纤蛇纹石吸附Cu(Ⅱ)的动力学及热力学研究
冯其明,王倩,刘琨,欧乐明,张国范,卢毅屏
(中南大学 资源加工与生物工程学院,湖南 长沙,410083)
摘要:研究纤蛇纹石对铜离子的吸附行为,探讨初始溶液pH、温度和铜离子初始浓度对吸附动力学的影响,进行吸附等温线的测定和热力学计算。研究结果表明:当温度为25~60 ℃,pH为2~4,铜离子初始浓度为10~100 mmol/L时,Cu(Ⅱ)的吸附动力学数据均符合准二级反应动力学模型;吸附量随反应温度、初始pH和溶液初始浓度的增加而增加;等温吸附曲线符合Langmuir等温吸附模型,吸附过程以单层吸附为主;反应的吉布斯自由能为负值,焓变为20.427 kJ/mol,熵变为109.424 J/(mol·K),说明吸附是一个自发进行的物理吸附过程。
关键词:纤蛇纹石;铜离子;动力学;吸附等温线;热力学
中图分类号:X70 文献标志码:A 文章编号:1672-7207(2011)11-3225-07
Adsorption kinetics and thermodynamics of
copper (Ⅱ) on chrysotile
FENG Qi-ming, WANG Qian, LIU Kun, OU Le-ming, ZHANG Guo-fan, LU Yi-ping
(School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: The adsorption behaviour of Copper (Ⅱ) on chrysotile was investigated. The effects of initial pH, temperature and initial concentration of Copper (Ⅱ) on the kinetics were researched, and the determination of adsorption isotherms and thermodynamic calculations were carried out. The results show that with the experimental parameters of temperature of 25-60 ℃, pH of 2-4, the initial concentration of Copper (Ⅱ) of 10-100 mmol/L, the adsorption processes agree with pseudo-second order rate equation. The copper adsorption capacity of chrysotile increases with the increase of the reaction temperature, initial pH and initial concentration. Adsorption isotherms correlate well with the Langmuir isotherm model and the adsorption process is mainly single-layer. The Gibbs free energy is negative, ΔH and ΔS are 20.427 kJ/mol, and 109.424 J/(mol·K), respectively, which indicates the adsorption is a spontaneous, endothermic and physical process.
Key words: chrysotile; copper ions; kinetics; isotherm; thermodynamics
