对Fe2O3-Cr2O3-NiO体系还原的影响
为了进一步考察温度和碳氧摩尔比(nC:nO)对碳还原Fe2O3-Cr2O3-NiO体系时各金属元素的还原率及产物中合金相(即图1中液相)所占比例的影响,由Factsage软件的计算数据可以得到如图2所示结果,其中温度为1 250~1 550 ℃,碳氧摩尔比(nC:nO)为0.5~1.2。
还原率(R)与失氧率(α)不同,某金属元素的还原率(Ri)是由生成的合金相(不包含生成的金属碳化物)中此金属元素的质量与体系中此金属元素总质量相比得到;产物中合金相所占比例(wl)是指还原生成的合金相在产物(包含合金相,金属碳化物相和未反应的氧化物等)中所占的质量分数。二者可分别由式(1)和式(2)得出:
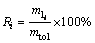 (1)
(1)
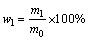 (2)
(2)
式中:Ri为元素i的还原率(i分别为Fe,Cr和Ni),%;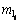 为合金相产物中i元素的质量,g;mtol为原样品中金属元素总质量,g;wl为反应后样品中合金相所占质量分数,%;ml为产物中合金总质量,g,且
为合金相产物中i元素的质量,g;mtol为原样品中金属元素总质量,g;wl为反应后样品中合金相所占质量分数,%;ml为产物中合金总质量,g,且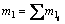 ;m0为还原后样品总质量,g。
;m0为还原后样品总质量,g。
当碳氧摩尔比为1.0时,温度对碳还原Fe2O3-Cr2O3-NiO体系时各金属元素的还原率及产物中合金相所占比例的影响如图2(a)所示。由图2(a)可以看出:当温度为1 250 ℃时,体系中Ni基本被完全还原,且Fe的还原率达到90%,而Cr的还原率只有40%左右;随着温度的升高,Fe在1 350 ℃时达到完全还原,还原率基本保持不变,而Cr的还原率还在不断增加,当温度为1 550 ℃时才能达到90%,说明Cr的还原率受温度的影响最大。热力学结果[16]也表明Cr2O3在3种氧化物中最难还原。此外,产物中合金相所占比例随着温度的升高而逐渐增加,表明温度越高越有利于合金相的生成。因为当温度升高后,低温条件下生成的碳化物(如Cr3C7等)与剩余的Cr2O3进一步反应生成了金属Cr,后者溶于铁液或最初生成的Fe-Ni熔体形成了Fe-Cr- Ni-C合金,从而使得产物中合金相所占比例增加。
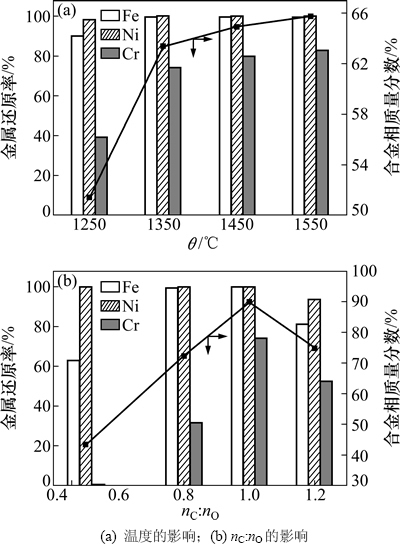
图2 各因素对Fe2O3-Cr2O3-NiO体系被碳还原的金属还原率及产物结构的影响
Fig. 2 Effect of factors on the reduction degree and carbothermal reduction products of Fe-Cr-Ni-O system
