文章编号:1004-0609(2016)-06-1303-08
葡萄糖在氧化锰矿浸出过程中的分解动力学
王雨红,雷作敏,屈欣轲,黄丽滢,侯佳敏,欧阳秋林,粟海锋
(广西大学 化学化工学院 广西高校资源化工应用新技术重点实验室,南宁 530004)
摘 要:研究在硫酸水溶液体系葡萄糖还原浸出氧化锰矿过程中的分解动力学,通过测定溶液中COD含量的变化,考察葡萄糖用量、硫酸浓度、氧化锰矿用量和反应温度对葡萄糖氧化分解速率的影响。结果表明:提高硫酸浓度、氧化锰矿用量和反应温度可以加快浸出反应过程葡萄糖的氧化分解速率。葡萄糖的氧化分解过程可以用指数经验模型来描述,属于扩散-化学反应混合控制。通过拟合动力学数据,获得葡萄糖氧化分解反应的活化能为41.80 kJ/mol,COD、硫酸和氧化锰矿的表观反应级数分别为2.0,0.927和0.976。
关键词:氧化锰矿;葡萄糖;分解;动力学
中图分类号:TQ028.96;TF803 文献标志码:A
我国是世界上最大的锰产品生产国和消费国[1]。由于多年的大量开采,碳酸锰矿的储量和品位大幅降低,而储量可观的低品位氧化锰矿的开发利用越来越受到科研工作者的重视。近年来,以蔗糖[2]、葡萄糖[3]、纤维素[4]、废糖蜜[5]、燕麦秸秆[6]、桔子皮[7]、木屑[8]、废茶叶[9]、商陆[10]等小分子有机物或农林副产物还原浸出氧化锰矿的研究,由于浸出工艺高效、节能、清洁、环保,成为研究的热门课题。然而,为了获得较高的锰浸出率,往往使用高于理论用量的有机还原剂,造成还原剂在逐步氧化分解的过程中,产生一些不彻底氧化降解的有机衍生产物。以葡萄糖为例,FURLANI等[11]研究了稀硫酸介质中使用葡萄糖还原浸出氧化锰矿过程的衍生产物,发现葡萄糖的碳链逐步被氧化拆解,大部分被彻底分解生成CO2和水,但还有少量降解产物依次生成葡萄糖酸、甘油酸、乙醇酸和甲酸等小分子有机物,残留在溶液中。葡萄糖在还原浸出氧化锰矿过程中的化学反应式如下式所示:
主反应[2],
C6H12O6+12MnO2+24H+=6CO2+12Mn2++18H2O (1)
副反应[11],
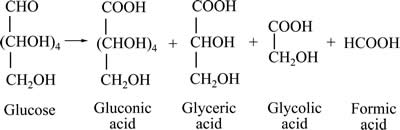 (2)
(2)
在实际生产过程中发现,这些残余有机物会影响电解锰的着板率和成品质量,且会腐蚀阳极板,给电解锰生产过程造成不良的影响。因此,研究氧化锰矿还原浸出过程中葡萄糖的氧化分解机理具有重要意义。
氧化锰矿中的MnO2在酸性介质中表现出较强的氧化性,可以与有机物逐步发生氧化还原反应,从而实现有机物的氧化降解。如废水处理过程中降解亚甲基蓝[12]、间苯胺黄[13]、酸性橙7[14]、聚乙烯薄膜[15]等。而在动力学研究方面,SU等[16]在硫酸介质中以软锰矿为氧化剂,研究了糖蜜酒精废液的氧化脱色降解规律。该过程属于扩散-化学控制,表观活化能为57.5 kJ/mol,糖蜜酒精废液浓度、硫酸浓度的反应级数分别是0.90及0.52。L?等[17]进一步对软锰矿降解废糖蜜酒精废液中美拉德色素的动力学行为进行研究,认为该过程符合收缩芯模型,表观活化能为56.2 kJ/mol,美拉德色素浓度、硫酸浓度的反应级数分别是0.93及0.54。WU等[18]对氧化锰矿降解纤维素的过程进行研究,认为反应前60 min硫酸浓度及纤维素用量的反应级数分别为2.102、0.660,表观活化能为46.487 kJ/mol,而后90 min硫酸浓度及纤维素用量的反应级数分别为3.642、0.724,表观活化能为62.29 kJ/mol。然而,这些动力学研究主要以锰的浸出率为目标,只考虑了有机物的初始浓度,并未对浸出过程中有机物的降解机理进行研究。因此,本文作者研究葡萄糖在硫酸水溶液中被锰矿氧化分解的规律,考察葡萄糖浓度、硫酸浓度、氧化锰矿用量和反应温度对葡萄糖氧化分解速率的影响,获得葡萄糖氧化分解过程的动力学数据,其研究成果不仅可为开发低有机物残余的氧化锰矿浸出工艺提供理论依据,也可为研究其它同类有机物的氧化分解规律提供参考。
