
Zinc removal from hyperaccumulator Sedum alfredii Hance biomass
YANG Jian-guang(杨建广)1, 2, PENG Chang-hong(彭长宏)1, TANG Chao-bo(唐朝波)1,
TANG Mo-tang(唐谟堂)1, ZHOU Ke-cao(周科朝)2
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 15 March 2009; accepted 25 May 2009
Abstract: Leaching of heavy metals from Sedum alfredii Hance biomass was studied with ammonia-ammonium chloride solution as leaching agent. The research was carried out in two phases: 1) a leaching study to determine the zinc extraction efficiency of this leaching agent, and 2) a thermodynamic analysis to identify the likely reactions and stable Zn(Ⅱ) species formed in the leaching systems. Taguchi orthogonal experiment, with four variable parameters, leaching temperature, molar ratio of NH4Cl to NH3, leaching time and solid-to-liquid(L/S) ratio, and each at three levels, was used to optimize the experiment parameters by the analysis of variances. The results indicate that leaching temperature has the most dominant effect on metal extraction performance, followed by molar ratio of NH4Cl to NH3, solid-to-liquid ratio and leaching time. The optimum condition was obtained as follows: temperature of 60℃, molecular ratio of NH4Cl to NH3 of 0.6, leaching time of 2 h and solid-to-liquid ratio of 5?1. The total zinc leaching efficiency under optimum conditions reaches 97.95%. The thermodynamic study indicates that the dominant species produced by the leaching process should be the soluble Zn(NH3)42+.
Key words: hyperaccumulator; Sedum alfredii Hance; phytoremediation; detoxication; leaching
1 Introduction
Phytoremediation of soil or water with hyperaccumulator has been extensively explored in recent years[1-4]. Living plants can clean up soils or waterways. This approach exploits the ability of various plant species to thrive in high metal environments where large amounts of toxic elements are accumulated, such as heavy metals, and is particularly appropriate when slow remediation of relatively low metal concentrations is acceptable. Advantages compared with existing remediation methods include the minimal site destruction and destabilization, low environmental impact, and favourable aesthetics; advantages compared with biosorption include continuous in situ regeneration of the biomass, and the ability of living plant cells to supplement passive sorption of metals with metabolic mechanisms of metal uptake and detoxification[5-8].
One of the main drawbacks of heavy-metal phytoremediation is related to handling and disposal of contaminated plant waste. In Ref.[9] it is mentioned that this biomass may be confined in landfills or used as compost. Nevertheless, these options are questionable because metals can be liberated to the surrounding environment by leaching and other natural processes, polluting soils, surface water and groundwater and threatening human and animal health. Some researchers have mentioned that the biomass can be dried, compacted, and incinerated to recover metals from the ash for recycling if they are valuable, in a similar way as is done for phytomining plants[10], or simply for confinement[11]. Others have proposed that biomass could be used as an energy source[12]. However, detailed studies related to the handling and uses of the biomass produced by heavy metals phytoremediation are scarce. SAS-NOWOSIELSKA et al[13] produced a review of phytoextraction crop disposal methods. They found little information to be available but suggested that incineration could be the preferred disposal method because it is economically feasible and environmentally sound. Recently, KELLER et al[12] investigated experimentally the thermal behaviour of two different plants used in heavy metal phytoextraction. They deter-mined that pyrolysis was better than incineration to recover Cd and Zn from plant biomass, but its effectiveness depends on the metal volatility, plant species growth form (i.e., herbs, shrubs or trees), and incineration scheme (i.e., incineration alone or co-incineration with other solid wastes).
Considering that biomass harvested at the end of heavy-metal phytoremediation can be quite abundant and that its disposal in landfills represents a potential risk to living beings, we are seeking an alternative method which would allow us on one hand to detoxify plant biomass in order to use it as bio-fertilizer or mulch and on the other hand to recover metals for confinement or recycling. The main objective of this research is to identify an efficient hydrometallurgical method for recovery of metal from heavy metals hyperaccumulator biomass, for instance, from Sedum alfredii Hance, an herbaceous plant with great potential for heavy-metal phytoremediation both in tropical and in subtropical environments, which has been identified as a hyperaccumulator of zinc[14], to recover zinc. We selected ammonia-ammonium chloride solution as the leaching agent because this compound has been successfully used to recovery heavy metal from low grade ores for a long time.
