文章编号:1004-0609(2015)-09-2588-08
硫酸介质中锂云母分解反应动力学
赵 寻,杨 静,马鸿文,刘梅堂,林 斐
(中国地质大学 材料科学与工程学院,北京 100083)
摘 要:以四川某地锂云母矿石经选矿预处理所得锂云母精矿为原料,研究在硫酸介质中锂云母的分解反应过程。结果表明:在硫酸浓度为4.2 mol/L、硫酸与锂云母液固质量比为3:1、温度为433 K、转速为30 r/min条件下反应4 h,Li2O的溶出率达96%。反应动力学实验结果表明:锂云母酸解反应属于固膜扩散控制,分解过程符合液-固单颗粒反应模型中的缩芯模型;反应的表观活化能为Ea为28.3 kJ/mol,阿伦尼乌斯常数k0为2.608,反应级数n为0.998。
关键词:锂云母;分解反应;动力学;硫酸介质
中图分类号:TD982 文献标志码:A
Kinetics of lepidolite decomposition reaction in sulfuric acid solution
ZHAO Xun, YANG Jing, MA Hong-wen, LIU Mei-tang, LIN Fei
(School of Materials Science and Technology, China University of Geosciences, Beijing 100083, China)
Abstract: The decomposition of lepidollite in sulfuric acid solution was studied and the lepidolite concentrate from Sichuan province was obtained by mineral separation pretreatment and its kinetics were studied. The results reveal that the dissolution rate of Li2O in the lepidolite sample reaches 96% when it was hydrothermal-treated at 433 K for 4 h, with the sulfuric acid concentration of 4.2 mol/L, the sulfuric acid lepidolite liquid to solid mass ratio of 3:1, and the stirring speed of 30 r/min, respectively. The kinetics research results indicate that the reaction of the lepidolite decomposition in sulfuric acid solution is controlled by solid membrane diffusion, and the decomposition conforms to the shrinking core model of the liquid-solid single particle reaction model, with the apparent activation energy (Ea) of 28.3 kJ/mol, the Arrhenius constant k0 of 2.608, and the reaction series n of 0.998.
Key words: lepidolite; decomposition reaction; kinetics; sulfuric acid solution