1 实验
1.1 材料与试剂
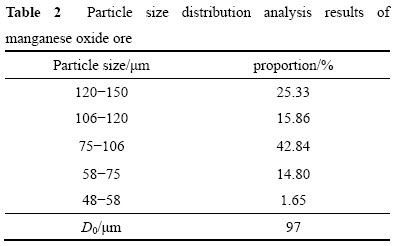
本研究中所用的氧化锰矿取自广西大新锰矿,主要成分见表1。锰矿经破碎,过(孔径150 μm),结果见表2,平均粒径D0为97 μm。烘干后置于干燥器中备用。试验所用一水葡萄糖、硫酸、硝酸等试剂均为分析纯,水为去离子水。
表1 氧化锰矿化学组成
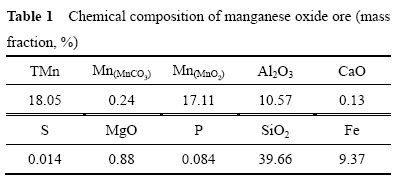
表2 氧化锰矿粒径分布情况及平均粒径
1.2 实验过程与步骤
动力学实验在1000 mL三口烧瓶中进行,置于恒温水浴中。三口烧瓶一侧口装有冷凝管防止水分蒸发,另一侧口装有温度计及取样器。首先加入葡萄糖和一定量的硫酸,开启搅拌装置,待溶液温度达到所需温度后加入氧化锰矿后开始计时,定期取样分析。葡萄糖的分解过程用溶液中有机物含量的变化来表示,因此,采用微波密封消解法[19]检测COD值。如无特别说明,浸出实验按下列条件操作:20.00 g氧化锰矿,温度70 ℃,硫酸浓度1.5 mol/L,葡萄糖浓度1.000 g/L,搅拌速率700 r/min。
2 结果与讨论
2.1 动力学模型选择
氧化锰矿降解美拉德色素[17]和焦糖色素[20]的研究表明,在硫酸溶液中氧化锰矿降解有机物的速率方程可用指数经验模型表示:
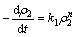 (3)
(3)
式中:k1是反应速率常数,表示除葡萄糖浓度以外其它因素对速率的影响;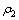 为反应浆液COD的含量,mg/L;t为反应时间,min;n为COD的表观反应级数。在葡萄糖被氧化锰矿分解的过程中,影响溶液中COD含量变化的因素除葡萄糖浓度外,还有硫酸浓度、氧化锰矿用量和反应温度。因此,葡萄糖被氧化锰矿分解过程中的动力学方程可表示为
为反应浆液COD的含量,mg/L;t为反应时间,min;n为COD的表观反应级数。在葡萄糖被氧化锰矿分解的过程中,影响溶液中COD含量变化的因素除葡萄糖浓度外,还有硫酸浓度、氧化锰矿用量和反应温度。因此,葡萄糖被氧化锰矿分解过程中的动力学方程可表示为
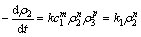 (4)
(4)
其中:
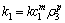 (5)
(5)
式中: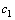 为硫酸浓度,mol/L;
为硫酸浓度,mol/L;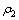 为反应浆液COD的含量,mg/L;
为反应浆液COD的含量,mg/L; 为氧化锰矿的浓度,g/L;m为硫酸的表观反应级数;n为COD的表观反应级数;p为氧化锰矿的表观反应级数。
为氧化锰矿的浓度,g/L;m为硫酸的表观反应级数;n为COD的表观反应级数;p为氧化锰矿的表观反应级数。
