文章编号:1004-0609(2011)11-2971-07
纳米TiO2对镉(Ⅱ)的吸附性能
刘雪岩1, 杨丽君1, 金燕利2, 张 蕾1, 徐天赐1, 李 娜1
(1. 辽宁大学 化学学院,沈阳 110036;2. 海军装备部 驻沈阳军事代表局,沈阳 110031)
摘 要:通过吸附实验研究纳米TiO2对Cd(Ⅱ)的吸附行为,考察溶液的pH值、吸附时间和温度等因素对吸附的影响。结果表明:在pH为 4~7范围内,纳米TiO2对Cd(Ⅱ)的吸附率均超过97%,吸附于纳米TiO2上的Cd(Ⅱ)可用7.0 mL 0.1 mol/L HCl于70 ℃恒温水浴中定量洗脱;该吸附过程符合准二级反应动力学模型,其反应的表观活化能为3.16 kJ/mol,粒子内部扩散过程是其吸附过程的速率控制步骤,但液相边界层向粒子表面的扩散过程亦不能忽略;等温吸附数据与Langmuir和D-R等温模型拟合较好。在室温下,纳米TiO2对Cd(Ⅱ)的饱和吸附容量为6.34 mg/g,平均吸附能为11.54 kJ/mol;吸附反应的焓变和熵变均为正值,自由能为负值,说明该吸附过程为自发的吸热过程。
关键词:Cd(Ⅱ);纳米TiO2;吸附;动力学;热力学
中图分类号:O 647.3 文献标志码:A
Adsorption properties of nano-TiO2 for Cd(Ⅱ)
LIU Xue-yan1, YANG Li-jun1, JIN Yan-li2, ZHANG Lei1, XU Tian-ci1, LI Na1
(1. College of Chemistry, Liaoning University, Shenyang 110036, China;
2. Military Representative Bureau in Shenyang, Navy Equipment Department, Shenyang 110031, China)
Abstract: The titanium dioxide nanoparticles were employed for the sorption of Cd(Ⅱ) ions from aqueous solution. The process was studied by varying the pH, sorption time and temperature. The adsorption rate of Cd(II) by the sorbent is over 97% at pH of 4-7. The adsorbed Cd(Ⅱ) are desorbed by 7.0 mL 0.1 mol/L HCl in constant temperature water bath at 70 ℃. The kinetic experimental data properly correlate with the second-order kinetic model. The apparent activation energy is 3.16 kJ/mol. The overall rate process is mainly controlled by the intraparticle diffusion, while the boundary layer resistance cannot be ignored. The sorption data can be well interpreted by the Langmuir and D-R type sorption isotherms. At room temperature, the static saturated adsorption capacity is 6.34 mg/g, and the average sorption energy is 11.54 kJ/mol. The thermodynamic parameters: ?HΘ>0, ?SΘ>0 and ?GΘ<0, which indicates a spontaneous and decalescence adsorption process.
Key words: Cd(Ⅱ); nano-TiO2; adsorption; kinetics; thermodynamics