锂是自然界最轻的金属,化学性质活泼,被誉为“能源金属”。锂及其化合物在化工、电子、冶金、医药、宇航、核能和能源等领域得到广泛应用,是传统工业领域与高新技术领域的重要材料[1]。锂在自然界中以化合物的形式存在于锂辉石、锂云母等矿物和盐湖卤水中[2-3]。现已查明,全球锂资源超过1300万t,中国储量350万t[4],居世界第二位。世界上主要从盐湖卤水提锂,但由于我国盐湖卤水镁离子含量高,生产电池级碳酸锂成本较高,其工业推广比较困难[5-6]。目前,我国主要从锂辉石(Li2O含量8%左右)中提取碳酸锂[7],而对低品位(Li2O含量3%~5%)锂云母的提锂技术研究较少[8]。
虽然我国锂资源的开发一直在不断推进,但大部分产能仍未能释放,锂盐供应量不足。2013年1~11月,我国碳酸锂净进口11675 t,氯化锂净进口1760 t[9],开发储量丰富的锂云母矿石可缓解锂盐短缺、严重依赖进口的现状[10]。
目前,以锂云母为原料生产锂盐的主要工艺有石灰石法[11]、氯化物法[12]、硫酸盐法[13],此类方法首先将锂云母与添加剂混合,在850~1050 ℃下焙烧,将锂转变为可溶性的硫酸盐形式[14]。但以上方法存在高污染、高能耗、高成本等问题。在此,本文作者采用硫酸分解锂云母的方法,对锂云母酸解过程反应动力学进行研究,以期为工业化应用提供理论依据。
1 实验
1.1 原料分析
实验所用原料为四川某地经选矿预处理的锂云母精矿,其化学成分分析结果、主要矿物相的晶格常数及电子探针分析结果分别如表1,2和3所列。其XRD谱如图1所示。其主要物相为锂云母、钠长石和石英。根据锂云母主要矿物相的电子探针分析结果,采用以阴离子为基准的氢当量法计算各矿物相的离子系数[15],矿物晶体的化学式分别如下:
锂云母(K0.93Na0.064Ca0.001)(Li1.327Al1.348Fe3+0.014Ti0.002- Mn0.034)[Al0.481Si3.519O10](OH)2;
钠长石(Na0.983K0.008Ca0.005Mg0.002Mn0.002)[Fe3+0.001- Ti0.001Al0.986Si3.007O8]
依据物质平衡原理[16]计算,锂云母精矿的物相组成为(质量分数):锂云母62.9%,钠长石22.2%,石英14.9%。

图1 锂云母精矿的XRD谱
Fig. 1 XRD pattern of lepidolite concentrate
1.2 实验原理与方法
锂云母在硫酸溶液中发生分解,化学反应为式如式(1)所示:
4K(Li1.5Al1.5)[AlSi3O10](OH)2+20H2SO4→
2K2SO4+3Li2SO4+5Al2(SO4)3+12H4SiO4↓ (1)
称取5 g锂云母精矿粉,置于聚四氟乙烯内胆中,加入一定量、一定浓度的硫酸,搅拌均匀后将其放入TL-JXF型水热反应釜,设置一定的转速和温度,待反应釜内达到设定温度开始计时,到达设定时间后,结束反应。采用SHB-Ⅲ循环水式多用真空泵对反应浆料进行抽滤,用蒸馏水洗涤滤饼4次(第四次洗液中Li2O浓度为1 mmol/L),洗涤时每次用水15 mL,将母液与洗液定容至200 mL。采用WFX-130型火焰原子吸收光谱仪分析滤液中Li2O的含量,计算实验原料中Li2O的溶出率。将所得滤饼置于101-1AB型电热鼓风干燥箱中,在110℃下烘干8h,采用D/Max-R型X射线衍射仪对产物进行表征。
表1 锂云母精矿的化学成分分析结果
Table 1 Chemical composition of lepidolite concentrate (mass fraction, %)
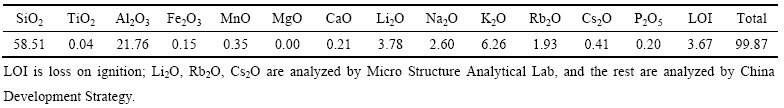
表2 主要矿物相的晶格常数