当温度为1 350 ℃时,碳氧比对碳还原Fe2O3-Cr2O3-NiO体系时各金属元素的还原率及产物中合金相所占比例的影响如图2(b)所示。从图2(b)可以看出:当碳氧摩尔比为0.5时,由于碳氧摩尔比不高,Cr的还原率几乎为0,生成的合金相主要为Fe-Ni合金;随着碳氧摩尔比升高,Cr的还原率逐渐增加,更多的Cr进入合金相中,从而使得产物中合金相所占比例逐渐升高;当碳氧摩尔比大于1.0时,由于更多金属碳化物的生成,所以各金属元素的还原率及产物中合金相所占比例有所降低。
2 实验
2.1 实验原料与装置
实验原料主要为化学纯试剂Fe2O3,Cr2O3,NiO(质量分数均大于99%)和石墨C(质量分数大于99.85%)。原料粉末均经75 μm筛子筛分(粒径均小于74 μm);炉管内保护气氛为高纯Ar(质量分数大于99.999%)。
实验装置如图3所示。该装置主要由以下部分组成:钼丝炉及温度控制器,用于提供实验所需温度;裂解炉,用于裂解NH3生成H2和N2,通入炉膛中保护钼丝;高纯Ar,用于通入炉管,为实验提供惰性气氛;天平,用于实时记录样品质量。
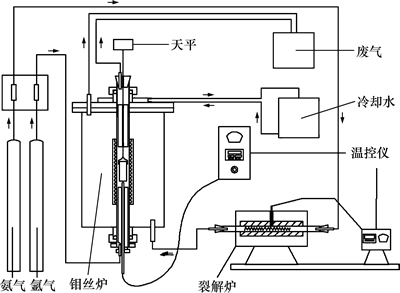
图3 实验装置示意图
Fig. 3 Schematic diagram of experimental apparatus
2.2 实验方案与步骤
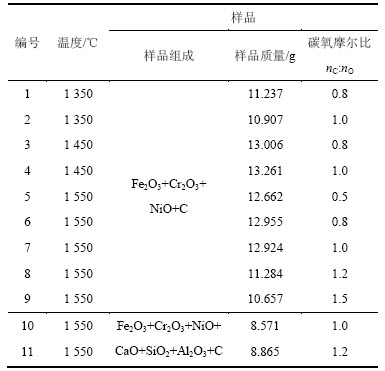
研究考察温度和碳氧摩尔比对Fe2O3-Cr2O3-NiO (n(Fe2O3):n(Cr2O3):n(NiO)=3:2:2)体系还原产物的影响。由于前期预备实验表明,还原时间为45 min时Fe2O3-Cr2O3-NiO体系样品的质量损失曲线已基本不变,反应趋于平衡,所以还原时间均控制在45 min。实验方案如表1所示。
实验时,首先按表1所示方案称取样品并混合均匀,取出一定量混合均匀的样品,用内径为16 mm的钢模进行压块(30 MPa),称质量后置于99瓷铝质坩埚中(直径×高为28 mm×50 mm)待用;待炉温升至目标温度后将坩埚放入炉内恒温区,并用天平连续记录样品质量(整个过程向炉管中通高纯Ar作为保护气);实验结束后,将样品随坩埚从钼丝炉中取出,室温下在强高纯Ar气流中快速冷却。
2.3 实验分析方法
2.3.1 样品失氧率的计算方法
由于实验温度较高(≥1 350 ℃),所以计算失氧率时,认为样品质量损失即为CO的生成量。样品的失氧率(α)计算式为:
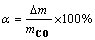 (3)
(3)
式中:Δm为样品实际质量损失,g;mCO为假设样品中的O全部转化为CO时的质量损失,g。
2.3.2 还原产物分析方法
采用M21X型超大功率X线衍射仪(日本玛珂科学仪器公司)分析还原产物的物相;采用JSM-6510型扫描电子显微镜(日本电子公司)观察微观形貌和成分分布;采用Genesis XMZ型附件能谱仪(美国热电公司)观察与分析。
表1 实验方案
Table 1 Experimental scheme
3 结果与讨论
3.1 碳还原Fe2O3-Cr2O3-NiO体系失氧曲线分析