金属镉及镉的化合物被广泛地应用于电镀工业、制造合金、蓄电池、颜料、聚氯乙烯稳定剂、核反应堆的中子吸收剂等。镉在自然界中以硫镉矿的形式存在,并常与锌、铅、铜、锰等矿共存,这些金属的精练过程都会排出大量镉,因此,镉以工业废水、废气、废渣的形式污染环境。镉是毒性较强的重金属元素,因此,快速、有效地去除镉具有十分重要的意义。目前,常用的去除镉的方法有共沉淀法[1]、浮选法[2]、溶剂萃取法[3]、离子交换法[4]、液膜法[5]、吸附法[6]等。沉淀法是一种传统的分离富集方法,在溶液中加入沉淀剂和少量金属离子作为载体,共沉淀溶液中的镉;离子交换分离法常用阳离子交换树脂或阴离子交换树脂去除镉,采用腐植酸树脂和螯合树脂去除镉的方法也有见报道;膜分离法处理含镉废水具有污染物去除率高、工艺简单等特点, 但膜在处理废水时的选择性和使用成本较高, 膜组件的设计也是一个难题,所以,膜法去镉的应用受到限制;吸附法是利用多孔性的固体物质作吸附剂去除镉的,常用于处理含镉废水的吸附剂有:活性炭、风化煤、磺化煤、高炉矿渣、沸石、壳聚糖、羧甲基壳聚糖、硅藻土、改良纤维、活性氧化铝和蛋壳等。纳米材料是近年来发展起来的一种新型功能材料,它具有比表面积大、表面原子数目多等优点,已受到研究工作者的广泛关注。纳米粒子对许多金属离子具有很强的吸附能力,因此,是去除有毒有害重金属污染物的理想吸附剂[7]。
梁沛等[8]利用ICP-AES研究了纳米TiO2对Cr(Ⅵ)/Cr(Ⅲ)的吸附性能,并将其用于水样中铬的形态分析。张东等[9]考察了纳米钛酸钙粉体对水中重金属铅和镉的吸附性能。MAT??等[10]利用ET-AAS和ICP-OES研究了纳米TiO2分离和富集痕量Al(Ⅲ)的可行性,探讨了纳米TiO2材料对Al(Ⅲ)的吸附行为。张蕾等[11]报道了纳米TiO2对钼(Ⅵ)吸附性能的研究。
有关金红石型纳米TiO2对镉的吸附性能研究鲜见报道,本文作者以金红石型纳米TiO2为吸附剂,探讨pH对吸附的影响及相关吸附机理,并对纳米TiO2吸附镉的热力学与动力学行为进行较系统的研究,该研究为纳米吸附剂有效去除镉提供了理论依据。
1 实验
1.1 实验仪器与试剂
1.1.1 实验仪器
AAnalyst-700原子吸收光谱仪(美国,PE公司),pHS-3C型精密pH计(上海雷磁),Malvern Nano ZS纳米粒度及Zeta电位分析仪(美国,Malvern公司),X 射线粉末衍射仪(D8 ADVACE 德国 BRUKER 公司),KQ-100B型超声波清洗器(昆山市超声仪器有限公司),TDL80-2B台式离心机(上海安亭科学仪器厂),DK-98-1型电热恒温水浴锅(天津市泰斯特仪器有限公司)
1.1.2 主要实验试剂
主要试剂如下:纯镉,硝酸,盐酸,氢氧化钠,金红石型纳米-TiO2(舟山明日纳米材料有限公司)。实验所用试剂均为分析纯,水为二次蒸馏水。
镉标准储备溶液(优级纯)为1 mg/mL:准确称取 1 g 镉置于烧杯中,加入20 mL HNO3(其中V(HNO3):V(H2O)=1:1)溶液,加热煮沸至溶液变为无色透明,冷却后转移至1 000 mL容量瓶中,以二次蒸馏水定容,摇匀。
1.1.3仪器工作条件
原子吸收分光光度计。工作参数如下:波长228.8 nm,狭缝宽0.7 H,灯丝电流4.0 mA,乙炔流量2.0 L/min,空气流量17 L/min。
1.2 实验方法
1.2.1 镉的测量方法
准确移取0、0.10、0.25、0.50、1.00、1.50、2.00、2.50、3.00 mL的镉标准溶液于25 mL容量瓶中,以0.5 mol/L HNO3稀释、摇匀。用AAnalyst700 原子吸收光谱仪,以乙炔-氧气作火焰,HNO3作空白溶液,测定吸光度A。
1.2.2 纳米二氧化钛等电点的测定
将0.059 9 g的金红石型纳米二氧化钛粉体于250 mL水溶液中,超声分散形成分散液,用HCl和NaOH调节一系列分散液的pH值,采用Zeta电位分析仪,测量分散液的Zeta电位,Zeta电位为零时所对应的pH值即是金红石型纳米二氧化钛的等电点。
1.2.3 镉的吸附方法
移取10 mL一定浓度Cd(Ⅱ)标准溶液置于50 mL具塞锥形瓶中,用HCl 和NaOH 溶液调节Cd(Ⅱ)标准溶液的pH 值,然后加入一定量的纳米TiO2,超声分散3 min,静置5 min,离心分离5 min (4 000 r/min),然后分析水相中Cd(Ⅱ)的浓度,按照1.2.1 测定方法,测定吸光度A,计算Cd(Ⅱ)含量和吸附率。