Table 2 Lattice parameters of principal minerals

表3 主要矿物相的电子探针分析结果
Table 3 Electron microprobe analysis of principal minerals

Li2O溶出率(x)的计算公式如下:
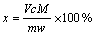 (2)
(2)
式中:m为锂云母精矿质量,g;w为锂云母精矿中锂云母的质量分数,%;V为溶出液体积,L;c为溶出液中Li2O的物质的量浓度,mol/L;M为Li2O的相对摩尔质量,g/mol。
2 结果与讨论
2.1 H2SO4浓度对Li2O溶出率的影响
称取5 g锂云母精矿粉,置于聚四氟乙烯内胆中,加入15 mL一定浓度的硫酸,控制转速为30 r/min,在160 ℃下反应4 h,考察H2SO4浓度对Li2O溶出率的影响,结果如图2所示。由图2可知,当H2SO4浓度由2.2 mol/L增至5.2 mol/L时,Li2O的溶出率随H2SO4浓度增大而提高;当H2SO4浓度由4.2 mol/L增加至5.2 mol/L时,Li2O溶出率增幅较小(不超过2%);当H2SO4浓度达6.2 mol/L时,Li2O溶出率有所降低。综合考虑,确定硫酸的浓度为4.2 mol/L。
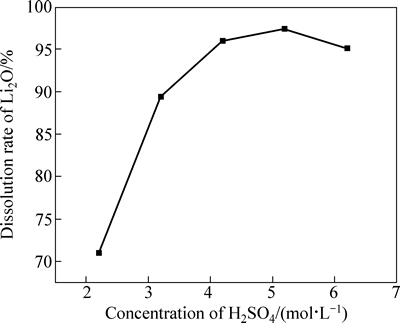
图2 H2SO4浓度对Li2O溶出率的影响
Fig. 2 Effect of H2SO4 concentration on dissolution rate of Li2O
2.2 液固质量比对Li2O溶出率的影响
控制2.1节中其他实验条件不变,当H2SO4浓度为4.2mol/L,考察液固质量比对Li2O溶出率的影响,结果见图3。由图3可知,Li2O溶出率随液固质量比增加而增大,液固质量比增大使固体颗粒在硫酸溶液中更分散,反应物混合更均匀,从而增加Li2O的溶出率。继续增加液固质量比,对Li2O的溶出率没有太大影响。因此,锂云母分解过程液固质量比选择为3:1。
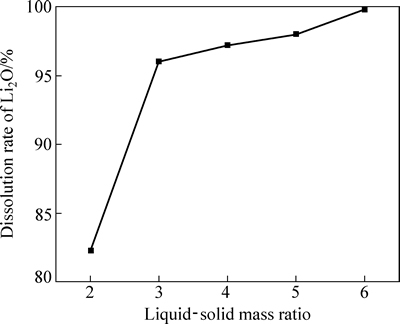
图3 液固质量比对Li2O溶出率的影响
Fig. 3 Effect of liquid-solid mass ratio on dissolution rate of Li2O
2.3 旋转速率对Li2O溶出率的影响
控制2.2节中其他实验条件不变,液固质量比为3:1,旋转速率对Li2O溶出率的影响见图4。由图4可知,在不同的旋转速率下,Li2O的溶出率随时间的延长而增加;当反应时间达到120 min后,Li2O的溶出率增加缓慢;在同一时刻,Li2O溶出率随旋转速率的增大而增加,但Li2O溶出率的增加幅度较小,可见旋转速率对Li2O溶出率的影响不大,这表明该过程不受外扩散控制。因此,锂云母分解过程旋转速率选择为30 r/min。
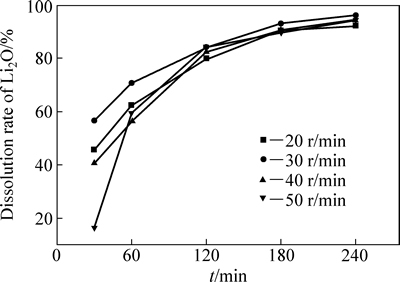
图4 旋转速率对Li2O溶出率的影响
Fig. 4 Effect of rotation speed on dissolution rate of Li2O
2.4 锂云母精矿颗粒直径对Li2O溶出率的影响
称取5 g粒径分别为50~75 μm、75~100 μm、100~125 μm的锂云母精矿粉,在160 ℃、液固质量比3:1、旋转速率为30 r/min的条件下反应0.5~4 h,粒径对Li2O溶出率的影响见图5。由图5可知,粒径50~75 μm的锂云母精矿反应2 h后,Li2O的溶出效果较好,溶出率达90%以上;而对于粒径75~100 μm、100~125 μm的锂云母精矿,Li2O的溶出率相对较低。由实验结果可知,在总质量一定的前提下,粒度越小,反应面积越大,从而有利于提高溶出率。