2 Materials and methods
2.1 Preparation of zinc-contaminated Sedum alfredii Hance
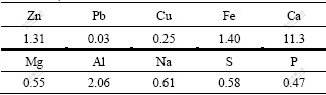
Sedum alfredii Hance biomass was received from Hunan Agricultural Research Center, China. More detailed introduction of this plant was shown in Ref.[15]. In this study, harvested Sedum alfredii Hance biomass was firstly rinsed with deionized water and dried in an oven at 35 ℃ for around 30 min to get rid of the exterior water. Later, the biomass was ground and analyzed at Chemical Analysis Center, Central South University, China. Table 1 shows the main chemical components of this biomass.
Table 1 Main chemical components of this biomass (mass fraction, %)
2.2 Leaching experiments
Leaching experiments were carried out under batch conditions using dried and ground biomass. In the leaching experiments, a hot plate with contact thermometer and a magnetic stirrer was used. The leaching was done in 250 mL glass balloons using Teflon coated magnets. A condenser was attached to the balloon to prevent the vaporization losses with the aid of the contact thermometer, and the temperature error of the system was controlled within ±2 ℃. At the end of each leaching experiment, the insoluble leach residue was separated from the pregnant leach solution by passing it through a filter paper and the solids were washed with distilled water. In the leaching tests, the other procedures were as follows. A measured amount of ammonia solution and calculated amount of analytical grade ammonium chloride were put into the glass balloon and heated to the desired temperature with magnetically stirring. Then, the weighted amount of ground Sedum alfredii Hance was added to the solution and the timing was initiated. The stirring speed that gave sufficient mixing was kept constant in all the experiments. At the end of leaching duration, solid and liquid were subjected to separation by filtration. The washed leach residues were dried, weighted and analyzed by Perkin Elmer Model 2380 AAS for zinc and other heavy metals. The leach recoveries were calculated using the analysis of the ground Sedum alfredii Hance, the leach residue and their respective masses.
2.3 Analytical methods
Solid and liquid samples were digested with HNO3 in a MARSX SEM microwave oven. Biomass was digested following the EPA Method 3052, while leachates and rinse water were treated according to EPA method 3015[16]. Metals concentration in the resultant liquid sample was determined by inductively coupled plasma atomic emission spectroscopy(ICP-AES) according to EPA method 6010B[16] using a Perkin Elmer Optimal 3300DV Emission Spectrophotometer.
2.4 Solution thermodynamics
A thermodynamic analysis was carried out by applying the principle of electronic charge neutrality (ECN) of the generalized species and equilibrium method (GSEM)[17], which are based on the electronic charge and chemical equilibria among species that can form in a given solution. The method makes it possible to construct predominance curves that help us to identify the most stable metal species that should form under specified conditions of pH, ligand concentration, etc. Simultaneously, these curves provide information concerning the chemical reactions among involved species, species stability, phases formation (i.e. soluble or insoluble species) and phases coexistence. Predominance curves are maps that show, in two or three dimensions, the regions of primary stability of each chemical species of analytical interest which can form in a given system. They are constructed by recognizing all chemical reactions involved and their corresponding equilibrium constants.
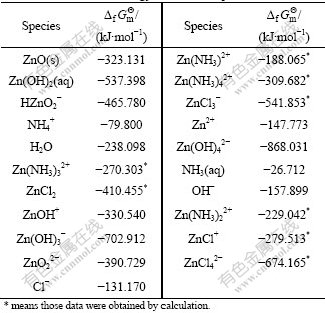
Tables 2 and 3 list the zinc-complexation reactions that are most likely to be significant in the leaching solutions, and the corresponding formation constants and standard freedom enthalpy, which were obtained from the International Union of Pure and Applied Chemistry Stability Constant Database (IUPAC SC-Data base). In most of the cases we choose the equilibrium constant reported at 25 ℃ (298 K) and at zero ionic strength.