随着工业生产的迅速发展,含重金属离子的废水排放量逐年增加。铜作为重金属之一,在有色、化工、钢铁、采选、冶炼等产生的废水中大量存在。人类对铜的摄入量过多会直接导致肝脏和肾脏受损、蔓延性毛细血管损伤、抑郁、肠胃发炎坏死等[1],因此,人们对含铜废水的处理日益重视。处理含铜废水的方法主要有化学沉淀法、絮凝法、反渗透法、离子交换法和吸附法[2],其中,吸附法因治理成本低、操作方便和回收利用率高被广泛采用。现有报道的天然吸附质有油页岩[3]、黏土[4]、泥炭[5]、海泡石[6]等。纤蛇纹石是典型的三八面体层状结构硅酸盐矿物,其晶体构造单元为硅氧四面体和氢氧镁石八面体。目前,对纤蛇纹石的研究主要集中在工艺方面,杨保俊等[7-10]利用纤蛇纹石酸浸工艺提取纳米氢氧化镁及白炭黑。纤蛇纹石因有很大的比表面积和大量的不饱和键,而具有一定的吸附性能[11]。杨志宽[12]通过研究发现煅烧后的蛇纹石对含铜废水有较好的吸附性能,郭继香等[13]用蛇纹石处理合成水样及石油污水。Fonseca等[14]由蛇纹石改性前后吸附电位的变化,推断了吸附机理。以上研究大都局限于将纤蛇纹石进行化学处理后再进行单一试验因素对吸附效果的影响,未涉及纤蛇纹石本身的吸附性能及吸附过程的动力学、热力学和相关吸附机理的研究,其研究结果缺乏理论性和系统性。针对这个问题,本文作者研究了不同温度、pH、铜离子初始浓度下纤蛇纹石对溶液中Cu(Ⅱ)的吸附动力学行为;通过测定吸附等温线得到吸附等温模型;计算吸附热力学参数,推断纤蛇纹石对Cu(Ⅱ)的吸附机理。
1 实验
1.1 实验材料
实验所用纤蛇纹石样品为青海祁连小八宝石棉矿产5-70级纤蛇纹石石棉,呈淡灰黄色的纤维状集合体。采用水洗提纯工艺对该石棉进行提纯处理,所得纤蛇纹石纯度(质量分数)达95%以上,仅含少量水镁石。所用硫酸铜、硫酸均为化学纯,实验用水为去离子水。
1.2 吸附动力学
取不同浓度Cu2+溶液100 mL置于250 mL锥形瓶中,用硫酸调节pH。加入1.0 g纤蛇纹石。将锥形瓶置于恒温振荡器中振荡吸附,振荡速度为100 r/min。在不同时间取样、过滤、洗涤,收集滤液和洗液并定容至500 mL,继而采用原子吸收光谱法测定液相中残留的Cu2+浓度。t时刻的吸附量qt按下式计算:
qt =(c0×0.1-ce×0.5)/1.0 (1)
其中:c0为初始溶液中Cu2+浓度,mmol/L;ce 为液相中残留Cu2+浓度,mmol/L。
1.3 等温吸附曲线
分别在温度为25,40和60 ℃下,将不同浓度的Cu2+溶液各100 mL加入到250 mL锥形瓶中,用1×10-4 mol/L硫酸调节pH为4.0。加入1.0 g纤蛇纹石,置于温度为40 ℃的恒温振荡箱中振荡20 h后,过滤、洗涤,并收集滤液和洗液进行定容,采用原子吸收光谱法测定液相中残留的Cu2+浓度。
1.4 吸附热力学
热力学参数包括吉布斯自由能变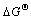 、焓变
、焓变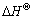 和熵变
和熵变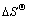 。Van’t Hoff 方程计算式如下:
。Van’t Hoff 方程计算式如下:
 (2)
(2)
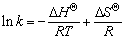 (3)
(3)
其中:k为等温吸附常数;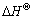 与
与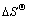 可分别由lnk与1 000/T的直线斜率和截距求得;R为摩尔气体常数(8.314 J/(mol·K));T为热力学温度,K。
可分别由lnk与1 000/T的直线斜率和截距求得;R为摩尔气体常数(8.314 J/(mol·K));T为热力学温度,K。
2 结果和讨论
2.1 温度对动力学的影响