吸附率η的计算公式为
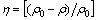 ×100% (1)
×100% (1)
式中:ρ0为原溶液中吸附质的质量浓度,mg/L;ρ为吸附平衡时溶液中剩余吸附质的质量浓度,mg/L。
1.2.4 动力学实验
移取10 mL浓度为20 mg/L的Cd(Ⅱ)溶液置于一系列50 mL具塞锥形瓶中,调溶液pH为6.0,加80 mg纳米TiO2,分别在273、293和313 K条件下,测定不同时间间隔下溶液中吸附质的浓度,直到吸附达到平衡。
1.2.5 热力学实验
在一系列50 mL具塞锥形瓶中,加入不同质量浓度的Cd(Ⅱ)溶液10 mL,用HCl和NaOH调溶液的pH为6.0,加入80 mg纳米TiO2,分别在273、293和313 K条件下进行吸附实验。
2 结果与讨论
2.1 纳米TiO2的表征
样品XRD的分析结果如图1所示。结果表明,样品衍射角在2θ= 27.4°有明显衍射峰,且其余各峰对应的面间距d值与 JCPDS 卡中 211276 号金红石型TiO2的d值完全一致,说明样品为金红石相结构。经测定样品的平均晶粒尺寸约为81.9 nm左右,皆处于纳米范围(见图2)。
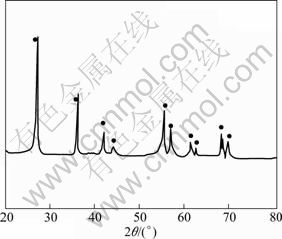
图1 纳米氧化钛的XRD谱
Fig.1 XRD pattern of nano-TiO2
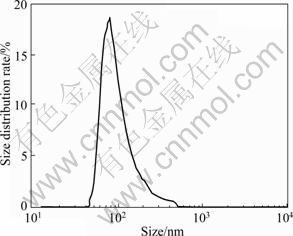
图2 纳米TiO2的粒度分布图
Fig.2 Particle diameter distributions of nano-TiO2
2.2 pH的影响
pH值对纳米TiO2吸附Cd(Ⅱ)起着重要的作用。在不同pH值条件下,纳米TiO2对Cd(Ⅱ)的吸附率的影响如图3所示,在pH值4~7范围内,纳米TiO2对Cd(Ⅱ)具有良好的吸附性能,吸附率可达97%以上,即可以被定量吸附。
Cd(Ⅱ)溶于酸性介质,不溶于中性和碱性介质中,在水溶液中,主要以Cd2+和Cd(OH)2两种形态存在。当pH<7时,主要以Cd2+形态存在;pH>7时,主要是Cd(OH)2形态存在(见图4),测定金红石型纳米TiO2的等电点pHZPC为3.8(见图4)。pH<3.8时,纳米TiO2表面带正电荷,不利于Cd2+的吸附。溶液pH在3.8~7之间,纳米TiO2表面带负电荷,有利于Cd2+吸附。故本实验选择pH 6.0为最佳吸附酸度。
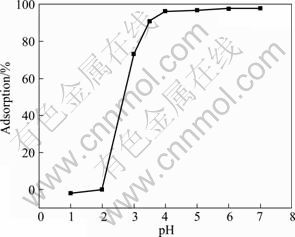
图3 pH值对纳米TiO2吸附Cd(Ⅱ)的影响
Fig.3 Effect of pH on adsorption rate of Cd(Ⅱ) for nano- TiO2
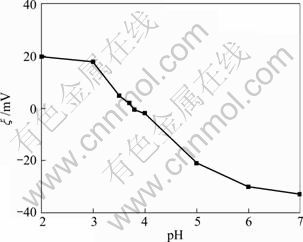
图4 不同pH值下金红石型纳米TiO2的Zeta电位
Fig.4 Zeta potential of rutile type nano-TiO2 at different pH
从图3可知,pH<2时,Cd(Ⅱ)的吸附率较低,本研究选择HCl溶液解析Cd(Ⅱ)。实验结果表明,7.0 mL 0.1 mol/L HCl 溶液于70 ℃恒温水浴中可定量解析Cd(Ⅱ),解析率为95%。
2.3 共存离子对吸附的影响
本研究也考察了常见的共存离子对纳米TiO2吸附镉的干扰情况。实验结果表明,对于 200 μg/mL 的Cd2+,1 mg/ mL 的 K+;1.5 mg/ mL Na+;3 mg/ mL 的Ca 2+,Mg 2+;3 mg/mL的Cl-;1.5 mg/mL的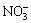 ; 1 mg/ mL 的
; 1 mg/ mL 的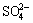 和
和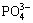 和 0.8 mg/ mL的
和 0.8 mg/ mL的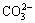 对测定结果无显著性影响。