当碳氧摩尔比为1.0时,不同温度下碳还原Fe2O3-Cr2O3-NiO体系的失氧曲线如图4(a)所示。从图4(a)可以看出:在样品失氧率达到40%之前,失氧曲线基本不受温度的影响,而后期受温度影响明显。由热力学分析结果可知:由于NiO在较低温度下即可被还原,所以其还原速率受温度的影响较小;相反,较难还原的Cr2O3还原速率受温度的影响最大。此外,对于本实验样品,理论上NiO全部还原时样品总的失氧率约为12%,而NiO和Fe2O3全部还原时的失氧率约为65%,所以,由以上分析可以得知,前期(失氧率≤40%)主要发生NiO及部分Fe2O3的还原,而后期主要发生Cr2O3的还原。郭文明等[17]的研究也得到了类似的结果。
当温度为1 550 ℃时,碳氧摩尔比为0.5~1.5时碳还原Fe-Cr-Ni-O体系失氧曲线如图4(b)所示。从图4(b)可以看出:当碳氧摩尔比在1.0~1.5变化时,失氧率变化不大,均能达到95%,且反应趋于平衡所需要的时间基本相同;而碳氧摩尔比在0.5~1.0变化时,样品最终失氧率变化较大,尤其在失氧率达到40%以后,失氧曲线受碳氧摩尔比的影响更为明显,由此推测初始碳氧摩尔比重点影响的是Cr2O3的还原。
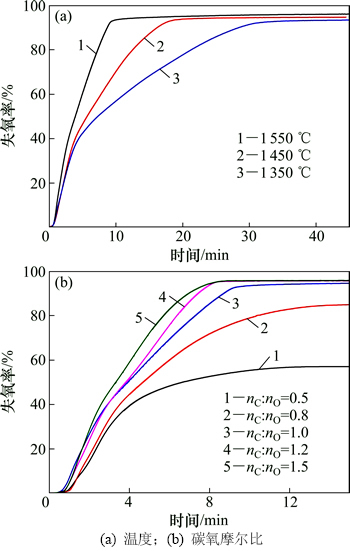
图4 不同条件下碳还原Fe2O3-Cr2O3-NiO体系失氧曲线
Fig. 4 Oxygen loss curves of Fe2O3-Cr2O3-NiO under different conditions
3.2 不同温度下体系碳还原产物分析
对碳氧摩尔比为0.8和1.0的样品进行3种温度 (1 350,1 450和1 550 ℃)下的还原实验,还原时间均为45 min,考察温度对Fe2O3-Cr2O3-NiO体系碳还原产物的影响。还原产物在不同实验温度条件下的宏观形貌如图5和图6所示,其中右上角的小图所示为产物经打磨抛光后的内部形貌。
从图5可以看出:当碳氧摩尔比为0.8,于1 350 ℃时的还原产物呈疏松块状(图5(a)),1 450 ℃时疏松块状物内部出现了致密球状物(图5(b)),1550℃时还原产物则大部分是致密块状,只是部分表面覆盖着一层粉状物质(图5(c))。而碳氧摩尔比为1.0时的还原产物(图6)基本都是致密块状,其中1 350 ℃时,致密块状外部附着有少量粉状物(图6(a));随着温度的升高,外部附着的粉状物基本消失。
图5所示粉末状物质经筛分后的XRD分析结果如图7所示。从图7可以看出:不同温度下产物中粉状物质的成分基本相同,主要为Cr2O3,Fe2O3和Fe-Ni合金,其中Cr2O3含量较多。这进一步表明:当碳氧摩尔比低于1.0时,C主要用于NiO和Fe2O3的还原,而碳氧摩尔比的不足导致Cr2O3还原不完全,这与热力学(图2(b))及失氧曲线(图4(b))的分析结果一致。
由于当碳氧摩尔比为1.0时的还原产物基本都呈致密块状,故推测其在实验温度下基本以液相形式存在,只是在冷却时才逐渐凝固成块。为了确定上述致密状物质组成,将其打磨抛光处理后进行SEM-EDS分析,结果如图8及表2所示。