Table 2 Formation constants of zinc-complex at 298 K
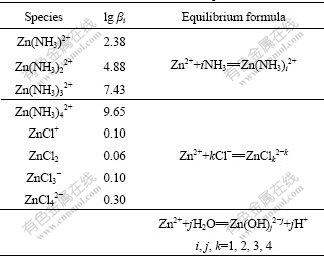
Table 3 Standard free energy of involved species at 298 K
According to the principle of electronic charge neutrality and the generalized species and equilibrium method, the sum concentration of zinc, ammonia and chloride can be expressed as Eqs.(1)-(4), respectively:
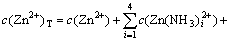
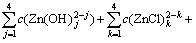
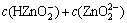 (1)
(1)
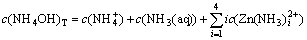 (2)
(2)
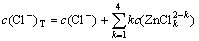 (3)
(3)
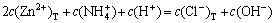 (4)
(4)
where c(NH4OH)T stands for the total concentration of ammonia and ammonium in the system; c(NH3(aq)) stands for the concentration of free ammonia in system; i, j and k are the numbers of ammonia, chloride and hydroxide ligands, respectively. The relationships among the six variables of c(Zn2+)T, c(Cl-)T, c(NH4OH)T, c(Cl-), c(NH3(aq)) and c(H+) are confined by the above listed equations. If two of them are fixed, the other four variables can be obtained by solving the simultaneous equations. In this study, we want to construct predominance curves that help us to identify the most stable metal species that should form under specified conditions of pH, ligands concentration, etc. Therefore, we supposed that the NH4Cl concentration was 5 mol/L, and initiative concentration of ammonia was various from 0 to 10 mol/L, and then solved the above mentioned simultaneous equations, at last some predominance curves were obtained.
Fig.1 and Fig.2 show that with increasing initiative concentration of ammonia, the total concentration of all zinc-chloride ligands, c(ZnCli2-i)T, and concentration of some zinc-ammonia ligands, i.e. c(Zn(NH3)2+), c(Zn(NH3)22+) and c(Zn(NH3)32+) decrease rapidly, whereas the concentration of zinc-hydroxyl ligands is quite low and nearly keeps constant. Simultaneously, Fig.1 and Fig.2 also show that with increasing initiative concentration of ammonia, the concentration of Zn(NH3)42+, c(Zn(NH3)42+), increases steadily. It is clearly seen that the zinc-ammonia ligand, Zn(NH3)42+, is predominant when ammonia concentration, c(NH3), is up to 0.7 mol/L; when c(NH3) is below 0.7 mol/L, the concentration of zinc-chloride, c(ZnCli2-i), is stable. At ammonia concentration in this study is 2-3 mol/L, it is safe to deduce that the predominant species in our leachate is Zn(NH3)42+; the equilibrium pH is 7.4-7.7; and free ammonia concentration is 0.03-0.05 mol/L.
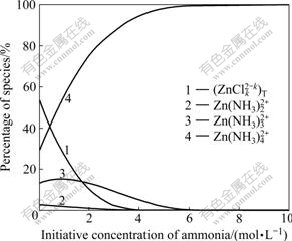
Fig.1 Relationship between initiative concentration of ammonia and percentage of zinc species in solution at c(NH4Cl) of 5 mol/L
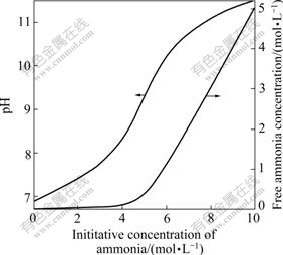
Fig.2 Relationship among initiative concentration of ammonia, equilibrium pH value and free ammonia concentration at c(NH4Cl) of 5 mol/L
3 Results and discussion
The ground Sedum alfredii Hance samples were treated with ammonia-ammonium chloride solution. The ground Sedum alfredii Hance used in the leaching experiments was previously ground to 86% less than 147 μm. Throughout the leaching experiments, molar ratio of NH4Cl to NH3, temperature of leaching, solid-to-liquid (L/S) ratio and leaching duration were examined in order to determine the optimum technical conditions. Stirring speed was kept constant in each test.
In the first leaching experiment, the leaching reagent was chosen to be water for dissolution of 1 kg Sedum alfredii Hance, while the other variables were 80℃ for leaching temperature, 9 h for duration of leaching and 1/5 for solid-to-liquid ratio. Leaching results show that the leach recoveries of both zinc and the other metals are substantially low. This is thought to result from the negligible solubility of the phases present in the samples in water. So, in all the following leaching experiments, ammonia-ammonium solution was chosen as the leaching reagent because this compound has been successfully used to recover some heavy metals from low grade ores for a long time[18-21].
3.1 Orthogonal experiments design
Taguchi’s parameter design provides a simple and systematic approach for optimization of design for quality, performance and cost[22]. The selection of control factors is the most important stage in the experimental design. It is possible to identify the non-significant variables at the early opportunity by including as many factors as possible. Taguchi creates a standard orthogonal array to address this requirement. Orthogonal experiment enables to investigate the relative importance of various factors and identify the best levels for different factors on a response and the results can be analyzed by using a common mathematical procedure. This method can significantly reduce experimental time and research cost by using orthogonal arrays.