2.5 反应动力学实验结果与分析
不同实验条件下锂云母精矿酸解反应实验所测定Li2O的溶出率如表4所列。
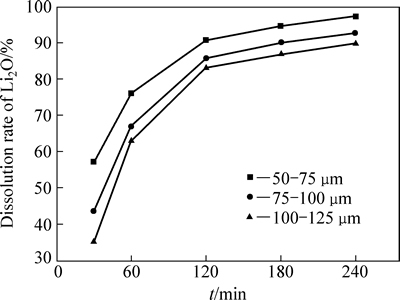
图5 颗粒直径对Li2O溶出率的影响
Fig. 5 Effect of particle diameter on dissolution rate of Li2O
由表4可知,在L-5实验条件下,Li2O溶出率达96%。在此条件下,锂云母酸解反应滤饼的XRD谱如图6所示,不同反应时刻Li2O溶出率关系曲线如图7所示。
由图6可知,锂云母完全分解,剩余物相为钠长石和石英相。由图7可知,反应30~120 min阶段锂云母酸解反应动力学曲线陡峭,表明初期锂云母颗粒表面的反应速率较快;反应120~240 min阶段曲线趋于平缓,原因是随反应时间的延长,在锂云母颗粒表面生成的原硅酸薄膜增厚,扩散阻力增大,反应速率降低。此外,在其他条件相同时,Li2O溶出率随温度升高而增大。其原因是随温度升高,粒子运动速度加快,反应体系液相黏度降低,有利于降低锂云母颗粒表面的薄膜包裹程度,减小扩散阻力[17],提高Li2O的溶出率。
2.6 反应动力学模型
锂云母精矿粉酸浸过程中,主要反应是锂云母与硫酸反应生成可溶性的硫酸盐,矿粉中的石英和长石相不与硫酸反应。锂云母与硫酸反应生成的原硅酸在锂云母颗粒表面形成一层薄膜,硫酸溶液必须穿过此薄膜,扩散到固体表面才能继续反应,因而其过程符合液-固单颗粒反应模型中的缩芯模型[18]。
2.7 锂云母酸解反应机理
表4 不同实验条件下锂云母精矿酸解反应实验结果
Table 4 Results of lepidolite concentrate decomposition reaction in sulfuric acid under different conditions


图6 酸解滤饼的XRD谱
Fig. 6 XRD pattern of acidolysis residue
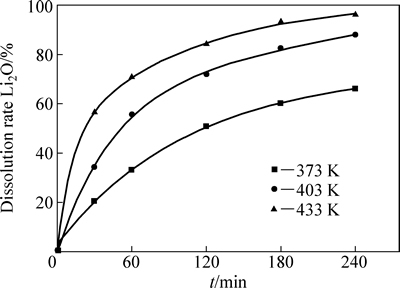
图7 不同温度下Li2O溶出率曲线图
Fig. 7 Leaching rate curve of Li2O at different temperatures
锂云母酸解反应包括以下5个过程:(1) H+从溶液向原硅酸薄膜层扩散(外扩散);(2) H+通过原硅酸薄膜层向锂云母颗粒表面扩散(内扩散);(3) H+与锂云母在颗粒表面发生反应,锂云母分解生成可溶性的硫酸盐;(4) 反应产物(K2SO4、Li2SO4、Al2(SO4)3)通过薄膜层向边界层扩散(内扩散);(5) 反应产物从边界层向溶液中扩散(外扩散)。其中,过程(1)和(5)属于液相传质控制;过程(3)属于化学反应控制;过程(2)和(4)属于固膜扩散控制[19-20]。本实验中在水热反应釜内旋转速率30 r/min条件下进行,固液间混合均匀,可忽略液相传质作用[21-22]。对于缩芯模型,其动力学方程[23]如下:
 (3)
(3)
式中:t为反应时间;b为反应物的摩尔比;ρp为固体颗粒密度(g/cm3);x为产物溶出率;Mp为含锂矿物相的相对摩尔质量(g/mol);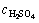 为硫酸的初始浓度(mol/mL);Rp为固体颗粒初始半径(cm);k0为化学反应速率常数;Dc为固膜扩散系数。
为硫酸的初始浓度(mol/mL);Rp为固体颗粒初始半径(cm);k0为化学反应速率常数;Dc为固膜扩散系数。
如酸浸反应属于固膜扩散控制过程,即化学反应速率常数k0非常大,式(3)可简化为
Y=1-2/3x-(1-x)2/3=kt (4)