从图8可以看出:碳还原Fe2O3-Cr2O3-NiO体系所得致密块状物主要包含连续分布的浅色相(区域1)和形状不一且分布不连续的深色相(区域2和3)。从表2可见:还原产物中浅色相Fe和Ni质量分数较多,而Cr质量分数较少;深色相中Cr质量分数约为60%,Fe质量分数约为30%,而Ni质量分数极少。可见Cr多出现在深色相中,而Fe和Ni多出现在浅色相中。此外,深色相中C质量分数更高。结合前期对Fe-Cr-O体系碳还原产物的研究[15]可以确定深色相主要为铁铬碳化物,而浅色相主要为Fe-Cr-Ni-C合金。

图5 当碳氧摩尔比为0.8时还原产物在不同温度条件下的宏观形貌
Fig. 5 Macroscopic morphologies of reduction products at nC:nO=0.8

图6 当碳氧摩尔比为1.0时还原产物在不同温度条件下的宏观形貌
Fig. 6 Macroscopic morphologies of reduction products at nC:nO=1.0
图9所示为不同温度下碳氧摩尔比为1.0时致密状还原产物的SEM图。从图9可以看出:当温度为1 350 ℃时,产物中深色相(碳化物相)多呈现出相对规则的形状且分布均匀;随着温度的升高,产物中碳化物相逐渐变为片状和棒状且分布较为杂乱。关于金属液相凝固过程的研究[18]表明:液态合金在冷却时,初晶相先于共晶相析出,当初晶相是化合物时,一般具有规则外形,而共晶相主要呈片状或棒状分布。由此可知,产物中呈规则形状的深色相是初生碳化物相,可能是在冷却过程中以液态合金相中含有的高熔点碳化物粒子为核心优先长大析出的;而产物中的片状和棒状深色相为共晶相,即由高温条件得到的液态合金相在冷却过程中达到共晶温度时析出的次生碳化物相。从图9可知:产物中片状和棒状的次生碳化物相含量随着温度的升高逐渐增加,表明高温条件下更多的Fe和Cr溶于Fe-Cr-Ni-C合金相中。由热力学分析结果(图2(a))也得出类似结论。
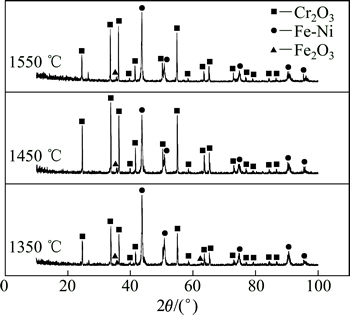
图7 当碳氧摩尔比为0.8时Fe2O3-Cr2O3-NiO体系还原产物中的粉末状物质XRD图像
Fig. 7 XRD patterns of powdery substance in reduction products at nC:nO=0.8
表2 各点成分EDS分析结果(质量分数)
Table 2 EDS results of points 1, 2 and 3 %
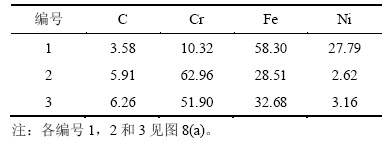
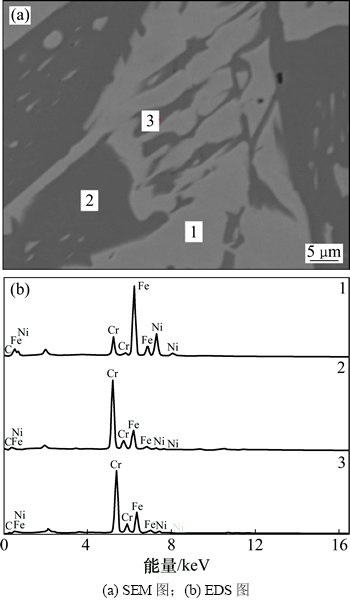
图8 Fe2O3-Cr2O3-NiO体系碳还原产物的SEM-EDS图 (θ=1 350 ℃,碳氧摩尔比1.0)
Fig. 8 SEM-EDS images for the carbothermal reduction products of Fe2O3-Cr2O3-NiO system at θ=1 350 ℃ and nC:nO=1.0
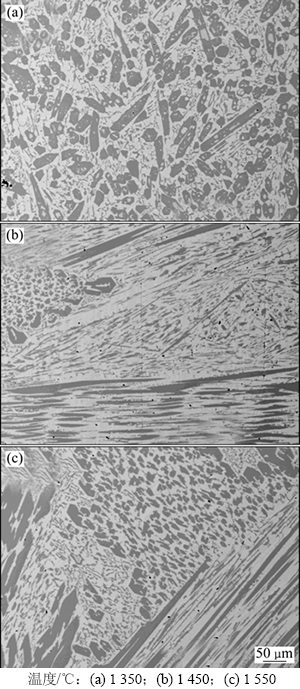