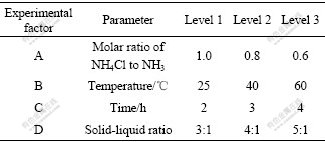
The number of trials chosen for an experimental design is based on the resolution desired and the number of the chosen experimental parameter levels is based on the range of operating conditions of the leaching process, resulting in three levels for each of the four controlled factors. Therefore, to investigate four controllable three level factors, L9 array including nine tests was selected for the leaching experiments with ammonia-ammonium chloride. Table 4 shows the factors and levels of the experiments for the leaching processes
Table 4 Experimental factors and levels selected for leaching of ground Sedum alfredii Hance
3.2 Analysis of mean value
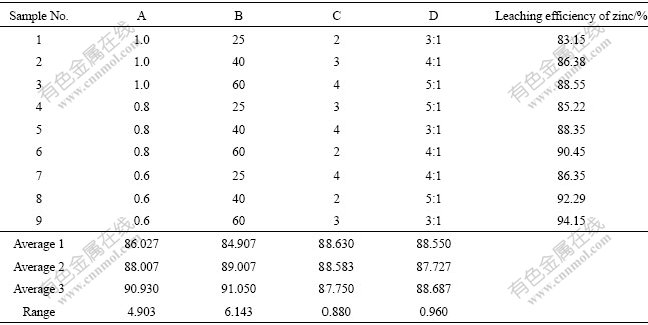
Leaching experiment results and the calculated values are given in Table 5. In this study, analysis of means (ANOM) was adopted. For each factor in the average row, the results of the three experiments consisting of level were added and then divided by 3, which gave the mean values of levels. The range for each factor was produced by subtracting the minimum value from the corresponding maximum value among the Average 1, Average 2 and Average 3. It is found that the ranges of A or B are higher than those of other factors, implying that these parameters (temperature and molar ratio of NH4Cl to NH3) have significant influences. The higher mean value is indicative of the better leaching process. Therefore, the optimal conditions for leaching are A3, B3, D3 and C1. In other words, the ideal experimental parameters are as follows: molar ratio of NH4Cl to NH3 of 0.6, temperature of 60 ℃, L/S of 5:1, and leaching duration of 2 h.
Table 5 Leaching experiment and analysis of means(ANOM) and range according to these obtained results
3.3 Analysis of variation(ANOVA)
Analysis of variance(ANOVA) in accordance with the Taguchi method represents the relationship between the operating parameters and the observed values. Calculation of the value of ANOVA for the four factors and their corresponding three levels (Table 6) was carried out with an L9 orthogonal array. Use of a full factorial design (3×3×3×3) reduced a total of 81 sets of experiments down to 9, thereby decreased cost, time and effort. The main purpose of ANOVA is to further investigate which factor significantly influences the leaching ratio of metals. The number of levels (3) subtracted 1 gave the degrees of freedom (2). The sum of squares was obtained by the following equation:
Table 6 Variation analysis of zinc leaching ratio
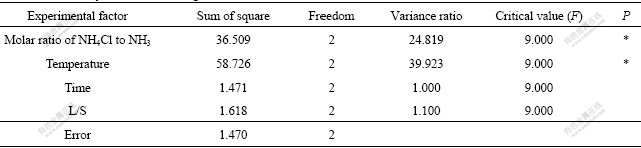
SSi=((M1i-M)2+(M2i-M)2+(M3i-M)2)×3 (i=A, B, C, D)where SSi stands for the sum of squares, and M stands for the mean of nine results presented in Table 6. Sum of squares was divided by degrees of freedom produced variance. The minimum sum of square 2.8 was chosen as error and other sums of squares divided by 2.8 gave the variance ratios. According to the Taguchi method, the leaching temperature has the highest contribution in the experiments. Factor contribution is the amount of improvement obtainable by setting the factor to the desired level. Based on the Taguchi method, this improvement was measured relative to the grand average of performance1.
3.4 Discussion
Table 5 shows that the ammonia-ammonium chloride solution can selectively leach Zn from the Sedum alfredii Hance facilely under some suitable condition.
3.4.1 Effect of temperature
Zinc leaching rate increases with increasing temperature. However, the higher leaching temperature will cause the quicker volatilization rate of ammonia. Considering the leaching efficiency and ammonia loss, 60 ℃ was determined as the leaching temperature.