为了研究葡萄糖浓度对溶液COD含量变化的影响,分别在0.600、0.800、1.000、1.200及1.400 g/L的葡萄糖浓度下进行反应,结果见图1。从图1可以看出,前20 min反应浆液中的COD含量快速减小,此后随着反应时间的延长,浆液中COD的含量缓慢下降。
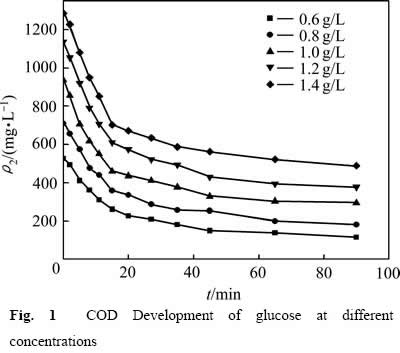
图1 不同葡萄糖浓度下COD的变化
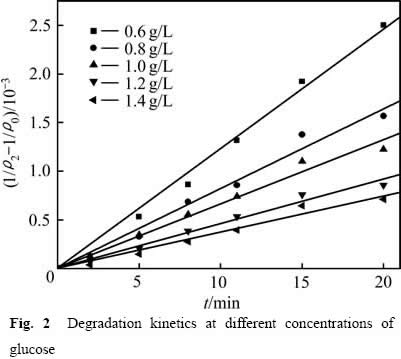
图2 不同葡萄糖浓度下COD的降解动力学
用式(4)和最小二乘法处理图1中的数据,结果如图2所示。图2中相关系数R2分别为:0.996(0.600g/L),0.983(0.800 g/L),0.983(1.000 g/L),0.984(1.200 g/L),0.977(1.4 00 g/L),表明当n=2.0时,式(3)可以较好地描述不同葡萄糖浓度下浆液中葡萄糖的降解规律,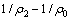 (
(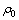 表示初始反应浆液COD的含量, mg/L)与反应时间t呈线性关系。根据图2的结果,将n=2.0代入方程(4)并积分,氧化锰矿氧化分解葡萄糖的动力学方程可进一步写成:
表示初始反应浆液COD的含量, mg/L)与反应时间t呈线性关系。根据图2的结果,将n=2.0代入方程(4)并积分,氧化锰矿氧化分解葡萄糖的动力学方程可进一步写成:
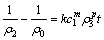 (6)
(6)
2.2 温度的影响
实验研究了50 ℃,60 ℃,70 ℃,80 ℃,90 ℃对葡萄糖氧化分解过程中COD浓度变化的影响,结果如图3所示。从图3中可以看出反应温度对葡萄糖的氧化氧化分解产生较大的影响。升高反应温度,浆液的黏度下降,反应传质速率加快,同时,活化分子的比例提升,单位时间单位空间内反应分子有效碰撞的几率增大,从而提高了反应速率和葡萄糖氧化分解的程度。
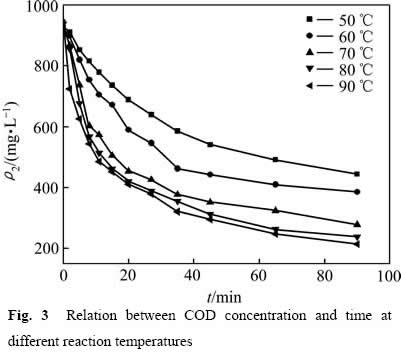
图3 不同反应温度下COD浓度与时间关系
采用式(6)处理图3中数据,结果如图4所示。不同温度下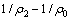 与t成线性关系,相关系数R2分别为:0.998(50 ℃),0.990(60 ℃),0.984(70 ℃),0.986(80 ℃),0.992(90 ℃)。得到不同温度下葡萄糖氧化分解过程的速率常数k1分别为1.854×10-5(50 ℃),2.896×10-5(60 ℃),5.818×10-5(70 ℃),7.763×10-5(80 ℃),9.451×10-5(90 ℃)。
与t成线性关系,相关系数R2分别为:0.998(50 ℃),0.990(60 ℃),0.984(70 ℃),0.986(80 ℃),0.992(90 ℃)。得到不同温度下葡萄糖氧化分解过程的速率常数k1分别为1.854×10-5(50 ℃),2.896×10-5(60 ℃),5.818×10-5(70 ℃),7.763×10-5(80 ℃),9.451×10-5(90 ℃)。
可用阿仑尼乌斯方程表示反应温度对葡萄糖氧化分解速率的影响,对方程两边取对数得:
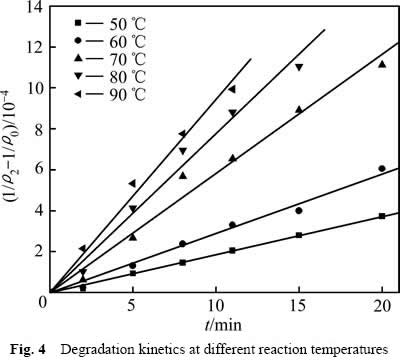
