高纯硼酸的制备及其影响因素
龚殿婷1,李凤华2,樊占国2,姜涛2
(1. 辽宁石油化工大学 职业技术学院,辽宁 抚顺,113001;
2. 东北大学 材料与冶金学院,辽宁 沈阳,110004)
摘 要:以分析纯硼砂为原料,通过对传统的硼酸制备方法加以改进,采用氯化氢气体代替盐酸溶液中和硼砂,从而减少杂质的引入。结合离子交换吸附树脂法与重结晶法,制备出纯度达到99.99%的高纯硼酸。考察制备过程中相关因素对硼酸成品纯度与结晶率的影响。得到中和反应最佳工艺条件为:加酸温度90 ℃,pH 3~4,结晶温度8 ℃,结晶时间8 h;离子交换吸附最佳工艺条件为:阴离子与阳离子体积比3?1,溶液流速8~10 mL/min;重结晶最佳工艺条件为:硼酸溶液中硼酸质量分数为28.5%~31.0%,结晶温度10 ℃,采用慢速搅拌结晶15 h。硼酸晶体呈细小颗粒状,其Fe,Cl-和SO42-杂质含量符合高纯硼酸中的杂质含量指标。
关键词:高纯硼酸;酸化;重结晶;离子交换
中图分类号:TQ016.1 文献标志码:A 文章编号:1672-7207(2010)05-1756-05
Preparation of high purity boric acid and its influencing factors
GONG Dian-ting1, LI Feng-hua2, FAN Zhan-guo2, JIANG Tao2
(1. Vocational Technology College, Liaoning Shihua University, Fushun 113001, China;
2. School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China)
Abstract:Boric acid with high purity of 99.99% was prepared from borax with modified traditional techniques, including adopting HCl gas instead of chlorhydric acid solution to acidize borax, thereby reducing the introduction of impurities, ion exchange adsorption resin method and recrystallization process. The related effects of the three procedures on the purity and crystallization ratio of boric acid were investigated. The results show that the optimal experimental parameters during neutralization reaction are as follows: acidolysis temperature is 90 ℃, pH is 3-4, crystallization is under the condition of 8 ℃ for 8 h. During ion exchange adsorption, the volume ratio of anion versus cation is 3?1, the current velocity of boric acid solution is 8-10 mL/min. During recrystallization process, the concentration of boric acid solution is 28.5%-31.0%, and crystallization is at 10 ℃ for 15 h associated with slow stirring. Using scanning electronic microscope the final boric acid crystals are observed to be granular and fine. The impurity contents of ferrum, chloride and sulphate in the finished product meet the index of high purity boric acid.