对测定结果无显著性影响。
2.4 吸附动力学研究
取10 mL 20 mg/L的Cd(Ⅱ)溶液,调溶液pH 6.0,加入80 mg纳米TiO2,以吸附量qt对时间t作图,得到Cd(Ⅱ)吸附量随温时间变化的吸附动力学曲线(见图5)。由图5可见,qt随时间的增长而增大,吸附3 min 基本达到平衡。
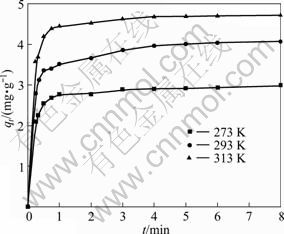
图5 不同温度时镉的吸附量随时间的变化曲线
Fig.5 Adsorption capacity of Cd(Ⅱ) on sorbent vs time at different temperature
将以上实验数据用于准二级动力学方程:
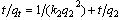 (2)
(2)
式中: k2为二级反应速率常数,g·mg-1·min-1;qt为t时刻的吸附量,mg·g-1;q2为平衡吸附量,mg·g-1。以t/qt对t作图,对所有数据进行线性回归分析,从斜率和截矩可得到不同温度下纳米TiO2吸附Cd(Ⅱ)的k2、q2以及相关系数r,实验数据如表1。从表1可看出,在3种温度下,动力学实验数据与二级反应动力学模型有较好的拟合(r2>0.999)。
根据不同温度下镉吸附动力学实验数据,用阿伦尼物乌斯(Arhenius)公式对吸附反应的活化能求解,求得纳米TiO2对Cd(Ⅱ)吸附的活化能Ea为3.16 kJ/mol。
表1 不同温度下纳米TiO2吸附Cd(Ⅱ)的动力学参数
Table 1 Kinetic parameters for Cd(Ⅱ) adsorption on nano-TiO2 at different temperatures

2.5 扩散模型
粒子内部扩散方程:
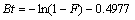 (3)
(3)
式中:F=qt /qe;qt和qe 分别为t 时刻的吸附容量和平衡吸附容量,mg·g-1;B=π2Di /d 2(Di 为内部扩散系数,d 为粒子半径)。将动力学数据用粒子扩散方程进行拟合,根据式(3),用-ln(1-F)-0.497 7对t做图(见图6)。图6中直线近似通过原点,表明粒子内部扩散是吸附过程的主要控制步骤[12]。
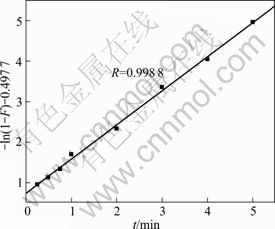
图6 粒子内部扩散拟合曲线
Fig.6 Fitted curve of intraparticle diffusion
Cd(Ⅱ) 在吸附剂内部的扩散过程可进一步用Weber-Morris 模型来描述[13],方程式如下:
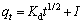 (4)
(4)
式中:Kd为内部扩散速度常数,mg·g-1·min-1/2;I 表示与边界层厚度有关的常数,I值越大,边界层对吸附的影响越大[14-15]。将Weber-Morris模型数据进行分段线性拟合,拟合曲线如图7所示,由模型得到的内部扩散速度常数和线性相关系数r 如表2 所列。从图7可以看出,Weber-Morris模型曲线的2段线性拟合情况较好,说明粒子内部扩散过程是吸附过程的控制步骤;但扩散模型拟合直线并不经过原点,说明纳米TiO2吸附Cd(Ⅱ)机理比较复杂,吸附剂周围液相边界层向粒子表面的扩散过程不可以忽略。
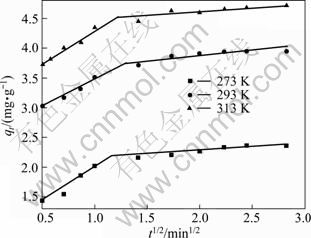
图7 在不同温度下Weber-Morris 模型拟合曲线
Fig.7 Fitted curves of Weber-Morris at different temperatures
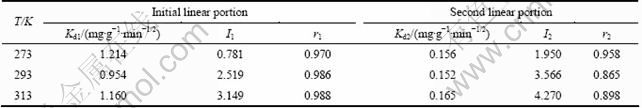
表2 不同温度下Weber-Morris模型多段线性拟合曲线系数及相关系数
Table 2 Calculated parameters for Cd(Ⅱ) adsorption on nano-TiO2 at different temperatures
2.6 吸附热力学研究
2.6.1 吸附等温模型和吸附容量
分别在不同温度下(0、20和40 ℃),按吸附方法(1.2.5)进行吸附实验,吸附等温线见图8。根据Langmuir吸附等温模型 [16],以ρe/qe对Ce做直线可得qm 和 b。