图4 不同反应温度下COD降解动力学
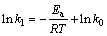 (7)
(7)
用式(7)处理数据,然后对1/T作图,结果如图5所示。由图5可知,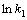 与
与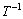 呈线性关系,相关系数R2为0.985,可得关系式:
呈线性关系,相关系数R2为0.985,可得关系式:
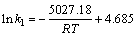 (8)
(8)
比较式(7)和(8)可得到氧化锰矿氧化分解葡萄糖的表观活化能为41.80 kJ/mol。
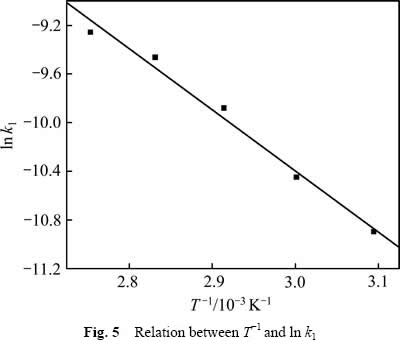
图5 T-1与ln k1关系
2.3 硫酸浓度影响
为了考察硫酸浓度对葡萄糖氧化分解过程的影响,分别在硫酸浓度为0.50、0.75、1.00、1.25及1.50 mol/L时测定葡萄糖氧化分解过程的动力学数据,结果见图6所示。
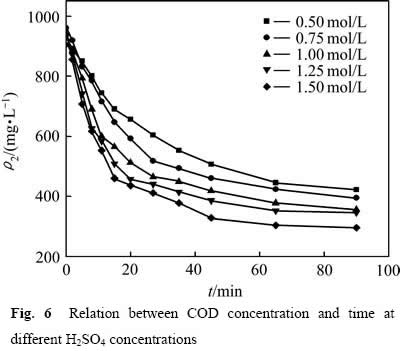
图6 不同硫酸浓度下COD浓度与时间关系
从图6可以看出,当硫酸浓度较低时,葡萄糖氧化分解反应温和,COD浓度下降较慢;提高硫酸浓度,COD浓度下降明显。这是因为提高硫酸浓度,反应体系中的H+浓度增大,氧化反应有效碰撞的几率增加,同时MnO2/Mn2+的氧化电位提高,增大了氧化反应的推动力,加快反应速率。但随着反应时间的延长,浆液中葡萄糖逐步分解,浓度降低,FURLANI等[11]经研究发现,随着反应时间的增加,溶液中葡萄糖完全分解,残余在溶液中的少量有机物主要以甲酸形式存在,还原性较弱,因此导致反应还原推动力下降,有机物氧化分解速率减缓,造成反应后期COD浓度下降缓慢。采用不同硫酸浓度下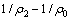 对反应时间t作图,结果如图7所示。
对反应时间t作图,结果如图7所示。
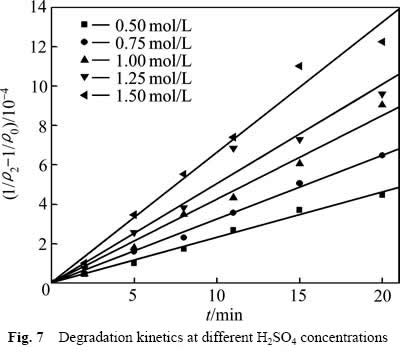
图7 不同硫酸浓度下COD降解动力学
从图7中可以看出,不同硫酸浓度的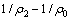 与t呈线性关系,相关系数R2分别:0.992(0.5 mol/L),0.997(0.75 mol/L),0.991(1.00 mol/L),0.973(1.25 mol/L),0.983(1.50 mol/L)。这表明式(6)可以很好地描述不同硫酸浓度下葡萄糖的氧化分解动力学。相应的速率常数k1分别为:2.319×10-5(0.5 mol/L),3.242×10-5(0.75 mol/L),4.269×10-5(1.00 mol/L),5.035×10-5(1.25 mol/L),6.629×10-5(1.50 mol/L)。
与t呈线性关系,相关系数R2分别:0.992(0.5 mol/L),0.997(0.75 mol/L),0.991(1.00 mol/L),0.973(1.25 mol/L),0.983(1.50 mol/L)。这表明式(6)可以很好地描述不同硫酸浓度下葡萄糖的氧化分解动力学。相应的速率常数k1分别为:2.319×10-5(0.5 mol/L),3.242×10-5(0.75 mol/L),4.269×10-5(1.00 mol/L),5.035×10-5(1.25 mol/L),6.629×10-5(1.50 mol/L)。