Key words: high purity boric acid; acidification; recrystallization; ion exchange process
硼酸是一种多功能的化工原料,在国民经济及工农业生产发展中占有重要的地位。由于传统制备工艺与低品位硼资源的限制,高纯硼酸在国内不易获得,主要依赖于进口。近年来,大量的进口高纯硼酸进入我国市场,与国内产品相比,国外硼酸各项指标均远远高于国内产品的同类指标。高纯硼酸用作高纯试剂及生产各种高纯硼酸盐晶体原料,在高科技领域中应用较广。在核工业中,大都使用核级天然硼酸代替同位素10B作为中子慢化剂、捕集剂和冷却剂,并且用量很大[1]。硼砂的酸化中和法技术成熟,是国内外传统的硼酸生产方法[2-3],但传统工艺制备的硼酸成品中杂质含量较高,硼酸的纯度只能达到试剂标准,远远不能满足于国防科技中核级硼酸对纯度和杂质指标的要求。从工艺控制方面出发,改进硼酸生产的技术水平,提高产品的结晶完整性,减少杂质吸附,对提高产品质量是很关键的。为此,本文作者在传统硼酸制备工艺的基础上,以分析纯硼砂为原料,通过酸化并结合离子交换吸附树脂法及重结晶法制备高纯硼酸。由于影响结晶过程与晶体形貌的因素很多,如溶液的过饱和度、酸度、结晶温度、结晶时间、搅拌速度、强磁场作用以及溶液中存在的杂质等[4-8],因此,本文作者着重探讨高纯硼酸制备过程中各步工艺相关因素对硼酸纯度与结晶率的影响。
1 实验
1.1 HCl气体中和反应法制备粗硼酸
准确称取一定量分析纯硼砂于去离子水中,磁力搅拌加热至95 ℃,完全溶解后,保持滤液温度在85 ℃左右,用石英砂漏斗快速过滤。利用恒温水浴对滤液加热至90 ℃,采用自制启普发生器,通入HCl气体中和硼砂反应,控制温度不超过102 ℃,反应3~5 min后,检测溶液酸度pH为3~4。将反应好的物料选取多种降温方式,最后放入8 ℃低温恒温槽中,恒温冷却结晶8 h后,离心分离,真空吸滤,洗涤,用精密pH试纸检测结晶体表面pH为3~4。将结晶体置于50 ℃真空干燥箱中烘干后即得硼酸粗品。母液中含有少量硼酸和钠盐,返回酸解中和工序,循环利用。
1.2 离子交换吸附法制备二次硼酸
将粗品硼酸配制成不饱和溶液,加热至50~60 ℃使硼酸溶解,溶解后趁热用石英砂过滤,冷却后将滤液注入处理过的强酸强碱混合床离子交换柱中,控制流速为8~10 mL/min。将经过离子交换的溶液低温加热浓缩,再经微孔滤膜过滤后置于密封塑料杯中,放入5 ℃低温恒温槽冷却,使硼酸结晶析出,经离心分离,真空吸滤,洗涤,真空干燥得二次硼酸粗品。
1.3 重结晶制备高纯硼酸
将二次硼酸粗品配制为饱和溶液,采用冷凝回流装置加热直至溶质全部溶解,先快速降温至50 ℃,再缓慢降温至10 ℃于低温恒温槽中冷却结晶。离心分离后,用石英砂抽滤,并用少量高纯水喷雾洗涤多次并抽干。将结晶的硼酸置于50 ℃真空干燥箱中,烘2 h。打开真空干燥箱,擦去箱内水分,并继续在30~40 ℃烘干样品,从而得到硼酸成品。
在扫描电镜下观察硼酸结晶体形貌,采用氢氧化钠电位滴定法检测硼酸产品的纯度,采用分光光度法测定硼酸中铁含量,采用光电比浊法测定氯化物及硫酸盐杂质含量[9]。
2 结果与讨论
2.1 中和反应制备粗硼酸过程中的影响因素
工业上采用盐酸或硫酸生产硼酸,粗产品纯度可达99.00%,经提纯可达分析纯(99.50%)级别。采用气相HCl取代液相盐酸减少了杂质污染,硼酸纯度可达99.70%。中和反应是化学试剂提纯的一种手段,在提纯过程中,要严格控制掌握中和反应的规律,主要是控制酸碱度即反应液pH、中和(酸解)初始温度、溶液的固液比、结晶温度、结晶时间、搅拌速度等反应 条件。
pH对反应进行与硼酸晶体的形状都有一定影响。若酸加入量不足,则减缓硼酸晶核的析出,容易形成大的鳞片状晶体,同时硼砂转化不完全,剩余的硼砂混入硼酸晶体中,将影响产品质量;若酸过量,则硼酸晶核的形成速度太快,结晶体细小,同时酸性太强易使设备受到腐蚀。本实验利用启普发生器,通入HCl气体进行中和反应,不仅可控制气体流量,还避免了传统工艺中由加酸酸化而引入酸中杂质。实验中控制反应溶液pH分别为1~2,2~3,3~4,4~5和5~6,所得硼酸纯度与结晶率如图1所示。
从图1可见:当反应溶液pH值为2~3和3~4时,硼酸的纯度均到达99.6%(质量分数)以上,结晶率大于85%(质量分数)。工厂生产硼酸时,在纯度达到要求后,主要考虑产品的产出率,通常会选择pH为2~3。实验以制备高纯硼酸为目的,因此,选取反应液最佳pH为3~4。