图9 不同温度下碳氧摩尔比为1.0时体系还原产物SEM图
Fig. 9 SEM images of reduction products at different temperatures and nC:nO=1.0
3.3 不同碳氧摩尔比条件下体系碳还原产物分析
对碳氧摩尔比为0.5~1.5的样品在1 550 ℃下进行还原实验,还原时间均为45 min,研究碳氧比对Fe2O3-Cr2O3-NiO体系碳还原产物的影响。
当温度为1 550 ℃时,不同碳氧摩尔比条件下还原产物的宏观形貌如图10所示。从图10可以看出:当碳氧摩尔比由0.5增加到1.0时,还原产物由疏松逐渐变得致密;当碳氧摩尔比为1.2时(图10(d)),还原产物中开始出现致密球状和粉末状物质;当碳氧摩尔比为1.5时,还原产物基本全为粉末状。对以上产物中致密块状或球状物质以及粉末状物质分别进行SEM和XRD分析。图11所示为1 550 ℃时不同碳氧摩尔比条件下还原产物的SEM图像。由图11可以看出:当碳氧摩尔比为0.8时,致密状产物中主要为浅色相(Fe-Cr-Ni-C合金相),深色相(金属碳化物相)很少且呈网状分布;当碳氧摩尔比为1.0时,致密状产物中开始出现形状规则的深色相,即初生碳化物相;当碳氧摩尔比达到1.2时,产物中初生碳化物相形状更大,质量分数更大。说明碳氧摩尔比增大会促进金属碳化物的形成,这与热力学分析(图2(b))及文献[12]中研究结果相吻合。
当碳氧摩尔比为0.5和温度1 550 ℃时,还原产物的XRD图像如图12所示。由于碳氧摩尔比达不到Fe2O3-Cr2O3-NiO体系完全还原的条件,故产物中主要以FeCr2O4,NiFe2O4和Cr2O3的形式存在的未被还原的金属氧化物,而产物中的Fe-Ni合金是由Ni和Fe氧化物优先被碳还原而生成的。
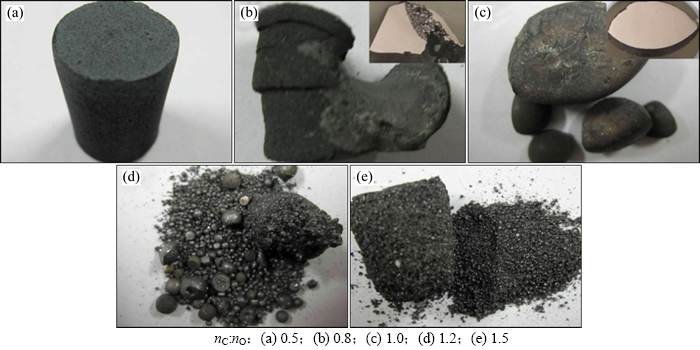
图10 不同碳氧摩尔比条件下还原产物的宏观形貌
Fig. 10 Macroscopic morphologies for carbothermal reduction products of Fe-Cr-O system
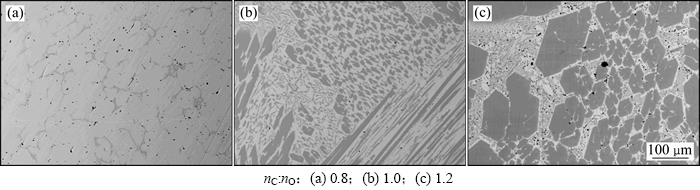
图11 1 550 ℃时不同碳氧摩尔比条件下体系还原产物SEM图
Fig. 11 SEM images of the reduction products at 1 550 ℃ and different nC:nO
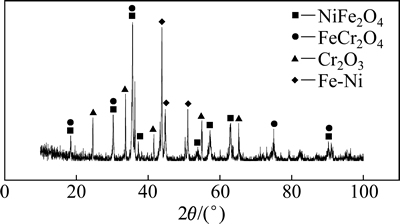
图12 当碳氧摩尔比为0.5、温度1 550 ℃时还原产物XRD图像
Fig. 12 XRD pattern of reduction products when temperature is 1 550 ℃ and nC:nO=0.5
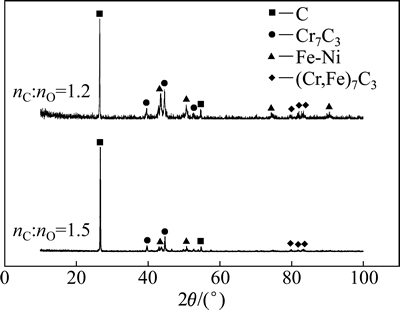
图13 当碳氧摩尔比分别为1.2和1.5时Fe2O3-Cr2O3-NiO体系碳还原产物粉状物XRD图像