当氧化锰矿用量和反应温度不变时,对式(5)两边取对数得:
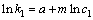 (9)
(9)
用式(9)处理不同硫酸浓度下所得速率常数k1,作图,结果如图8所示。由图8可知, 与
与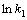 呈线性关系,相关系数R2为0.990,其关系式:
呈线性关系,相关系数R2为0.990,其关系式:
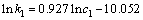 (10)
(10)
由式(10)可知,反应中硫酸浓度的表观反应级数为0.927。
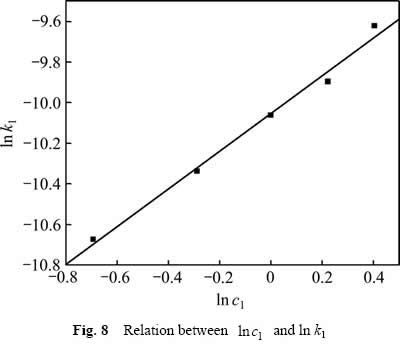
图8  与ln k1关系
与ln k1关系
2.4 氧化锰矿用量的影响
分别考察氧化锰矿用量为12、16、20、24、26 g/L对葡萄糖氧化分解过程的影响。结果如图9所示。从图9中可以看出,不同氧化锰矿用量下的COD的浓度均随着时间的延长而不断降低。提高氧化锰矿的用量,由于两相接触面积的增加,反应有效碰撞的几率不断增加,加快了反应速率,同时使葡萄糖与氧化锰矿发生的氧化还原反应进行更充分,增大了葡萄糖的转化率。
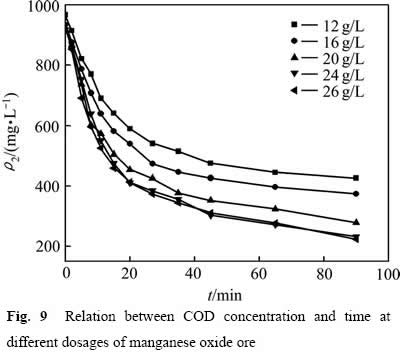
图9 不同氧化锰用量下COD浓度与时间关系
采用式(6)处理图9中数据,结果如图10所示。不同氧化锰矿用量下的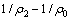 与t呈线性关系,相关系数R2分别为:0.993(12.00 g/L),0.992(16.00 g/L),0.994(20.00 g/L),0.995(24.00 g/L),0.998(26.00 g/L)。获得不同氧化锰矿用量下葡萄糖氧化分解过程的速率常数k1分别为:3.435×10-5(12.00 g/L),4.137×10-5(16.00 g/L),5.116×10-5(20.00 g/L),6.577×10-5(24.00 g/L),7.231×10-5(26.00 g/L)。
与t呈线性关系,相关系数R2分别为:0.993(12.00 g/L),0.992(16.00 g/L),0.994(20.00 g/L),0.995(24.00 g/L),0.998(26.00 g/L)。获得不同氧化锰矿用量下葡萄糖氧化分解过程的速率常数k1分别为:3.435×10-5(12.00 g/L),4.137×10-5(16.00 g/L),5.116×10-5(20.00 g/L),6.577×10-5(24.00 g/L),7.231×10-5(26.00 g/L)。
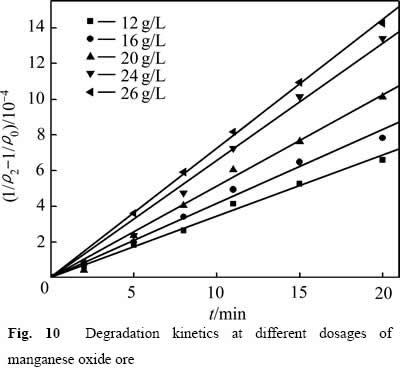
图10 不同氧化锰矿用量下COD降解动力学
在硫酸浓度和反应温度不变的条件下,对式(5)两边取对数得:
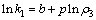 (11)
(11)
用式(11)处理不同氧化锰矿用量下所得速率常数k1,作图,结果如图11所示,从图11中可知,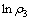 与