在酸解反应过程中,提高浸出温度,有利于反应的进行。由于硼酸具有挥发性,工业上将中和初始温度选择75~85 ℃。实验中选取酸解温度分别为20,75,80,85,90和95 ℃,酸解初始温度对硼酸结晶率和纯度的影响见图2。从图2可以看出,当中和初始温度为90 ℃时,结晶率超过85%(质量分数),硼酸纯度达到99.7%以上,相对于工业选取温度,结晶率稍低,但纯度有所提高。
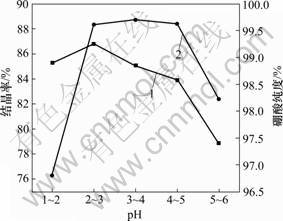
1—结晶率;2—硼酸纯度
图1 不同pH对硼酸结晶率和纯度的影响
Fig.1 Effects of different pH values on crystallization of boric acid
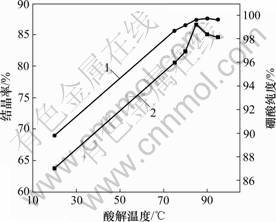
1—结晶率;2—硼酸纯度
图2 酸解初始温度对硼酸结晶率和纯度的影响
Fig.2 Effects of acidolysis temperature on crystallization of boric acid
固液相的溶解反应为一个逐步的过程,当溶液处于饱和状态时,结晶物质才从水溶液中析出[10]。通常溶液可处于3种状态:稳定态、介稳态和不稳定态。在理想状态下,硼酸的结晶过程是在介稳态区域进行的。选取合适的固液比有利于硼酸晶体的析出,提高硼酸产出率。实验中选取硼砂与水质量比为1?2与1?3,所得硼酸纯度分别为99.74%与99.75%,相差仅为0.01%,而所得硼酸结晶率分别为85.86%与68.42%,二者相差17.44%。因此,确定最佳固液比为1?2。
结晶温度的选择主要依据物质在不同温度下的溶解度变化。硼酸的溶解度随温度升高而增大,而氯化钠的溶解度受温度影响很小,因此,可通过控温冷却分离硼酸和氯化钠。实验分别选取结晶温度为5,8,10和15 ℃,所得实验结果见图3。从图3可见:在5~15 ℃时,硼酸纯度均在99.6%以上,结晶率相差不大;在10 ℃以下时,结晶率超过85%,本实验选择结晶温度为5~8 ℃。
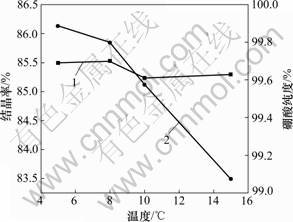
1—结晶率;2—硼酸纯度
图3 不同结晶温度对硼酸结晶率和纯度的影响
Fig.3 Effects of different crystallization temperatures on crystallization of boric acid
随着温度的降低,过饱和度在介稳区不断上升,晶核的形成与生长同时进行。实验中选取2种降温方式:自然冷却与快速降温。结果发现:不同的降温方式对硼酸的结晶率影响不大[11]。在光学显微镜下观察结晶体,自然冷却得到的晶体为大的鳞片状,而快速冷却得到的晶体中有细小颗粒状,但粒径很小。可见冷却速度对晶体的形状影响很大。实验中发现采用高温时快速冷却,待降至一定温度后,再中速降温,最后缓慢冷却,可以保证硼酸结晶体多数为具有一定粒度的颗粒。
浓度随时间的变化在一定程度上反映了结晶动力学。在结晶过程中,溶液中存在浓度梯度,在开始的某段时间内,浓度实际上保持不变或变化较小。若结晶时间短,则溶液中呈固液溶解平衡状态,晶粒较小;若时间足够长,则自然将有利于晶粒的持续增长。若结晶时间过度延长(如几天),则已析出的硼酸晶体则会有结块趋势[12],而且会增大制冷能耗。实验中当结晶时间超过8 h时,结晶率达到85%以上,晶体无结块。
搅拌溶液,可加快晶核出现。搅拌速度过快,会有一些晶体受到磨损而产生破碎微粒,但不改变晶型;搅拌速度过慢,晶粒易结成晶簇,将母液包藏在晶粒间,会降低产品的纯度。在晶相大量析出阶段,应停止搅拌,否则,聚集体将被破坏,同时会增加能耗。 Sahin等[13]研究了50~200 r/min的搅拌速度对硼酸结晶的影响,发现当搅拌速度为200 r/min时,硼酸的结晶率及晶体形状比较理想。本实验将搅拌速度增加为300~1 200 r/min。通过实验发现:慢速搅拌(约300 r/min)有助于硼酸晶体的生成,结晶率在99.70%附近,在光学显微镜下观察,晶体呈细小颗粒状。
2.2 离子交换树脂吸附提纯过程中的影响因素
实验采用混合床进行离子交换,交换装置中添加滤膜过滤,可去除漂浮于溶液表面的浮尘以及树脂预处理时残留的大孔径有机污染物等杂质。离子交换树脂选用732号强酸性阳离子与717号强碱性阴离子交换树脂。当硼酸溶液流过离子交换树脂柱时,溶液中的Fe3+,Al3+,Ca2+,Mg2+,Cu2+,Pb2+,Zn2+,Na+和NH4+等被强酸性树脂所交换,而硼酸溶液中的杂质阴离子(如SO42-,NO3-和Cl-等)被强碱性树脂所交换。虽然强碱性阴树脂对硼酸也有一定的吸附作用,但是较SO42-和Cl-等解离度大的酸根离子来说要弱得多。若使用弱碱性阴离子交换树脂,虽不吸附硼酸,但是,对中强度和较弱的酸根(如PO43-和S2-等)除去能力较小[14]。考虑实验原料的纯度及高纯硼酸中各杂质含量指标,离子交换的重点放在对溶液中阴离子的吸附,故实验中选用阴、阳离子树脂体积比为3?1。