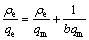 (5)
(5)
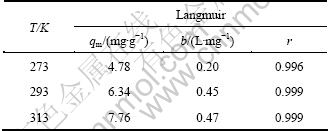
式中:qm为饱和吸附量,mg·g-1;ρe为Cd(Ⅱ)吸附平衡时的质量浓度,mg·L-1;b为吸附平衡常数,L·mg-1。Langmuir 吸附等温模型拟合结果如表3所列。由表3可知,在3种温度下,实验数据与Langmuir 等温吸附线有较好的拟合(r≥0.99),饱和吸附容量随着温度的升高而增加,说明纳米TiO2 对Cd(Ⅱ)的吸附是吸热过程。
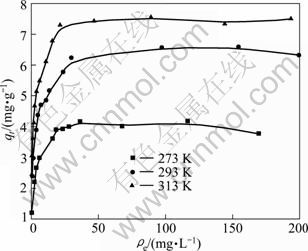
图8 不同温度下的吸附等温线
Fig.8 Isotherm of Cd(Ⅱ) adsorption on nano-TiO2 at different temperatures
表3 不同温度下Langmuir等温吸附曲线的参数
Table 3 Langmuir isotherm constants at different temperatures
2.6.2 吸附过程中的热力学参数
实验考察了在273、293和313 K下不同初始浓度的Cd(Ⅱ)的吸附情况。热力学函数计算,从吸附等温线求出吸附等量线,以ln ρ对1/T作图,根据Clausius-Clapeyron方程可得[17]:
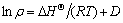 (6)
(6)
式中:ρ为不同温度时Cd(Ⅱ)的吸附平衡浓度(mg·L–1)。用线性回归法求出斜率,求标准吸附焓?HΘ,根据如下关系计算其它热力学函数值,计算结果列于表4。
 (7)
(7)
 (8)
(8)
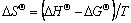 (9)
(9)
式中:ρBe和ρAe分别是Cd(Ⅱ)在吸附剂中的平衡浓度(mg·L–1)和在溶液中的平衡浓度(mg·L –1);KC为平衡常数。
由表4可知,纳米TiO2吸附Cd(Ⅱ)离子的吸附是自发过程(?GΘ<0),且?GΘ的绝对值随着温度的升高而增加,这与等温吸附线表现出的温度升高,吸附量增加的实验结果一致,此吸附反应为吸热过程(?HΘ>0),且熵变皆为正值(?SΘ>0)。
2.6.3 平均吸附能
平均吸附能E 是判断吸附类型的重要指标,其计算式为[18]
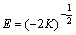 (10)
(10)
根据D-R模型的线性形式(见式(11)),以lnqe对ε2作直线(见图9),由直线斜率求得K:
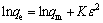 (11)
(11)
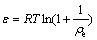 (12)
(12)
式中:E为平均吸附能,kJ/mol;K为与吸附能有关的常数;ε为Polanyi势能,kJ。根据式(10),在273、293和313 K条件下,纳米TiO2对Cd(Ⅱ)的平均吸附能分别为13.36、11.54和10.82 kJ/mol,离子交换反应的能量范围为8~16 kJ/mol,故该吸附反应属于离子交 换[19]。
表4 镉吸附于纳米TiO2的热力学表观参数
Table 4 Thermodynamic parameters for adsorption of Cd(Ⅱ) on nano-TiO2

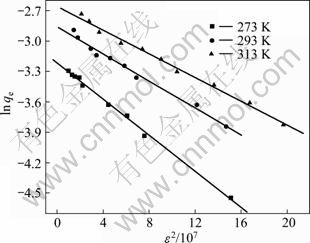
图9 在不同温度下的D-R吸附等温线
Fig.9 D-R adsorption isotherms at different temperatures
3 结论
1) 在pH 4~7 范围内,纳米TiO2对Cd(Ⅱ)的吸附率可达97%以上,强酸性条件不利于Cd(Ⅱ)的吸附,7.0 mL 0.1 mol/L HCl 溶液可定量解析Cd(Ⅱ),解析率为95%。
2) 该吸附过程符合准二级反应动力学模型,常温下,其反应速率常数为1.89 g·mg-1·min-1,反应活化能为3.16 kJ/mol,吸附机理比较复杂,粒子内部扩散过程是其吸附控制步骤,但液相边界层向粒子表面的扩散过程亦不可忽略。
