硫铁矿烧渣水热法合成形貌可控的氧化铁
刘昭成,郑雅杰
(中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘要:以硫铁矿烧渣硫酸浸出液与氨水反应制备的Fe(OH)3胶体为前驱体,采用水热法合成不同形貌的氧化铁粒子。在中性介质中考察反应温度、物质的量比(n(Fe2+)/n(Fe3+))、水热体系总Fe浓度及晶种量对水热法氧化铁物相、形貌和粒径的影响。采用X线衍射仪、扫描电镜、透射电镜及选择区域电子衍射对水热产物物相和形貌进行研究。研究结果表明:反应温度为230 ℃,n(Fe2+)/n(Fe3+)为0.1以及水热体系总Fe浓度为1.25 mol/L和晶种量为2 g时,水热产物为片状α-Fe2O3粒子;控制上述其他条件不变,当温度在140~260 ℃时,随着温度的升高,水热法产物由α-FeOOH相向α-Fe2O3相转变,α-Fe2O3粒子形貌由球形向小圆饼状和片状依次转变;当n(Fe2+)/n(Fe3+)在0~0.12时,随着n(Fe2+)/n(Fe3+)的增加,水热产物由α-Fe2O3相向α-Fe2O3和Fe3O4相转变,α-Fe2O3粒子形貌由球形逐渐向片状转变,其粒径由小变大;当水热体系中总Fe浓度为0.625 mol/L和1.875 mol/L时,水热法氧化铁分别为不均一形貌和球形;当没有加入晶种及晶种量为6 g时,水热法氧化铁分别为椭球状和不均一形貌。
关键词:硫铁矿烧渣;氧化铁;α-Fe2O3;水热法;形貌可控
中图分类号:TQ138.1 文献标志码:A 文章编号:1672-7207(2011)09-2563-08
Morphology controllable iron oxide synthesized from pyrite cinders by hydrothermal method
LIU Zhao-cheng, ZHENG Ya-jie
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Iron oxide particles with different morphologies were synthesized by hydrothermal treatment using ferric hydroxide gel as precursor, prepared by adding ammonia to sulfuric acid leaching solution of pyrite cinders. In addition, the effects of reaction temperature, n(Fe2+)/n(Fe3+), total iron concentration and seed crystals adding amount on the phase, shape and size of the prepared iron oxide in the neutral medium were investigated. The phase and shape of the synthesized iron oxide particles were characterized by X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscopy (TEM) and selected area electron diffraction (SAED). The results show that platelet-type α-Fe2O3 particles are prepared via hydrothermal route when the temperature is 230 ℃, n(Fe2+)/n(Fe3+) is 0.1, total iron concentration is 1.25 mol/L and seed crystal adding amount is 2 g. With the increase of temperature, when it is in the range of 140-260 ℃, the hydrothermal products transfer from α-FeOOH to α-Fe2O3 and their shapes change from sphere to round pie and then to platelet. With the increase of n(Fe2+)/n(Fe3+), in the range of 0-0.12, the hydrothermal products are transferred from α-Fe2O3 to α-Fe2O3 and Fe3O4, and the shapes of α-Fe2O3 particles gradually change to platelet from sphere. Furthermore, the particle size also increases with the increase of n(Fe2+)/n(Fe3+). The obtained iron oxide particles become heterogeneous when the total Fe concentration is 0.625 mol/L, while it becomes sphere when the total Fe concentration is 1.875 mol/L. The products are elliptical without adding any seed crystal, while it becomes heterogeneous when the crystal seed adding amount is 6 g.