Fig. 13 XRD patterns of powdery substance in reduction products when nC:nO=1.2 and nC:nO=1.5
当温度为1 550 ℃、碳氧摩尔比分别为1.2和1.5时,Fe2O3-Cr2O3-NiO体系碳还原所得粉状物的XRD图像如图13所示。从图13可以看出:二者成分基本相同,由于初始样品中的碳过量,所以,组成主要为未反应的C、金属碳化物(主要铁铬碳化物)以及少量Fe-Ni合金。
3.4 模拟不锈钢粉尘碳还原产物渣铁分离情况
实验进一步探讨碳氧比对模拟不锈钢粉尘还原产物渣铁分离情况的影响。样品组成如表1所示(编号10和11),其中CaO,SiO2和Al2O3质量占样品总质量的20%,其组成是基于CaO-SiO2-Al2O3渣系熔点最低的原则设计的(相图显示40%CaO-42%SiO2- 18%Al2O3渣系,熔点约为1 300 ℃),实验温度为1 550 ℃,初始碳氧摩尔比按1.0和1.2配制。
图14所示为不同碳氧摩尔比条件下模拟不锈钢粉尘碳还原产物的宏观形貌。从图14可以看出:当碳氧摩尔比为1.0时(图14(a)),渣铁分离效果良好。图14(a)所示中间部分致密状物质的SEM分析结果如图15所示。从图14(a)可知:主要为Fe-Cr-Ni-C合金相(深色相为冷却过程中析出的碳化物相)。而从图14(b)可知:在高碳氧摩尔比条件下(碳氧摩尔比为1.2),渣铁分离效果不好。其原因主要是生成了大量高熔点的碳化物,金属相流动性不好,导致渣铁分离困难。
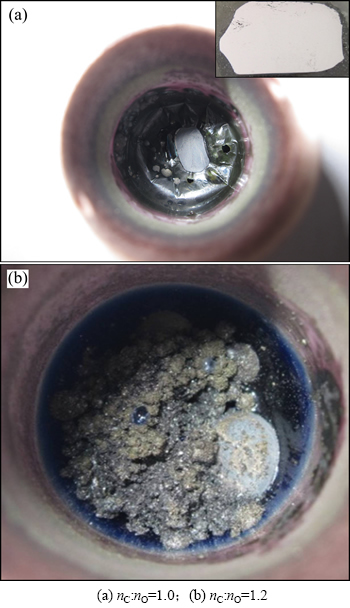
图14 模拟不锈钢粉尘碳还原产物宏观形貌
Fig. 14 Macroscopic morphologies for carbothermal reduction products of simulative stainless steel dust
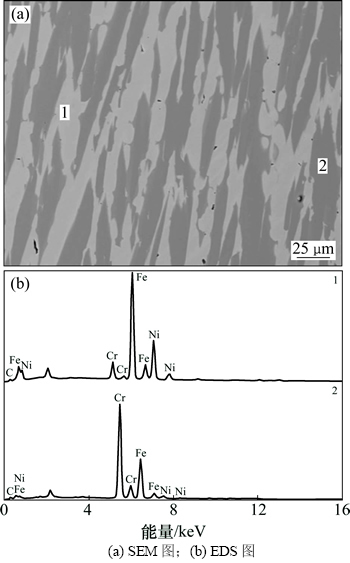
图15 模拟不锈钢粉尘碳还原产物的SEM-EDS图(θ=1 550 ℃,碳氧摩尔比1.0)
Fig. 15 SEM-EDS images for carbothermal reduction products of simulative stainless steel dust when temperature is 1 550 ℃ and nC:nO=1.0
4 结论
1) 随着温度的升高,Fe2O3-Cr2O3-NiO体系碳还原产物中首先出现金属碳化物相,当温度达到一定值后,才会出现液态合金相;当碳氧摩尔比为1.0时,样品中金属元素还原率及合金相所占比例均随温度的升高而逐渐增加;当温度为1 350 ℃时,随着碳氧摩尔比的增加,金属元素还原率及产物中合金相所占比例均有先升高后降低的趋势。
2) 还原前期基本不受温度的影响,而还原后期受温度影响明显;当碳氧摩尔比为1.0~1.5时,样品的最终失氧率与失氧速率均变化不大,而当碳氧摩尔比为0.5~1.0时,样品的最终失氧率随碳氧比的增加逐渐增大;确定前期(失氧率≤40%)主要发生的是NiO及部分Fe2O3还原,而后期主要发生Cr2O3还原且初始碳氧摩尔比重点影响的是Cr2O3的还原。