与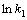 呈直线关系,直线的相关系数R2为0.977,可得关系式:
呈直线关系,直线的相关系数R2为0.977,可得关系式:
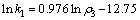 (12)
(12)
由式(12)可知,反应中氧化锰矿的表观反应级数为0.976。
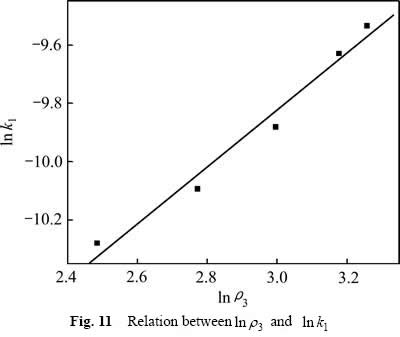
图11 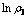 与
与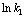 关系
关系
2.5 动力学总方程
用上述计算的结果,代入式(6),经最小二乘法回归即可得到葡萄糖的氧化分解动力学方程,即:
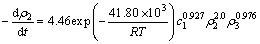 (13)
(13)
图12所示为利用式(13)计算得到的反应液COD浓度值与实验值的比较图,计算值和实验值的偏差基本落在±10%的范围内。动力学模型采用F检验[21]:
 (14)
(14)
式中:P为模型参数个数;N为实验数据点数值;SR为回归平方和;SE为剩余平方和;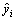 为模型计算值;yi为实验值;
为模型计算值;yi为实验值;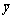 为实验点平均值。
为实验点平均值。
模型的F检验值为:F=753.64>Fα=3.96(α=0.01)[22],可见,该动力学模型的可信度显著,式(13)可以较好地描述硫酸介质中葡萄糖被氧化锰矿分解的动力学行为。
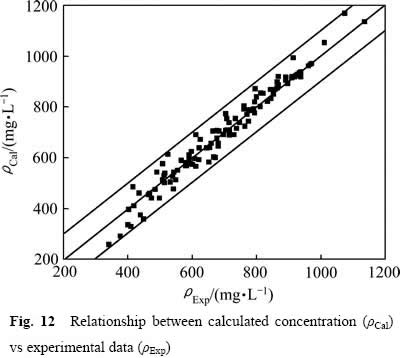
图12 反应液CDO浓度计算值与理论值的关系
3 结论
1) 葡萄糖被氧化锰矿分解过程可以用指数经验模型来描述,其反应过程属于扩散-化学反应控制。
2) 提高硫酸浓度、氧化锰矿用量和反应温度可以加快氧化锰矿浸出过程葡萄糖的氧化分解速率。
3) 硫酸介质中葡萄糖被氧化锰矿分解反应的表观活化能为41.80 kJ/mol,COD、硫酸和氧化锰矿的表观反应级数分别为2.0,0.927和0.976。
4) 硫酸介质中葡萄糖被氧化锰矿分解反应的动力学方程为:
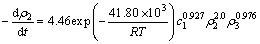
REFERENCES
[1] ZHANG Hong-lei, ZHU Guo-cai, YAN Hong, LI Tian-cheng, FENG Xiu-juan. Thermogravimetric analysis and kinetics on reducing low-grade manganese dioxide ore by biomass[J]. Metallurgucal and Materials Transactions B, 2013, 878(44B): 878-888.
[2] BEOLCHINI F, PETRANGELI PAPINI M, TORO L. Acid leaching of manganiferous ores by sucrose: Kinetic modelling and related statistical analysis[J]. Minerals Engineering, 2001, 14(2): 175-184.