3.4.2 Effect of leaching time
In the leaching process, the treatment time has the smallest effect in comparison with the other controllable factors, especially at high temperatures, where its influence is lower due to the high reaction rate at these temperatures. Considering the leaching efficiency and energy cost, 2 h was determined as the treatment time.
3.4.3 Effect of molar ratio of NH4Cl to NH3
In the present work, three ratios of NH4Cl to NH3 levels were chosen from 0.6 to 1.0. The ratio of n(NH4Cl)/n(NH3) was also found to be an effective parameter on recovering zinc from the Sedum alfredii Hance. In the leaching process, with n(NH4Cl)/n(NH3) increasing, a noticeable increase in the Zn leaching efficiency was observed. The reason may be explained that the chelate reaction between ammonia and zinc present in the Sedum alfredii Hance resulted in Zn removal. Therefore, the ammonia concentration and n(NH4Cl)/n(NH3) showed a direct influence on the reaction rate. On the other hand, with the ammonia- ammonium chloride-treated Sedum alfredii Hance, the removal of zinc may be related to an increase in diffusion of ammonia into the Sedum alfredii Hance plant particles with the increase in concentration, which results in solubilization of the plant matter.
3.4.4 Effect of liquid-solid ratio (L/S)
In the leaching process, the L/S ratio has not apparent effect in comparison with the other controllable factors. The higher leach recoveries of metals with decreasing solids percent were thought to be due to the higher chance of solid particles to contact with the leach reagent when the solids percent was low. Considering the leaching efficiency and cost, 5:1 was determined as the liquid-to-solid ratio.
3.4.5 Confirmation experiments
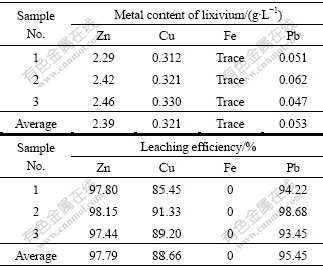
The confirmation leaching experiments were carried out three times under the optimized leaching condition, temperature of 60 ℃, n(NH4Cl)/n(NH3) of 0.6, leaching time of 2 h and solid-to-liquid ratio of 5:1, and the main metal contents of lixivium and the average leaching efficiency of zinc and other metals are presented in Table 7. It can be seen that this lixivium is suitable for final recovery of zinc by electrolysis, replacement or salt precipitation, and the leaching residue can be safely used as fertilizer because the detoxified plant biomass can completely satisfy the limit set by China legislation related to biosolids disposal. There was also reasonable agreement among the zinc concentrations of leach liquor in this case, but the observed zinc extraction was about 8% higher than the predicted extraction. This difference confirms the existence of the interaction effect of parameters that need to be considered in process optimization.
Table 7 Confirmation experiment results
4 Conclusions
1) Based on the above mentions studied, ammonia- ammonium chloride was found to be effective leaching agent to extract zinc from Sedum alfredii Hance. Its zinc extraction efficiency was considerably higher (97.79%). In addition, copper and lead were also leached coherently (88.66% and 95.45% respectively). The heavy metals (zinc, copper and lead) concentration in biomass leached by this leaching agent was reduced significantly enough to satisfy the limit set by China legislation related to biosolids disposal.
2) The optimized leaching condition was obtained as follows: temperature of 60 ℃, molar ratio NH4Cl to NH3 of 0.6, leaching time of 2 h and solid/liquid of 5?1.
References
[1] KR?MER U. Phytoremediation: Novel approaches to cleaning up polluted soils [J]. Current Opinion in Biotechnology, 2005, 16: 133-141.
[2] GONZ?LEZ R C, GONZ?LEZ-CH?VEZ M C A. Metal accumulation in wild plants surrounding mining wastes [J]. Environmental Pollution, 2006, 144: 84-92.
[3] MIN Y, BOQING T, MEIZHEN T, AOYAMA I. Accumulation and uptake of manganese in a hyperaccumulator Phytolacca Americana [J]. Minerals Engineering, 2007, 20: 188-190.
[4] BROWN S L, CHANCY R L, ANGLE J S. Phytoremediation potential of Thlaspi caerulescens and bladder campion for zinc- and cadmium-contaminated soil [J]. Journal of Environmental Quality, 1994, 23: 1151-1157.
[5] DUSHENKOV V, KUMAR P B A N, MOTTO H. The use of plants to remove heavy metals from aqueous streams [J]. Environmental Science and Technology, 1995, 29: 1239-1245.