图1所示为不同温度对纤蛇纹石吸附Cu2+动力学的影响,所考察的浓度为50 mmol/L,pH为4.0,温度分别为25,40和60 ℃。从图1可见:总体上,随着温度的升高,吸附量逐渐增大;在初始20 min内,吸附量增加很快;随着时间延长,吸附量增速逐渐减慢,最后达到吸附平衡;在不同温度下,达到吸附平衡的时间基本相同。
吸附过程直接用准二级动力学方程拟合[15]。准二级吸附速率方程的线性表达式[16]为:
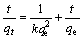 ,
, (4)
(4)
其中:qt为t时刻的吸附量,mmol/g;qe为平衡时刻的吸附量,mmol/g;K为准二级反应吸附速率常数,g/(mmol·min);H为初始吸附速率常数,mmol/(g·min)。
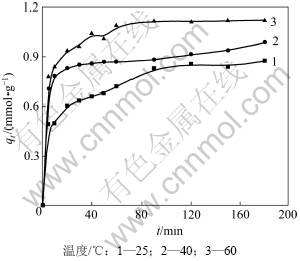
图1 温度对吸附动力学的影响
Fig.1 Effect of temperature on adsorption kinetics
利用式(4)对实验数据进行线性拟合,结果见图2和表1。由表1可以看出:用准二级动力学方程拟合的相关性系数较高(拟合系数R2>0.99),故吸附过程符合准二级动力学吸附模型,即二级动力学模型相关的所有过程中,外部液膜扩散、表面吸附和颗粒内部扩散都能用于描述纤蛇纹石对Cu2+的吸附过程。当温度由25 ℃增加到60 ℃时,初始速率常数H由9.860× 10-2 mmol/(g·min)增加到34.130×10-2 mmol/(g·min),准二级反应吸附速率常数K由0.122 g/(mmol·min)增加到0.263 g/(mmol·min)。这主要是因为升温加快了Cu2+的热运动,提高了Cu2+在溶液中的扩散速率[17];另外,在升温过程中,铜离子溶液的黏度会降低[18],使溶液中Cu2+透过液膜吸附到纤蛇纹石表面和接触活性位点的速率加快,单位时间内吸附的量增加。温度升高后吸附量由0.901 mmol/g增加到1.140 mmol/g,这与实际测得的吸附量相差很小,说明反应过程是一个吸热的过程,升温有利于吸附的进行。
综合上述分析可以看出:吸附温度通过影响铜离子的热运动,达到提高初始反应速率和平均反应速率的目的,使吸附量随着温度的升高而增大。
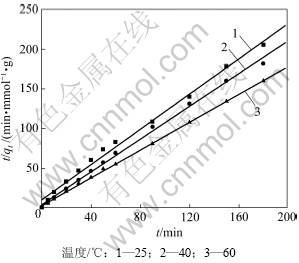
图2 准二级动力学方程的线性拟合
Fig.2 Linear fit of pseudo-second order equation
表1 温度对纤蛇纹石吸附铜离子的影响参数
Table 1 Effect parameters of temperature on copper ion adsorption

2.2 pH对动力学的影响
溶液pH是影响吸附速率的一个重要因素。本研究所考察的pH分别为2,3和4,初始溶液浓度为50 mmol/L,温度为40 ℃,所得pH对Cu2+吸附动力学的影响见图3。由图3可看出:随pH升高,吸附量由0.5 mmol/g增加到0.988 mmol/g。说明高pH利于吸附的进行。在同一pH下,吸附量在初始20 min内基本达到饱和;而后随着时间延长,吸附量增大趋势缓慢。
用式(4)对数据进行线性拟合,曲线和拟合参数分别见图4和表2。由表2可以看出:线性相关系数R2为0.997,表明吸附符合准二级动力学方程;Cu2+吸附到纤蛇纹石表面后,直接与纤蛇纹石外羟基上的氢质子发生离子交换[14],释放氢质子;溶液pH越低,溶液中存在的H+量越多,反应向左移动,不利于离子交换的进行,所以,平均反应速率常数随着pH减小而减小。在反应初始阶段,纤蛇纹石表面的OH-和Mg2+先部分解离进入弱酸性水溶液中,使溶液pH略微升高,利于Cu2+与氢质子交换,此时,吸附在短时间内基本达到饱和,所以,初始反应速率常数H随着pH增大而增大。随着置换出的氢质子浓度增加,溶液pH降低到初始pH附近,吸附速度逐渐减小。
2Si—O—Mg—OH+Cu2+=(Si—O—Mg—O)2Cu+2H+
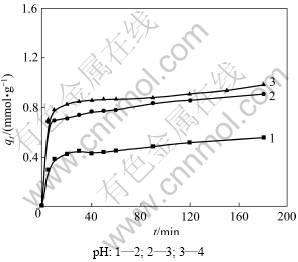