3) 纳米TiO2对Cd(Ⅱ)的吸附符合Langmuir、D-R等温式,在273、293 和313 K 条件下,其饱和吸附容量分别为4.78、6.34、7.76 mg·g-1,平均吸附能分别为13.36、11.54、10.82 kJ·mol-1,饱和吸附容量随体系温度升高而增大,说明此吸附反应为吸热过程,平均吸附能范围为8~16 kJ·mol-1,故该吸附反应属于离子交换过程。
4) 纳米TiO2对Cd(Ⅱ)的吸附热力学参数分别为?HΘ>0,?GΘ<0,?SΘ>0,表明吸附反应为自发的吸热过程。
REFERENCES
[1] 卢莲英, 邹光中, 叶宋娣. 铁氧体与镉共沉淀的试验研究[J].化学与生物工程, 2004, 6: 44-45.
LU Lian-ying, ZOU Guang-zhong, YE Song-di. Experiment research on concomitance deposition of cadmium and ferrite[J]. Chemistry & Bioengineering, 2004, 6: 44-45.
[2] 易晓明, 李建文. 铁置换-硫酸铵-碘化钾-丁基罗丹明B浮选分离镉[J]. 岩矿测试, 2007, 26(2): 126-128.
YI Xiao-ming, LI Jian-wen. Separation of Cadmium(II) with replacement -flotation[J]. Rock and Mineral Analysis, 2007, 26(2): 126-128.
[3] 刘 阁, 周 凯. Tween80-(NH4)2SO4-H2O体系萃取镉的研究[J].湿法冶金, 2007, 26(2): 92-95.
LIU Ge, ZHOU Kai. Extraction of Cd(Ⅱ) in Tween80- (NH4)2SO4-H2O system[J]. Hydrometallurgy of China, 2007, 26(2): 92-95.
[4] 杨莉丽, 康海彦, 李 娜, 张德强. 离子交换树脂吸附镉的动力学研究[J]. 离子交换与吸附, 2004, 20(2): 138-143.
YANG Li-li, KANG Hai-yan, LI Na, ZHANG De-qiang. Study on the kinetics of adsorption of cadmium by ion-exchange resin[J]. Ion Exchange and Adsorption, 2004, 20(2): 138-143.
[5] 刘新芳, 张晓宇, 何鼎胜. 组合液膜分离镉锌的动力学分析[J]. 电镀与涂饰, 2009, 28(6): 44-47.
LIU Xin-fang, ZHANG Xiao-yu, HE Ding-sheng. Dynamic analysis on the separation of cadmium and zinc with hybrid liquid membrane[J]. Electroplating & Finishing, 2009, 28(6): 44-47.
[6] 侯 明, 刘振国. 接枝壳聚糖对铅, 镉吸附行为研究及应用[J].分析试验室, 2006, 25(10): 1-6.
HOU Ming, LIU Zhen-guo. Study on adsorption properties of carboxyl-containing grafted chitosan polymer toward Pb(II) and Cd(Ⅱ) and its application[J]. Chinese Journal of Analysis Laboratory, 2006, 25(10): 1-6.
[7] 张中太, 林元华, 唐子龙, 张俊英. 纳米材料及其技术的应用前景[J]. 材料工程, 2000, 3: 42-48.
ZHANG Zhong-tai, LIN Yuan-hua, TANG Zi-long, ZHANG Jun-ying. Nanometer materials & nanotechnology and their application prospect[J]. Materials Engineering, 2000, 3: 42-48.