式中:Y和k分别为固膜扩散控制过程反应动力学函数和反应速率常数,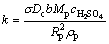 。
。
如酸浸反应属于化学反应控制过程,即固膜扩散系数Dc非常大,式(3)又可简化为
Y′=1-(1-x)1/3=k′t (5)
式中:Y'和k'为化学反应控制过程反应动力学函数和反应速率常数,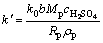 。
。
将表4中实验测定的Li2O溶出率x分别代入式(4)和式(5),分别求出Y和Y'值[24]。将不同温度下的动力学函数Y、Y'对时间t作图,用最小二乘法回归各直线方程。结果显示:Y与时间t呈良好的线性关系,在截距误差0.004范围内,各直线通过原点;Y'与时间t线性关系较差。说明Li2O浸出过程符合固膜扩散控制,其实验结果如图8所示,等温反应速率常数k及其相关系数r见表5。
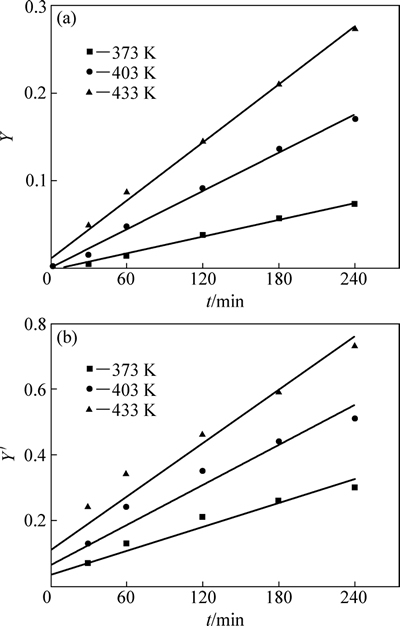
图8 不同温度下反应动力学函数Y和Y'与反应时间t关系图
Fig. 8 Relationship between reaction kinetic function Y, Y'and reaction time t at different temperatures
表5 反应速率常数k及其相关系数r
Table 5 Reaction rate constant k and correlation coefficient r

由图8和表5可知,动力学实验结果较好地符合公式Y=1-2/3x-(1-x)2/3,相关系数r大于0.993,表明所选动力学模型与实验结果吻合较好。
2.8 锂云母酸解反应表观活化能Ea
反应速率常数k是温度的函数,温度对其影响可用Arrhenius公式[25]表示
k=Aexp[-Ea/(RT)] (6)
式中:A为指前因子(其因次与k相同);Ea为反应活化能;T为反应温度;R为摩尔气体常数(R=8.314 J/(K·mol))。对式(6)取对数可得到
lnk=lnA-Ea/(RT) (7)
以lnk对1/T作图,得到图9。由图9可知,lnk与1/T呈良好的线性关系,直线斜率为-Ea/R,截距为lnA。

图9 锂云母酸浸反应速率常数与温度关系
Fig. 9 Relationship between reaction rate constant and temperatures of cepidolite during acid leaching process
由图9可知,直线斜率为-3.4,计算得锂云母分解反应表观活化能Ea=28.3 kJ/mol。研究表明,受化学反应控制的反应过程,按Arrhenius方程计算的表观活化能应大于41.8 kJ/mol[26],说明在硫酸介质中锂云母分解的动力学过程符合扩散控制。根据公式1-2/3x-(1-x)2/3,可计算出433 K下Li2O溶出率达到84%时,理论上需要127 min,而实际反应时间为120 min。实际反应时间比理论计算值少7 min,原因是水热反应釜由室温升至反应温度过程中,锂云母已发生分解反应,可见实验结果与理论预测基本吻合。
2.9 反应级数
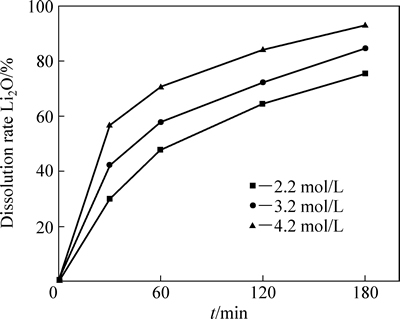
图10 不同硫酸浓度下Li2O溶出率曲线
Fig. 10 Leaching rate curves of Li2O at different H2SO4 concentrations

图11 精矿浸出的lnk-lnc(H2SO4)关系图
Fig. 11 Relationship between lnk and lnc(H2SO4) for concentrate leaching