图3 pH对铜离子吸附动力学的影响
Fig.3 Effect of solution pH on copper (Ⅱ) adsorption kinetics
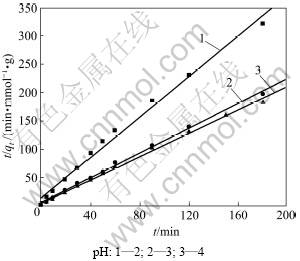
图4 准二级动力学方程的线性拟合
Fig.4 Linear fit of pseudo-second order equation
表2 pH对铜离子吸附的影响参数
Table 2 Effect parameters of pH on copper ion adsorption
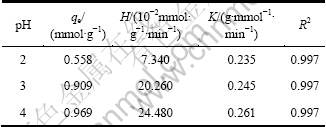
李学军等[11]认为在弱酸到中性条件下,产生氢氧化铜沉淀是纤蛇纹石对铜离子吸附的主要原因。溶液化学理论[19]计算结果指出:试验所用浓度为50 mmol/L Cu2+溶液产生沉淀时pH为4.99,而试验过程中测得的pH一直在4.85以下,没有达到沉淀产生所需pH,氢氧化铜沉淀也不可能生成。所以,“纤蛇纹石对铜离子吸附应以离子交换为主,产生氢氧化铜沉淀”的观点还有待研究。
由表2可看到:随着pH增大,理论上的饱和吸附量从0.558 mmol/g增加到0.969 mmol/g,这与试验结果基本吻合;溶液pH降低,纤蛇纹石表面的羟基和Mg2+更易解离进入溶液,能参加反应的羟基数量减少,造成吸附量减小。
2.3 Cu2+初始浓度对动力学的影响
图5所示为Cu2+初始浓度对吸附动力学的影响。考察的初始浓度为10,50和100 mmol/L,温度为40 ℃,pH为4.0。由图5可以看出:纤蛇纹石平衡吸附量随初始浓度的增加而增加。
用式(4)拟合,所得拟合结果见图6及表3。由表3可见:R2均大于0.990,表明与准二级动力学模型有良好的拟合性;在初始浓度为10~100 mmol/L时,初始反应速率常数和平均反应速率常数与初始浓度的变化趋势一致。这主要是因为溶液初始浓度为铜离子克服液相和固相阻力提供驱动力[20]。当溶液浓度增大时,单位铜离子通过液相、透过滤膜的速度加快,使初始反应速率增大;当纤蛇纹石开始接触铜离子溶液时,它表面的铜离子浓度也一直在增加。因加入的纤蛇纹石量一定,故纤蛇纹石表面能提供的进行离子交换的外羟基数量也有限。化学计量学表明:1 mol Cu2+能置换2 mol H+。当初始浓度低时,纤蛇纹石可以提供充足的氢质子用于吸附反应,吸附量逐渐增加。但当铜离子浓度较高时,活性位点则相对不足,吸附迅速达到饱和后,吸附量不再增长,吸附曲线最终也趋于平缓。
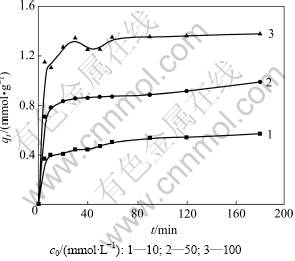
图5 铜离子初始浓度对吸附动力学的影响
Fig.5 Effect of Cu(Ⅱ)initial concentration on adsorption kinetics
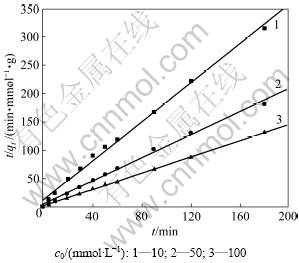
图6 准二级动力学方程的线性拟合
Fig.6 Linear fit of pseudo-second order equation
表3 初始浓度对铜离子吸附的影响参数
Table 3 Effect parameters of solution initial concentration on copper ion adsorption

2.4 吸附等温线
等温吸附曲线指在一定温度下,溶质分子在两相界面上进行的吸附过程达到平衡时,留在两相中浓度之间的关系曲线。利用吸附等温线有助于了解吸附现象的本质,通过等温吸附模型可求得纤蛇纹石在特定铜离子浓度下的吸附容量。
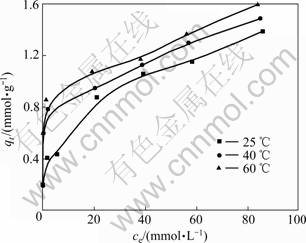
图7 等温吸附曲线
Fig.7 Adsorption isotherm