[8] 梁 沛, 李春香, 秦永超, 胡 斌, 江祖成. 纳米二氧化钛分离富集和ICP-AES 测定水样中Cr(VI)/ Cr(III)[J]. 分析科学学报, 2000, 16(4): 300-303.
LIANG Pei, LI Chun-xiang, QIN Yong-chao, HU Bin, JIANG Zu-cheng. Selective adsorption on nanometer size TiO2 powders for the chromium speciation using ICP-AES[J]. Journal of Analytical Science, 2000, 16(4): 300-303.
[9] 张 东, 侯 平. 纳米钛酸钙粉体的制备及其对水中铅和镉的吸附行为[J]. 化学学报, 2009, 12(67): 1336-1342.
ZHANG Dong, HOU Ping. Preparation of nano-calcium titanate powder and its adsorption behavior for lead ion and cadmium ion in water[J]. Acta Chimica Sinica, 2009, 12(67): 1336-1342.
[10] MAT?? P, HAGAROV? I, BUJDO? M, DIVI? P, KUBOV? J. Determination of trace amounts of total dissolved cationic aluminium species in environmental samples by solid phase extraction using nanometer-sized titanium dioxide and atomic spectrometry techniques[J]. Journal of Inorganic Biochemistry, 2009, 103: 1473-1479.
[11] 张 蕾, 刘雪岩, 姜晓庆, 李 绮, 康平利. 纳米TiO2对钼(Ⅵ)的吸附性能[J]. 中国有色金属学报, 2010, 20(2): 301-307.
ZHANG Lei, LIU Xue-yan, JIANG Xiao-qing, LI Qi, KANG Ping-li. Adsorption properties of nano-TiO2 for Mo(Ⅵ) [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(2): 301-307.
[12] AKSU Z. Application of biosorption for the removal of organic pollutants: A review[J]. Process Biochemistry, 2005, 40(3/4): 997-1026.
[13] SHAUHA M S, SARWAR M I, QADEER R. Adsorption of strontium ions from aqueous solution on Pakistani coal[J]. Journal of Radioanalytical and Nuclear Chemistry, 2005, 265(1): 73-79.
[14] ?ZER A, AKKAYA G, TURABIK M. The biosorption of acid red 337 and acid blue 324 on enteromorpha prolifera: The application of nonlinear regression analysis to dye biosorption[J]. Chemical Engineering Journal, 2005, 112(1/3): 181-190.
[15] MALL I D, SRIVASTAVA V C, AGARWAL N K, MISHRA I M. Removal of congo red from aqueous solution by bagasse fly ash and activated carbon: Kinetic study and equilibrium isotherm analyses[J]. Chemosphere, 2005, 61(4): 492-501.
[16] 邹卫华, 陈宗璋, 韩润平, 谢 霜, 石 杰. 锰氧化物/石英砂 (MOCS)对铜和铅离子的吸附研究[J]. 环境科学学报, 2005, 25(6): 779-784.
ZOU Wei-hua, CHEN Zong-zhang, HAN Run-ping, XIE Shuang, SHI Jie. Removal of copper cation and lead cation from aqueous solution by manganese-oxide-coated-sand [J]. Acta Scientiae Circumstantiae, 2005, 25(6): 779-784.
[17] BEREKET G, AROGUZ A Z, ?ZEL M Z. Removal of Pb(Ⅱ), Cd(Ⅱ), Cu(Ⅱ) and Zn(Ⅱ) from aqueous solutions by adsorption on bentonite [J]. Journal of Colloid and Interface Science, 1997, 187: 338-343.
[18] KILISLIOGLB A, BILGIN B. Thermodynamic and kinetic investigations of uranium adsorption on amberlite IR-118H resin[J]. Applied Radiation and Isotopes, 2003, 58(2): 155-160.
[19] EL-SHAHAWI M S, NASSIF H A. Retention and thermodynamic characteristics of mercury(Ⅱ) complexes onto polyurethane foams[J]. Analytica Chimica Acta, 2003, 481(1): 29-39.
(编辑 李艳红)
基金项目:辽宁省教育厅科研创新团队项目(2007T053);辽宁省“百千万人才工程”资助项目; 辽宁省高等学校优秀人才支持计划(2009R30)
收稿日期:2010-11-01;修订日期:2011-05-23
通信作者:张蕾,教授,博士;电话:024-62207809;传真:024-62202380; E-mail: zhanglei63@126.com