根据实验结果,浸出动力学方程可表示为
1-2/3x-(1-x)2/3=k0exp[-Ea/(RT)] t/Rp2 (8)
t/Rp2 (8)
式中:k0为阿伦尼乌斯常数,n为反应级数。根据图9直线截距求得k0=2.608。为进一步确定硫酸的表观反应级数n,进行不同浓度硫酸、不同时间的酸溶反应实验,图10所示为Li2O溶出率结果。反应级数n可由lnk和lnc(H2SO4)作图求出(见图11),由图11可得硫酸的表观反应级数n=0.998。将k0和n分别代入浸出动力学方程,可得[27]
1-2/3x-(1-x)2/3=2.608exp[-28.3/(RT)] t/Rp2
t/Rp2
在公式1-2/3x-(1-x)2/3= kt中,k=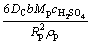 ,其中DC、b、Mp、
,其中DC、b、Mp、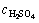 、ρp均为定值,故k∝1/Rp2。
、ρp均为定值,故k∝1/Rp2。
3 结论
1) 锂云母在硫酸溶液中发生分解反应,在硫酸浓度为4.2 mol/L、硫酸与锂云母的液固质量比为1:3、温度433 K、转速30 r/min条件下反应4 h,锂云母完全分解,Li2O溶出率达96%。
2) 反应动力学实验结果表明:锂云母酸解过程中Li2O溶出率随温度升高而增大。相同温度下,在0~3 h内,随反应时间的延长而增大;反应3 h后,Li2O的溶出率变化不明显。锂云母酸解反应过程可以用液-固单颗粒反应模型中的缩芯模型描述,动力学方程符合Y=1-2/3x-(1-x)2/3,拟合的线性相关系数r>0.993。按照Arrhenius方程计算,在硫酸介质中,锂云母分解反应的表观活化能Ea=28.3 kJ/mol,阿伦尼乌斯常数k0=2.608,反应级数n=0.998,反应过程受固膜扩散控制。
REFERENCES
[1] 郭春平, 周 健, 文小强, 普 建, 王玉香, 袁德林. 锂云母硫酸盐法提锂研究[J]. 无机盐工业, 2014, 46(3): 41-44.
GUO Chun-ping, ZHOU Jian, WEN Xiao-qiang, PU Jian, WANG Yu-xiang, YUAN De-lin. Extraction of lithium from lepidolite by sulfate process[J]. Inorganic Chemicals Industry, 2014, 46(3): 41-44.
[2] 何启贤. 世界锂金属资源开发利用现状及其市场前景分析[J]. 轻金属, 2011(9): 3-8.
HE Qi-xian. Develoment status of lithium metal resources in the world and its prospect[J]. Light Metals, 2011(9): 3-8.
[3] 纪志永, 焦鹏鹏, 袁俊生, 王 阳. 锂资源的开发利用现状与发展分析[J]. 轻金属, 2013(5): 1-5.
ZHU Zhi-yong, JIAO Peng-peng, YUAN Jun-sheng, WANG Yang. The exploitation and utilization of lithium resources and its development[J]. Light Metals, 2013(5): 1-5.
[4] U.S.Geological Survey, 2015, Mineral commodity summaries 2015: U.S. Geological Survey, 196p.