利用式(1)计算绘制平衡浓度与平衡吸附量之间关系曲线,得到纤蛇纹石吸附铜离子的等温曲线,结果如图7所示。由图7可知:在考察的温度25,40和60 ℃时,平衡吸附量随着平衡浓度的增加而增加;在不同温度下,平衡吸附量随着温度升高而增加。在稀溶液中,常用Langmuir等温方程和Freundlich等温方程来描述吸附过程。Langmuir方程的线性表达 式为:
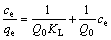 (5)
(5)
Freundlich方程的线性表达式为:
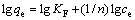 (6)
(6)
式中:qe为平衡时的吸附量;ce为平衡时的浓度; Q0为与饱和吸附量有关的常数;KL和KF为与最大吸附能有关的常数。
将吸附等温线按式(5)和(6)进行回归,所得线性关系见图8和图9,相关性系数如表4所示。从表4可见: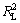 >
>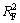 ,即纤蛇纹石对Cu2+的吸附数据更符合Langmuir等温吸附模型。Langmuir等温吸附方程假定:吸附是单分子层,溶质与溶剂分子的体积近似相等或有相同的吸附位;将溶质的吸附看作是溶液中溶质分子与吸附层中被吸附的溶剂分子交换的结果[21]。根据Langmuir理论,推测 Cu2+是均匀地吸附在纤蛇纹石表面活性位点—Mg—O-上,当活性点都吸附上Cu2+后,吸附量达到饱和,吸附处于平衡状态[22]。表4中Langmuir吸附常数KL和吸附量Q0均随温度的升高而增大,表明升温促进吸附进行。
,即纤蛇纹石对Cu2+的吸附数据更符合Langmuir等温吸附模型。Langmuir等温吸附方程假定:吸附是单分子层,溶质与溶剂分子的体积近似相等或有相同的吸附位;将溶质的吸附看作是溶液中溶质分子与吸附层中被吸附的溶剂分子交换的结果[21]。根据Langmuir理论,推测 Cu2+是均匀地吸附在纤蛇纹石表面活性位点—Mg—O-上,当活性点都吸附上Cu2+后,吸附量达到饱和,吸附处于平衡状态[22]。表4中Langmuir吸附常数KL和吸附量Q0均随温度的升高而增大,表明升温促进吸附进行。
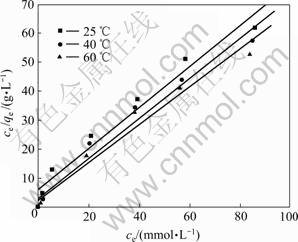
图8 Langmuir等温吸附曲线拟合
Fig.8 Linearized Langmuir isotherms obtained from Copper (Ⅱ) adsorption on chrysotile
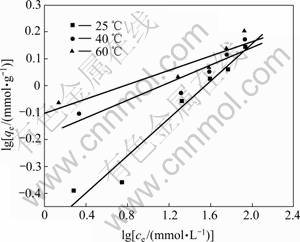
图9 Freundlich等温吸附曲线拟合
Fig.9 Linearized Freundlich isotherms obtained from Cu (Ⅱ) adsorption on chrysotile
表4 等温曲线拟合参数
Table 4 Linear fit parameters isotherm adsorption
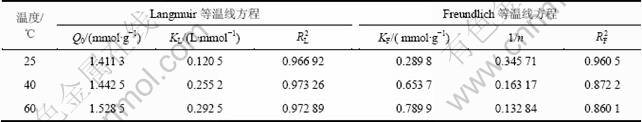
根据图(8)中Langmuir拟合直线的斜率和截距,求得纤蛇纹石在25,40和60 ℃时的等温吸附方程,分别为:ce/qe=5.880+0.709ce,ce/qe=2.716+0.693ce,ce/qe=2.237+0.654ce。
Langmuir等温吸附的本质特征是能表示成一个无量纲的常数PL[23]。它能表征吸附性能,预测吸附质与吸附剂的结合力:

其中:KL为Langmuir等温吸附常数;c0为溶液初始浓度(mmol/L)。
当0<PL<1时在研究的试验条件下吸附容易进行。选取每个温度的初始浓度,计算得PL分别为0.077~0.805 8,0.038~0.662 1和0.033~0.630 9。可以看出PL都在0~1之间,且同一温度下PL随Cu2+浓度的升高而降低,说明吸附溶液Cu2+初始浓度的增加有利于吸附的进行,在整个试验条件下,吸附都是有利的。
2.5 吸附热力学分析