[3] PAGNANELLI F, FURLANI G, VALENTINI P, et al. Leaching of low-grade manganese ores by using nitric acid and glucose: optimization of the operating conditions[J]. Hydrometallurgy, 2004, 75(1/4): 157-167.
[4] ZHANG Xiao-yun, TIAN Xue-da, ZHANG Dong-fang. Separation of silver from silver-manganese ore with cellulose as reductant[J]. Transactions of Nonferrous Metals Society of China, 2006, 16(3): 705-708.
[5] SU Hai-feng, WEN Yan-xuan, WANG Fan, SUN Ying-yun, TONG Zhang-fa. Reductive leaching of manganese from low-grade manganese ore in H2SO4 using cane molasses as reductant[J]. Hydrometallurgy, 2008, 93(3/4): 136-139.
[6] 满瑞林, 常 伟, 吴 奇, 薛建荣. 燕麦秸秆还原浸出低品位软锰矿及其动力学[J]. 中国有色金属学报, 2014, 24(8): 2158-2163.
MAN Rui-lin, CHANG Wei, WU Qi, XUE Jian-rong. Reductive leaching and leaching kinetics of low-grade pyrolusite ores using oat straw as reductant[J]. The Chinese Journal of Nonferrous Metals, 24(8): 2158-2163.
[7] 牛莎莎, 王志兴, 郭华军, 李新海, 彭文杰, 胡启阳, 张云河. 电解锰阳极渣还原浸出锰[J]. 中国有色金属学报, 2012, 22(9): 2662-2666.
NIU Sha-sha, WANG Zhi-xing, GUO Hhua-jun, LI Xin-hai, PENG Wen-jie, HU Qi-yang, ZHANG Yun-he. Reductive leaching of manganese from manganese anode slag[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(9): 2662-2666.
[8] EKMEKYAPAR A, ASIN C, DEMIRKIRAN N, K?NK?L A. Reductive leaching of pyrolusite ore by using sawdust for production of manganese sulfate[J]. Russian Journal of Non-Ferrous Metals, 2012, 53(2): 211-217.
[9] TANG Qing, ZHONG Hong, WANG Shuai, LI Jin-zhong, LIU Guang-yi. Reductive leaching of manganese oxide ores using waste tea as reductant in sulfuric acid solution[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(3): 861-867.
[10] XUE Jian-rong, ZHONG Hong, WANG Shuai, LI Chang-xin, LI Jin-zhong, WU Fang-fang. Kinetics of reduction leaching of manganese dioxide ore with Phytolacca americana in sulfuric acid solution[J]. Journal of Saudi Chemical Society, 2014: 1-6.
[11] FURLANI G, PAGNANELLI F, TORO L. Reductive acid leaching of manganese dioxide with glucose: Identification of oxidation derivatives of glucose[J]. Hydrometallurgy, 2006, 81(3/4): 234-240.
[12] KUAN Wen-hui, CHAN Yen-chuan. pH-dependent mechanisms of methylene blue reacting with tunneled manganese oxide pyrolusite[J]. Journal of Harzadous Materials, 2012, 239/240: 152-159.
[13] CLARKE C E, KIELAR F, JOHNSON K L. The oxidation of acid azo dye AY 36 by a manganese oxide containing mine waste[J]. Journal of Harzardous Materials, 2013, 246/247: 310-318.
[14] CLARKE C E, KIELAR F, JOHNSON K L. Oxidative breakdown of acid orange 7 by a manganese oxide containing mine waste: Insight into sorption, kinetics and reaction dynamics[J]. Applied Catalysis B: Environment, 2010, 101: 13-20.
[15] LIU Guang-long, LIAO Shui-jiao, ZHU Duan-wei, CUI Jing-zhen, ZHOU Wen-bing. Solid-phase photocatalytic degradation of polyethylene film with manganese oxide OMS-2[J]. Solid State Sciences, 2011, 13: 88-94.
[16] SU Hai-feng, WEN Yan-xuan, WANG Fan, LI Xuan-hai, TONG Zhang-fa. Leaching of pyrolusite using molasses alcohol wastewater as a reductant[J]. Minerals Engineering, 2009, 22: 207-209.