[6] HOLAN Z R, VOLESKY B. Accumulation of cadmium, lead, and nickel by fungal and wood biosorbents [J]. Applied Biochemistry and Biotechnology, 1995, 53: 133-146.
[7] HUANG J W, CUNNINGHAM S D. Lead phytoextraction: Species variation in lead uptake and translocation [J]. New Phytologist, 1996, 134: 75-84.
[8] HUGHES J B, SHANKS J, VANDERFORD M, LAURITZEN J, BHADRA R. Transformation of TNT by aquatic plants and planl tissue cultures [J]. Environmental Science and Technology, 1997, 31: 266-271.
[9] GHOSH M, SINGH S P. A review on phytoremediation of heavy metals and utilization of its byproducts [J]. Applied Ecological Environment Research, 2005, 3: 1-18.
[10] BROOKS R R, CHAMBERS M F, NICKS L J, ROBINSON B H. Phytomining [J]. Trends Plant Science, 1998, 3: 359-362.
[11] DUSHENKOV V, KUMAR P B A N, MOTTO H, RASKIN I. Rhizofiltration: The use of plants to remove heavy metals from aqueous streams [J]. Environment Science and Technology, 1995, 29: 1239-1245.
[12] KELLER C, LUDWIG C, DAVOLI F, WOCHELE J. Thermal treatment of metalenriched biomass produced from heavy metal phytoextraction [J]. Environment Science and Technology, 2005, 39: 3359-3367.
[13] SAS-NOWOSIELSKA A, KUCHARSKI R, MA?KOWSKI E, POGRZEBA M, KUPERBERG J M, KRY?NSKI K. Phytoextraction crop disposal—An unsolved problem [J]. Environment Pollutant, 2004, 128: 373-379.
[14] NI W Z, SUN Q, YANG X. Growth and zinc accumulation of Sedum alfredii Hance—A Zn hyperaccumulator as affected by phosphorus application[J]. Bulletin of Environment Contamination and Toxicology, 2004, 72: 756-762.
[15] YANG Xiao-e, LONG Xing-xian, NING Wu-zhong, FU Cheng-xin. Sedum alfredii: A new zinc-accumulating ecotype [J]. Science Bulletin, 2002, 47(13): 1003-1006. (in Chinese)
[16] Environmental Protection Agency (EPA). Methods for analytes and properties [M]. United States Environmental Protection Agency, USA, 1998.
[17] JU Shao-hua, TANG Mo-tang, YANG Sheng-hai. Thermodynamics and technology of extracting gold from low-grade gold ore in system of NH4Cl-NH3-H2O [J]. Trans Nonferrous Met Soc China, 2006, 16(1): 203-208.
[18] YANG Sheng-hai, TANG Mo-tang, CHEN Yi-feng, TANG Chao-bo, HE Jing. Anodic reaction kinetics of electrowinning system of Zn(Ⅱ)-NH3-NH4Cl-H2O [J]. Trans Nonferrous Met Soc China, 2004, 14(3): 626-630.
[19] TANG Mo-tang, YANG Jian-guang, YANG Sheng-hai. Thermodynamic calculation of Sn(Ⅳ)-NH4+-Cl--H2O system [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(4): 802-806. (in Chinese)
[20] YANG Jian-guang, TNAG Mo-tang, YANG Sheng-hai, TANG Chao-bo. Transparent conductive oxide ATO powders prepared by complex-co-precipitation method [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(6): 966-974. (in Chinese)
[21] YANG Jian-guang, TANG Mo-tang, YANG Sheng-hai. Thermodynamics analysis of Sn(Ⅳ)-Sb(Ⅲ)-NH3-NH4Cl-H2O system and its application [J]. Journal of Central South University, 2005, 36(4): 582-586. (in Chinese)
[22] TAGUCHI G, YOKOYAMA Y, WU Y. Taguchi methods design of experiments [M]. USA: American Supplier Institute Press, 1993.
Foundation item: Project(KY20080577000002) supported by the Hi-tech Research and Development Program of China; Project(20080431028) supported by China Postdoctoral Science Foundation; Project(50804056) supported by the National Natural Science Foundation of China
Corresponding author: YANG Jian-guang; Tel: +86-731-88830470; E-mail: Jianguang_yang@hotmail.com
DOI: 10.1016/S1003-6326(08)60449-5
(Edited by YANG Hua)