由热力学参数吉布斯自由能变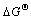 、焓变
、焓变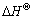 和熵变
和熵变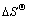 能判断吸附反应进行的方向和难易程度。用式(2)和(3)计算得出纤蛇纹石吸附水中铜离子时的热力学参数,见表5,其中 k取值为Langmuir等温吸附常数KL[24]。
能判断吸附反应进行的方向和难易程度。用式(2)和(3)计算得出纤蛇纹石吸附水中铜离子时的热力学参数,见表5,其中 k取值为Langmuir等温吸附常数KL[24]。
表5 不同温度下的吸附热力学参数
Table 5 Thermodynamic parameters for adsorption of Cu(Ⅱ) onto chrysotile at different temperatures

由表5可以看出:焓变?H为正,说明在自然条件下,反应是吸热的,这与实验中升温有利于吸附的进行相符合。当ΔH为2.1~20.9 kJ/mol时,为物理吸附;当ΔH为20.9~418.4 kJ/mol时,为化学吸附[25]。ΔH小于且非常接近20.9 kJ/mol,因此,整个吸附过程以物理吸附为主;离子交换的焓变通常小于8.4 kJ/mol[26],ΔH不在这个范围内,说明吸附反应还存在离子交换之外的吸附机理。推测的机理之一是吴清 辉[27]提出的在纤蛇纹石断裂面上,存在大量不饱和 Si—O—Si悬挂键,具有很高的化学活性,不饱和键中的Si能将金属离子固着在矿物表面。由Oepen[28]检测到的几种作用力引起的吸附焓变的范围(范德华力作用时为4~10 kJ/mol,疏水键力作用时为5 kJ/mol,氢键力作用时为2~40 kJ/mol,配位基交换作用时为40 kJ/mol,偶极力作用时为2~29 kJ/mol,化学键力作用时大于60 kJ/mol),推断吸附过程的作用力是氢键力和偶极作用力为主。熵变ΔS>0,表明在吸附过程中,液固表面的自由度增加,整个固液系统的无序性增大。这可能是因为:铜离子从液相进入纤蛇纹石表面交换位点时失去了部分自由度,但交换出的氢质子重新进入水相界面,恢复相对自由的状态使总熵变值增加。吉布斯自由能ΔG为负值,说明在自然条件下反应能自发进行。
3 结论
(1) 升高温度、提高pH和铜离子的初始浓度能增加纤蛇纹石对铜离子的吸附容量。
(2) 准二级动力学方程拟合试验数据,线性相关性系数均大于0.99,拟合得出的饱和吸附量与实验结果基本吻合。准二级动力学方程的吸附过程都能用于描述纤蛇纹石对铜离子的吸附。
(3) 吸附符合Langmuir等温吸附模型,在25,40和60 ℃时,最大吸附量分别为1.411 3,1.442 5和 1.528 5 mmol/g。等温吸附方程分别为:ce/qe=5.880+ 0.709 ce, ce/qe=2.716+0.693ce,ce/qe=2.237+0.654ce。分离因子为0~1,在整个反应体系下吸附都是有利的。
(4) 反应吉布斯自由能变ΔG<0,表明纤蛇纹 石纤维吸附金属铜离子在自然条件下能自发进行, ΔS>0 kJ/mol,吸附发生后,整个固液系统无序性加大;ΔH>0 J/(mol·K),吸附反应吸热,升温有利于反应的进行。吸附机理以Cu2+与纤蛇纹石纤维表面的外羟基中的氢质子进行离子交换为主,交换的作用力主要是偶极作用力和氢键力。
参考文献:
[1] Demirbas E, Dizge N, Sulak M T, et a1. Adsorption kinetics and equilibrium of copper from aqueous solutions using hazelnut shell activated carbon[J]. Chemical Engineering Journal, 2009, 148(2/3): 480-487.