3) 温度升高会促进合金相的生成,相反会促进碳化物相的生成;当碳氧摩尔比小于1.0时,体系中的金属氧化物还原不完全,而当碳氧摩尔比大于1.0时,还原产物中容易出现金属碳化物。
4) 当碳氧摩尔比为1.0时,模拟不锈钢粉尘碳还原产物中渣铁分离效果良好,获得了流动性较好的Fe-Cr-Ni-C合金相;而当碳氧摩尔比为1.2时,由于高熔点碳化物的生成,渣铁分离不理想。
参考文献:
[1] 马国军, 范巍, 徐之浩, 等. 不锈钢厂烟尘中铬及其他元素的分布规律[J]. 过程工程学报, 2010, 10(S1): 68-71.
MA Guojun, FAN Wei, XU Zhihao, et al. Distribution behavior of chromium and other elements in the stainless steel plant dust[J]. The Chinese Journal of Process Engineering, 2010, 10(S1): 68-71.
[2] 彭兵, 张传福, 彭及. 电弧炉粉尘球团非等温还原的动力学研究[J]. 北方工业大学学报, 2000, 12(3): 52-58.
PENG Bing, ZHANG Chuanfu, PENG Ji. Research of non-isothermal reduction kinetics of EAF dust pellets[J]. Journal of North China University of Technology, 2000, 12 (3): 52-58.
[3] ZHANG Huaiwei, HONG Xin. An overview for the utilization of wastes from stainless steel industries[J]. Resources, Conservation & Recycling, 2011, 55(8): 745-754.
[4] Ma G, Garbers-Craig A M. Cr (VI) containing electric furnace dusts and filter cake from a stainless steel waste treatment plant. Part 1. Characteristics and microstructure[J]. Ironmaking and Steelmaking, 2006, 33(3): 229-237.
[5] Laforest G, Duchesne J. Characterization and leachability of electric arc furnace dust made from remelting of stainless steel[J]. Journal of Hazardous Materials, 2006, 135(1): 156-164.