[17] L? Yi-ju, SU jing, WEN Yan-xuan, SU Hai-feng, YANG Ke-di, LV Xiao-yan. Leaching kinetics of pyrolusite by macromolecular melanoidins of molasses alcohol wastewater in H2SO4[J]. Procedia Engineering, 2011, 18: 107-115.
[18] WU Fang-fang, ZHONG Hong, WANG Shuai, LAI Su-feng. Kinetics of reductive leaching of manganese oxide ore using cellulose as reductant[J]. Journal of Central South University, 2014, 21: 1763-1770.
[19] 孙英云. 废糖蜜还原浸出低品位软锰矿的工艺及动力学研究[D]. 南宁: 广西大学, 2008: 49-50.
SUN Ying-yun. Technology and kinetics study on reductive acid leaching of low-grade pyrolusite with cane molasses[D]. Nanning: Guangxi University, 2008: 49-50.
[20] 粟海锋, 崔勍焱, 文衍宣, 童张法. 低品位软锰矿降解糖蜜酒精废液中焦糖色素的脱色动力学[J]. 过程工程学报, 2009, 9(3): 480-485.
SU Hai-feng, CUI Qing-yan, WEN Yan-xuan, TONG Zhang-fa. Decolorizing kinetics of caramel in molasses alcohol wasterwater degraded by low-grade pyrolusite[J]. The Chinese Journal of Process Engineering, 2009, 9(3): 480-485.
[21] 崔益顺. 硫酸分解开阳磷矿动力学特性研究[J]. 四川轻化工报, 2002, 14(4): 34-36.
CUI Yi-shun. Study on acidolysis dynamics of Kaiyang phosphate rock by sulfuric acid decomposition[J]. Journal of Sichuan Institute of Light Industry and Chemical Technology, 2002, 14(4): 34-36.
[22] 汪荣鑫. 数理统计[M]. 西安: 西安交通大学出版社, 2003: 266-277.
WANG Rong-xin. Mathematical Statistics[M]. Xi’an: Xi’an Jiao Tong University Press, 2003: 266-277.
Oxidative breakdown kinetics of glucose in process of leaching mangaese oxide ore
WANG Yu-hong, LEI Zuo-min, QU Xin-ke, HUANG Li-ying, HOU Jia-min, OUYANG Qiu-lin, SU Hai-feng
(Guangxi Colleges and Universities Key Laboratory of New Technology and Application in Resource Chemical Engineering, School of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, China)
Abstract: The kinetics of decomposing of glucose during the reductive leaching process of manganese oxide ore in dilute sulfuric acid was examined. The change of COD concentration remained in solution was measured to quantify the effects of glucose dosage, sulfuric acid concentration, manganese oxide ore dosage and leaching temperature on decomposing rate of glucose. The results show that the decomposing rate of glucose increases with the increase of the leaching temperature, sulfuric acid concentration and manganese oxide ore dosage. The leaching process follows the kinetics of index empirical model, hybrid controlled by diffusion-chemical reaction and the apparent activation energy is 41.80 kJ/mol. The reaction orders of COD, sulfuric acid, manganese oxide ore are 2.0, 0.927 and 0.976, respectively.
Key words: manganese oxide ore; glucose; decomposing; kinetics
Foundation item: Project(51164002) supported by the National Natural Science Foundation of China; Project(2013GXNSFAA019290) supported by the Province Natural Science Foundation of Guangxi, China; Project(1598015-4) supported by the Province Science and Technology Key Projects of Guangxi, China; Project(YCBZ2014014) supported by the Innovation Project of Guangxi Graduate Education, China
Received date: 2015-09-06; Accepted date: 2016-01-12
Corresponding author: SU Hai-feng; Tel: +86-771-3233718; E-mail: suhfeng@gxu.edu.cn
(编辑 王 超)
基金项目:国家自然科学基金资助项目(51164002);广西自然科学基金资助项目(2013GXNSFAA019290);广西科技开发计划课题(桂科攻1598015-4);广西研究生教育创新计划项目(YCBZ2014014)
收稿日期:2015-09-06;修订日期:2016-01-12
通信作者:粟海锋,教授,博士;电话:0771-3233718;E-mail: suhfeng@gxu.edu.cn