离子交换过程主要包括:离子从溶液通过树脂颗粒表面液膜扩散到树脂颗粒的表面,离子从颗粒表面扩散到颗粒内部交换基团,基团上的离子交换、被交换下来的离子从基团处向树脂颗粒表面扩散,离子通过表面液膜向溶液中扩散[14]。流速是离子交换过程中的重要因素。流速过低,树脂外层液膜加厚,离子穿透液膜的时间增长,影响交换速度,不利于交换反应的进行;过高的流速虽然使液膜减薄,缩短了液相与树脂的接触时间,但增加了工作层厚度,降低成树脂工作交换容量,使交换时间过长,会造成水质恶化和树脂破损。实验中选择流速为8~10 mL/min,交换时间在40 min之内,交换层高度为30 mm左右,始漏量大于96%,既提高膜扩散速度,又使离子与树脂有足够的反应时间,可保证交换的顺利进行。
2.3 重结晶过程中的影响因素
在中和反应过程中单因素实验的基础上设计了正交实验,研究不同影响因素同时作用时硼酸溶液结晶率的变化规律及各因素的影响主次顺序,从而确定硼酸溶液重结晶实验的最佳实验条件。分别选取硼酸溶液中硼酸质量分数(26.0%,28.5%和31.0%)、结晶温度(5,10和15 ℃)、结晶时间(10,15和20 h)、搅拌强度(0,300和1 000 r/min)为正交因素,设计了4因素3水平正交实验[15]。在选定的实验条件下,对硼酸重结晶影响因素正交实验进行的单指标极差分析。结果表明:4因素的极差顺序为:R浓度>R结晶温度>R结晶时间>R搅拌强度,即影响硼酸重结晶的主要影响因素为硼酸溶液中硼酸的质量分数和结晶温度,结晶时间次之,搅拌强度的影响最小。该结果与单因素实验结果一致。同时,正交实验结果表明:硼酸溶液中硼酸质量分数为28.5%~31.0%,最终结晶温度为10 ℃,硼酸的结晶时间为15 h,采用慢速搅拌为重结晶的最优条件,最终所得硼酸产品纯度可达到99.99%。
3 硼酸的实验检测
实验制备的高纯度硼酸与分析纯硼酸的SEM像如图4所示。由图4可以看出:分析纯硼酸呈大而密集的片状或块状,而本实验制得的高纯硼酸则呈现分散的颗粒状,但颗粒粒径不均匀。
将进口高纯硼酸中主要杂质含量指标与分析纯硼酸比较,发现仅铁(Fe)、氯化物(以Cl-计)和硫酸盐(以

(a) 分析纯硼酸;(b) 实验制备高纯度硼酸
图4 硼酸的SEM像
Fig.4 SEM images of boric acid
SO42-计)的含量远小于分析纯指标。由于实验用水为去离子水,实验中没有引入其他杂质,因此,对照高纯硼酸中各项杂质指标,本研究主要检测硼酸产品的纯度以及硼酸中铁、氯化物、硫酸盐杂质含量。硼酸纯度采用氢氧化钠电位滴定法测定,Fe含量采用分光光度法测定,Cl-和SO42-采用光电比浊法测定。经测定,硼酸产品纯度可达到99.99%,Fe含量小于0.000 2%,Cl-含量小于0.000 1%,SO42-含量小于 0.000 3%,结果均在高纯硼酸出厂标签所示含量范 围内。
4 结论
(1) 通过改变现有硼砂酸化过程中的反应工艺条件,采用HCl气体代替传统工艺中加入盐酸中和,避免了直接加入盐酸溶液引入的杂质,实验中无废气排出。得到最佳工艺条件如下:加酸温度为90 ℃,溶液pH为3~4,酸化浸出温度为95~100 ℃,硼酸结晶温度为8 ℃,结晶时间为8 h。制备的粗硼酸纯度可提高到99.70%以上。
(2) 采用强酸强碱树脂混合床提纯硼酸,其中717号强碱性阴离子与732号强酸性阳离子体积比为3?1,流速为8~10 mL/min,产品的纯度可提高至99.90%。
(3) 在重结晶过程中,控制硼酸溶液中硼酸质量分数为28.5%~31.0%,硼酸结晶率可达88%以上,降低结晶温度至10 ℃,延长结晶时间至15 h附近,采用慢速搅拌结晶并选取适当的降温方式,所得硼酸晶体呈细小颗粒状。重结晶后硼酸产品纯度可达到99.99%,其中所测Fe,Cl-和SO42-杂质含量在高纯硼酸出厂标签所示含量范围内。
参考文献:
[1] Kumar S D, Venkatesh K, Maiti B. Determination of chloride in nuclear-grade boron carbide by ion chromatography[J]. Chromatographia, 2004, 59(3/4): 243-245.