[6] Tang M T, Peng J, Peng B, et al. Thermal solidification of stainless steelmaking dust[J]. Transactions of Nonferrous Metals Society of China, 2008, 18(1): 202-206.
[7] Sofilic T, Rastovcan-Mioc A, Cerjan-Stefanovic S, et al. Characterization of steel mill electric-arc furnace dust[J]. Journal of Hazardous Materials, 2004, 109(1): 59-70.
[8] 魏文洁, 张延玲, 魏芬绒, 等. Fe-Ni-O体系中Fe/Ni的还原行为[J]. 北京科技大学学报, 2013, 35(3): 288-296.
WEI Wenjie, ZHANG Yanling, WEI Fenrong, et al. Reduction behavior of Fe/Ni in the Fe-Ni-O system[J]. Journal of University of Science and Technology Beijing, 2013, 35(3): 288-296.
[9] Abdel-Halim K S, Khedr M H, Nasr M I, et al. Carbothermic reduction kinetics of nanocrystallite Fe2O3/NiO composites for the production of Fe/Ni alloy[J]. Journal of Alloys and Compounds, 2008, 463(1): 585-590.
[10] Mori T, Yang J, Kuwabara M. Mechanism of carbothermic reduction of chromium oxide[J]. ISIJ International, 2007, 47(10): 1387-1393.
[11] Khedr M H. Isothermal Reduction kinetics of Fe2O3 mixed with 1-10% Cr2O3 at 1173-1473 K[J]. ISIJ International, 2000, 40(4): 309-314.
[12] Gornerup M, Lahiri A K. Reduction of electric arc furnace slags in stainless steelmaking: Part 1 Observations[J]. Ironmaking and Steelmaking, 1998, 25(4): 317-322.
[13] Ma P, Lindblom B, Bjorkman B. Mechanism study on solid-state reduction in the Fe2O3-NiO-Cr2O3-C system using thermal analyses[J]. Scandinavian Journal of Metallurgy, 2005, 34(1): 22-30.
[14] 林万明, 陈津. 不锈钢粉尘选择性还原镍铬的研究[J]. 环境与可持续发展, 2010, 35(3): 49-51.
LIN Wanming, CHEN Jin. The investigation of selectively reduction of Ni and Cr from stainless steel dust[J]. Environment and Sustainable Development, 2010, 35(3): 49-51.
[15] ZHANG Yanling, LIU Yang, WEI Wenjie. Carbothermal reduction process of the Fe-Cr-O system[J]. International Journal of Minerals, Metallurgy and Materials, 2013, 20(10): 931-940.
[16] 郭汉杰. 冶金物理化学[M]. 北京: 冶金工业出版社, 2008: 16-17.
GUO Hanjie. Metallurgical physical chemistry[M]. Beijing: Metallurgical Industry Press, 2008: 16-17.
[17] 郭文明, 张延玲, 刘洋, 等. Fe-Cr-Ni-O体系碳还原动力学[C]//冶金反应工程学会议论文集. 太原, 2013: 537-549.
GUO Wenming, ZHANG Yanling, LIU Yang, et al. Reduction kinetics of Fe-Cr-Ni-O system with carbon[C]//Metallurgical Reaction Engineering Conference of China. Taiyuan, 2013: 537-549.
[18] 丁建生. 金属学与热处理[M]. 北京: 机械工业出版社, 2010: 42-43.
DING Jiansheng. Metal science and heat treatment[M]. Beijing: China Machine Press, 2010: 42-43.
(编辑 罗金花)
收稿日期:2014-10-15;修回日期:2014-12-15
基金项目(Foundation item):国家自然科学基金资助项目(51474021);北京科技大学钢铁冶金新技术自主研发课题(41602005)(Project (51474021) supported by the National Natural Science Foundation of China; Project (41602005) supported by Independent Research and Development of Advanced Metallurgy of University of Science and Technology Beijing)
通信作者:张延玲,博士,副教授,从事冶金二次资源综合利用研究;E-mail:zhangyanling@metall.ustb.edu.cn