[2] Amarasinghe B M W P K, Williams R A. Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater[J]. Chemical Engineering Journal, 2007, 132(1/3): 299-309.
[3] Pimentel P M, Melo M A F, Melo D M A, et al. Kinetics and thermodynamics of Cu(Ⅱ) adsorption on oil shale wastes[J]. Fuel Processing Technology, 2008, 89(1): 62-67.
[4] Ceils R, Hermosin M C, Cornejo J. Heavy metal adsorption by functionalized clays[J]. Environmental Science and Technology, 2000, 34: 4593-4599.
[5] Chen X H, Gosset T, Thévenot D R. Batch copper ion binding and exchange properties of peat[J]. Water Research, 1990, 24(12): 1463-1471.
[6] Brigatti M F, Lugli C, Poppi L. Kinetics of heavy-metal removal and recovery in sepiolite[J]. Applied Clay Science, 2000, 16(1/2): 45-57.
[7] 杨保俊, 于少明, 单承湘. 蛇纹石硫酸浸出过程动力学研究[J]. 硅酸盐学报, 1999, 27(1): 65-70.
YANG Bao-jun, YU Shao-ming, SHAN Cheng-xiang. Study on kinetics of serpentine leaching sulfuric acid[J]. Journal of the Chinese Ceramic Society, 1999, 27(1): 65-70.
[8] 刘琨, 冯其明, 杨艳霞, 等. 纤蛇纹石制备氧化硅纳米线[J]. 硅酸盐学报, 2007, 35(2): 164-169.
LIU Kun, FENG Qi-ming, YANG Yan-xia, et al. Preparation of silica nanowires derived from chrysotile[J]. Journal of the Chinese Ceramic Society, 2007, 35(2): 164-169.
[9] 段涛, 彭同江. 天然纤蛇纹石制备纤维状纳米氧化硅研究[J]. 非金属矿, 2008, 31(1): 25-27.
DUAN Tao, PENG Tong-jiang. Preparation of nano-fibrous silica from natural chrysotile[J]. Non-Metallic Mines, 2008, 31(1): 25-27.
[10] Fonseca M G, Oilveira A S, Claudio A. Silylating agents grafted onto silica derived from leached chrysotile[J]. Journal of Colloid and Interface Science, 2001, 240(2): 533-538.
[11] 李学军, 王丽娟, 鲁安怀. 天然蛇纹石活性机理初探[J]. 岩石矿物学杂志, 2003, 22(4): 386-390.
LI Xue-jun, WANG Li-juan, LU An-huai. A discussion on activation mechanism of atom groups in serpentine[J]. Acta Petrologica Et Mineralogica, 2003, 22(4): 386-390.
[12] 杨志宽. 用蛇纹石处理含铜废水的研究[J]. 环境科学与技术, 1997, 20(2): 17-19.
YANG Zhi-kuan. Study on disposing of wastewater containing copper by serpentine[J]. Environmental Science and Technology, 1997, 20(2): 17-19.
[13] 郭继香, 袁存光. 蛇纹石吸附处理污水中重金属的实验研究[J]. 精细化工, 2000, 17(10): 586-589.
GUO Ji-xiang, YUAN Cun-guang. Study of adsorption of heavy metals in wastewater by serpentine[J]. Fine Chemicals, 2000, 17(10): 586-589.