[2] Mergen A, Demirhan M H, Bilen M. Processing of boric acid from borax by a wet chemical method[J]. Advanced Powder Technol, 2003, 14(3): 279-293.
[3] 钟耀荣. 硫酸法制硼酸过程中提高回收率的研究[J]. 化工矿山技术, 1996, 25(4): 35-37.
ZHONG Yao-rong. Study on improving recovery rate of sulfuric acid process to prepare boric acid[J]. Chemical Mining Technology, 1996, 25(4): 35-37.
[4] Li L, Chen S. A study on role of physical fields in sucrose crystallization[J]. South China University of Technology, 1994, 22(3): 50-59.
[5] 赵龙涛, 李入林, 张明海. 影响硼酸结晶形状的因素[J]. 海湖盐与化工, 2003, 32(5): 15-17.
ZHAO Long-tao, LI Ru-lin, ZHANG Ming-hai. Impact factors on the morphology of boric acid crystal[J]. Sea Lake Salt and Chemical, 2003, 32(5): 15-17.
[6] Sahin ?, Bulutcu A N. Effect of electrical field on dendritic growth of boric acid[J]. Crystal Research and Technology, 2003, 38 (1): 47-55.
[7] Sahin ?, ?zdemir M, Kendirci H, et al. Determination of growth and dissolution mass rate of boric acid[J]. Journal of Crystal Growth, 2000, 219(1/2): 75-82.
[8] Tuna H H,Epslein A,Sowa M,et al. Proceedings of international symposium on industrial crystallization[M]. Beijing:Academic Press, 1998: 138-141.
[9] GB 628—93. 化学试剂-测定通用方法[S].
GB 628—93. Chemical reagent-general method of determination [S].
[10] Bechtlff B. The kinetics of heterogeneous solid-liquid reaction crystallizations-an overview and examples[J]. Chemie Ingenieur Technik, 2001, 73(5): 453-460.
[11] 龚殿婷, 刘湘倩, 李凤华, 等. 硼砂酸化法制备硼酸过程中影响因素研究[J]. 硅酸盐通报, 2008, 27(5): 1051-1054.
GONG Dian-ting, LIU Xiang-qian, LI Feng-hua, et al. Study of effect factors in boric acid preparation by borax acidification[J]. Bulletin of the Chinese Ceramic Society, 2008, 27(5): 1051-1054.
[12] Cleaver J A S, Karatzasa G, Louisa S, et al. Moisture-induced caking of boric acid powder[J]. Powder Technology, 2004, 146(1/2): 93-101.
[13] Sahin ?. Effect of borax on the crystallization kinetics of boric acid[J]. Journal of Crystal Growth, 2002, 236(1/3): 393-399.
[14] 王广珠, 汪德良, 崔焕芳. 离子交换树脂使用及诊断技术[M]. 北京: 化学工业出版社, 2004: 72-74.
WANG Guang-zhu, WANG De-liang, CUI Huan-fang. Application of ion exchange resin and diagnostic techniques[M]. Beijing: Chemical Industry Press, 2004: 72-74.
[15] 龚殿婷, 李凤华, 樊占国, 等. 硼酸重结晶过程影响因素研究[J]. 东北大学学报: 自然科学版, 2008, 29(12): 1734-1737.
GONG Dian-ting, LI Feng-hua, FAN Zhan-guo, et al. Studies on influence factors of H3BO3 recrystallization[J]. Journal of Northeastern University: Natural Science, 2008, 29(12): 1734-1737.
(编辑 陈爱华)
收稿日期:2009-10-23;修回日期:2010-01-15
基金项目:国家高技术研究发展计划(“863”计划)项目(2006AA06Z368)
通信作者:李凤华(1977-),女,湖北荆门人,博士,讲师,从事生态环境材料研究;电话:024-83687727,13700049607;E-mail: lifh@smm.neu.edu.cn