[5] 司秀芬, 张伟光, 何利华, 梁新星, 赵中伟. 尖晶石锰酸锂卤水提锂热力学[J]. 中国有色金属学报, 2013, 23(12): 3410-3422.
SI Xiu-fen, ZHANG Wei-guang, HE Li-hua, LIANG Xin-xing, ZHAO Zhong-wei. Thermodynamics of Li-extraction from brine using spinel LiMn2O4[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(12): 3410-3422.
[6] 赵中伟, 梁新星, 刘旭恒, 何利华, 陈星宇, 司秀芬, 陈爱良. 磷酸铁离子筛卤水提锂热力学分析[J]. 中国有色金属学报, 2013, 23(2): 559-567.
ZHAO Zhong-wei, LIANG Xin-xing, LIU Xu-heng, HE Li-hua, CHEN Xing-yu, SI Xiu-fen, CHEN Ai-liang. Thermodynamics analysis of Li-extraction from brine using FePO4 ion-sieve[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(2): 559-567.
[7] KESLER S E, GRUBER P W, MEDINA P A, KOOLEIAN G A, EVERSON M P, WALLINGTON J J, KESLER S E. Global lithium resources: Relative importance of pegmatite, brine and other deposits[J]. Ore Geology Reviews, 2012, 48: 55.
[8] OGORDOVA L P, KISELEVE I A, MELCHAKOVA L V, SCHURIGA T N. Thermodynamic properties of lithium mica: Lepidolite[J]. Thermochimica Acta, 2005, 435(1): 68.
[9] 李冰心. 2014年锂市场发力前行[J]. 中国战略新兴产业, 2014(10): 74-75.
LI Bing-xin. Lithium-market made a big progress in 2014[J]. China Strategic Emerging Industry, 2014(10: 74-75.
[10] 李冰心. 我国锂市场2013年回顾与2014年展望[J]. 新材料产业, 2014(2): 44-47.
LI Bing-xin. 2013 review and 2014 outlook of Lithium-Market in China[J]. Advanced Materials Industry, 2014(2): 44-47.
[11] 虞宝煜, 胡永海, 辛文芳. 锂云母石灰石烧结中碳硅灰石的生成及作用[J]. 中国有色金属学报, 1995, 5(4): 47-50.
YU Bao-yu, HU Yong-hai, XIN Wen-fang. Formation and role of carbon wollastonite in reaction of lepidolite limestone sintering[J]. The Chinese Journal of Nonferrous Metals, 1995, 5(4): 47-50.
[12] YAN Qun-xuan, LI Xin-hai, WANG Zhi-xing, WANG Jie-xi, GUO Hua-jun, HU Qi-yang, PENG Wen-jie, WU Xi-fei. Extraction of lithium form lepidolite using chlorination roasting-water leaching process[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(7): 1753-1759.
[13] 舒启溢, 胡炜华, 邱祖民. 用硫酸盐法从锂云母中浸取锂[J]. 南昌大学学报(工科版), 2011, 33(4): 325-327.
SHU Qi-yi, HU Wei-hua, QIU Zu-min. Lithium leaching form lithium-mica using sulfate[J]. Journal of Nanchang University (Engineering and Technology), 2011, 33(4): 325-327.
[14] LUONG V T, DONG J K, AN J W, DAO D A, KIM M J, TRAN T. Iron sulphate roasting for extraction of lithium from lepidolite[J]. Hydrometallurgy, 2014, 141: 8-16.
[15] 马鸿文. 结晶岩热力学概论[M]. 2版. 北京: 高等教育出版社, 2001: 1-17.
MA Hong-wen. Introduction to thermodynamics in crystalline petrology[M]. 2nd ed. Beijing: Higher Education Press, 2001: 1-17.
[16] 马鸿文, 杨 静, 刘 贺, 李贺香, 白志民, 王庆华. 硅酸盐体系的化学平衡: (1)物质平衡原理[J]. 现代地质, 2006, 2: 329-339.