[14] Fonseca M G, Airoldi C. Thermodynamics date of interaction of copper nitrate with native and modified chrysotile fibers in aqueous solution[J]. Journal of Colloid and Interface Science, 2001, 240(1): 229-236.
[15] Aharoni C, Sparks D L, Levinson S. Kinetics of soil chemical reaction: relationships between empirical equations and diffusion models[J]. Soil Science Society of America Journal, 1991, 55(9/10): 1307-1313.
[16] Ho Y S, Mckay C. The kinetic of sorption of divalent metal ions onto sphagnum moss peat[J]. Water Research, 2000, 34(3): 735-742.
[17] HU Xiao-jun, LI Yu-shuang, WANG Yan. Adsorption kinetics, thermodynamics and isotherm of thiacalix arene-loaded resin to heavy metal ions[J]. Desalination, 2010, 259(1/3): 76-83.
[18] WANG Shao-bin, ZHU Zhong-hua. Effect of acidic treatment of activated carbons on dye adsorption[J]. Dyes and Pigments, 2007, 75(2): 306-314.
[19] 王淀佐, 胡岳华. 浮选溶液化学[M]. 长沙: 湖南科学技术出版社, 1988: 132-134.
WANG Dian-zuo, HU Yue-hua. Solution chemistry of flotation[M]. Changsha: Science and Technology Press of Hunan, 1988: 132-134.
[20] Alkan M, Demirbas O, Dogan M. Adsorption kinetics and thermodynamics of an anionic dye onto sepiolite[J]. Microporous and Mesoporous Materials, 2007, 101(3): 388-396.
[21] Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum[J]. Journal of the American Chemical Society, 1918, 40(9): 1361-1403.
[22] 彭长宏, 程晓苏, 曹金艳. 离子液体负载型碳纳米管吸附除砷研究[J]. 中南大学学报: 自然科学版, 2010, 41(2): 416-421.
PENG Chang-hong, CHENG Xiao-su, CAO Jin-yan. Removal performance of arsenic by carbon nanotube adsorbents loaded with ionic liquid[J]. Journal of Central South University: Science and Technology, 2010, 41(2): 416-421.
[23] Weber T W, Chakkravorti R K. Pore and solid diffusion models for fixed-bed adsorbers[J]. AICHE Journal, 1974, 20(2): 228-238.
[24] Rogas G, Silva J, Flores J A, et al. Adsorption of chromium onto cross-linked chitosan[J]. Separation and Purification Technology, 2005, 44(1): 31-36.
[25] Kilic M, Yazici H, Solak M. A Comprehensive study on removal and recovery of copper(Ⅱ) from aqueous solutions by NaOH-pretreated marrubium globosum sp.globosum leaves powder: Potential for utilizing the copper(Ⅱ) condensed desorption solutions in agricultural applications[J]. Bioresour Technology, 2009, 100(7): 2130-2137.
[26] Lyubchik S I, Lyubchik A I, Galushko O L, et al. Kinetics and thermodynamics of the Cr (Ⅲ) adsorption on the activated carbon from co-mingled wastes[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2004, 242(1/3): 151-158.
[27] 吴清辉. 表面化学与多相催化[M]. 北京: 化学工业出版社, 1991, 144-149.
WU Qing-hui. Surface chemistry and multiphase catalyze[M]. Beijing: Chemical Industry Press, 1991: 144-149.
[28] von Oepen B, Kordel W, Klein W. Sorption of nonpolar and polar compounds to soils: Processes, measurement and experience with the applicability of the modified OECD-guideline106[J]. Chemosphere, 1991, 22(3/4): 285-304.
(编辑 陈灿华)
收稿日期:2010-11-15;修回日期:2011-02-28
基金项目:国家自然科学基金资助项目(50574102)
通信作者:刘琨(1979-),男,湖南长沙人,博士,讲师,从事新型矿物材料与无机功能材料研究;电话:0731-88877203;E-mail: kliu@csu.edu.cn