MA Hong-wen, YANG Jing, LIU He, LI He-xiang, BAI Zhi-min, WANG Qing-hua. Chemical equilibrium in silicate system: Part Ⅰ Mass balance principle[J]. Geoscience, 2006, 2: 329-339.
[17] 唐晓宁, 陈肖虎, 薛 安. 赤泥中钪的浸出动力学研究[J]. 湿法冶金, 2010, 29(3): 155-158.
TANG Xiao-ning, CHEN Xiao-hu, XUE An. Leaching kinetics of scandium from red mud [J]. Hydrometallyrgy of China, 2010, 29(3): 155-158.
[18] 占寿祥, 郑雅杰. 硫铁矿烧渣酸浸反应动力学研究[J]. 化学工程, 2006, 34(11): 36-39.
ZHAN Shou-xiang, ZHENG Ya-jie. Study on leaching kinetics of pyrite cinder[J]. Chemical Engineering, 2006, 34(11): 36-39.
[19] 徐耀兵, 骆仲泱, 王勤辉, 周劲松, 施正伦. 石煤灰渣酸浸提钒工艺中钒的浸出动力学[J]. 过程工程学报, 2010, 10(1): 60-63.
XU Yao-bing, LUO Zong-yang, WANG Qin-hui, ZHOU Jin-song, SHI Zheng-lun. Leaching kinetics of vanadium from acid leaching of stone coal[J]. The Chinese Journal of Process Engineering, 2010, 10(1): 60-63.
[20] AL-SALEH H M. A Kinetic study of leaching process of raney silver catalyst[J]. Chemical Engineering Journal, 1999, 72(2): 229-233.
[21] JEFFREY M I. Kinetic aspects of gold and silver leaching in ammonia-thiosulfate solutions[J]. Hydrometallurgy, 2001, 60(1): 7-16.
[22] 郑雅杰, 陈昆昆. 采用Na2SO3溶液从硒渣中选择性浸出Se及其动力学[J]. 中国有色金属学报, 2012, 22(2): 585-590.
ZHENG Ya-jie, CHEN Kun-kun. Selective leaching Se from selenium residue by Na2SO3 solutions and leaching kinetics[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(2): 585-590.
[23] ABDEL L E A, RASHAD M M. Kinetic study on the leaching of spent nickel oxide catalyst with sulfuric acid[J]. Hydrometallurgy, 2004, 74(2): 189-194.
[24] LEVINE I N. Physical Chemistry[M]. 5th ed. New York: McGraw-Hill, 2003: 528-597.
[25] FENG Xing-liang, LONG Zhi-qi, CUI Da-li, WANG Liang-shi, HUANG Xiao-wei, ZHANG Guo-cheng. Kinetics of rare leaching from roasted ore of bastnaesite with sulfuric acid[J]. Transaction of Nonferrous Metals Society of China, 2013, 23: 849-854.
[26] 李洪桂. 湿法冶金学[M]. 长沙: 中南大学出版社, 2005: 69-123.
LI Hong-gui. Hydrometallurgical[M]. Changsha: Central South University Press, 2005: 69-123.
[27] 张晓伟, 李 梅, 柳召刚, 胡艳宏, 王觅堂, 刘 佳, 阳建平. 包头稀土精矿的配合浸出及动力学[J]. 中国有色金属学报, 2014, 24(8): 2137-2143.
ZHANG Xiao-wei, LI Mei, LIU Zhao-gang, HU Yan-hong, WANG Mi-tang, LIU Jia, YANG Jian-ping. Complex leaching and kinetics of Baotou mixed rare earth concentrate[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(8): 2137-2143.
(编辑 龙怀中)
基金项目:中央高校基本科研业务费资助项目(2652014017)
收稿日期:2014-12-01;修订日期:2015-06-02
通信作者:杨 静,副教授,博士;电话:010-82320512;E-mail:yjcl@cugb.edu.cn