Key words: pyrite cinders; iron oxide; α-Fe2O3; hydrothermal method; morphology-controlled
硫铁矿烧渣是工业硫酸生产过程中产生的副产品,其主要成分是赤铁矿为主的Fe氧化物,并伴生一些有害重金属,其传统处理过程会产生潜在的环境危害[1-2],也不利于其作为炼铁原料[3]。鉴于环境保护、处理成本及有限的可利用填埋土地,近年来寻求具有经济效益性处理硫铁矿烧渣的新方法越来越多。如利用硫铁矿烧渣生产染色砖、屋面陶瓦[4]、水泥[5-6]及铁系产品[7-9]等。目前因为硫铁矿烧渣制备高性能氧化铁具有生产成本低、产品附加值高及环境污染少等特点,国内进行了广泛的研究。如杨喜云等[9]对硫铁矿烧渣制备静电显影剂Fe3O4进行了研究;阳征会等[10-11]研究了利用硫铁矿烧渣制备的硫酸亚铁为原料经碳铵沉淀后煅烧制备α-Fe2O3;徐旺生等[12]研究了以硫铁矿烧渣酸浸液为反应液经空气氧化法制备高纯氧化铁;Chen等[13]利用沉淀分离和液压评级的方法从硫铁矿烧渣中回收微米级氧化铁。近年来, 本课题组一直研究硫铁矿烧渣水热法制备氧化铁,并已研制磁用氧化铁[14-15]和陶瓷釉料用氧化铁红颜料[16]。由于氧化铁粒子形貌及粒径对其性能具有很大的影响, 因此,有必要对硫铁矿烧渣水热法氧化铁形貌和粒径进行可控研究。在此,本文作者以硫铁矿烧渣酸浸液为原料,用氨水中和后经水热法制备氧化铁,考察反应温度、物质的量比n(Fe2+)/n(Fe3+)、水热体系总Fe浓度及晶种量对氧化铁形貌和粒径的影响,并合成了不同粒径的球状、椭球状、片状氧化铁粒子,获得了各形貌氧化铁粒子合成的适宜条件。
1 实验
1.1 原料与试剂
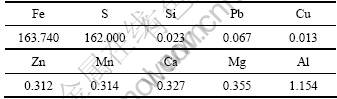
试剂有:H2SO4,H2O2及NH3·H2O(天津开通化工公司),均为分析纯。水热反应晶种超细氧化铁粒子(湖南三环颜料厂)形貌为球形,颗粒均匀,粒径约为0.3 ?m。硫铁矿烧渣(广东云浮硫铁矿企业集团公司)作为铁源。采用X线荧光光谱(XRF)分析其化学成分,如表1所示。
硫铁矿烧渣经硫酸酸浸可制备得到硫酸亚铁和硫酸铁混合溶液[17]。取6.5 L质量分数为50%的硫酸加入到10 L的三颈烧瓶后,在搅拌作用下,向其缓慢地加入3 kg上述硫铁矿烧渣。在115 ℃,浸出4 h后过滤,并稀释至9.0 L。经分析可知:滤液主要由硫酸铁和硫酸亚铁混合液组成(如表2所示),并含有Al,Mg和Ca等微量杂质。所得的酸浸液作为制备氧化铁的初原料。
表1 硫铁矿烧渣的化学成分(质量分数)
Table 1 Chemical composition of pyrite cinders investigated by XRF %

表2 硫铁矿烧渣酸浸液的化学成分(质量浓度)
Table 2 Chemical composition of acid leaching solution of pyrite cinders g/L
1.2 步骤
取一定量上述酸浸液加H2O2溶液调节酸浸液中物质的量比n(Fe2+)/n(Fe3+),随后加入25%氨水至体系中pH为7,形成胶体溶液加入一定量的超细氧化铁粒子,并加入去离子水稀释至400 mL;然后,将制备好的溶液转移到高压釜(SUS316, 0.5 L)进行水热反应;设置搅拌速度为300 r/min,在一定温度下,反应30 min后采用内管冷却水快速冷却反应器;最后,将所得水热产物从反应釜中取出后、过滤、洗涤,并于105 ℃干燥12 h得到产品。滤液硫酸铵经蒸发结晶进行回收利用。
1.3 分析与检测
根据GB 1863—2008采用化学滴定法分析溶液中Fe2+和Fe3+浓度;采用X线荧光光谱仪(XRF,S4 PI0NEER)分析原料及固体产物中元素含量;采用X线衍射仪(XRD,Rigaku D/max-TTR III)分析水热产物晶相(发光源为Cu Kα靶,管压为25 kV,管流为400 mA,λ=0.154 056 nm,步长为0.02°,2θ为10.0°~70.0°);在扫描电镜(SEM,FEI Quanta 200)下观察水热产物表面形貌及颗粒平均粒径;采用透射电子显微镜(TEM,Hitachi H-800)观察水热产物形貌,使用选择区域电子衍射(SAED)进一步确定样品结晶度。
2 结果与讨论
2.1 反应温度对水热法氧化铁物相及形貌的影响
取200 mL经调节后n(Fe2+)/n(Fe3+)=0.1,初始液总铁浓度为2.5 mol/L的酸浸液加氨水中和至pH为7,添加2 g氧化铁晶种稀释至400 mL即水热体系中Fe3+浓度为1.25 mol/L时进行水热反应30 min,考察反应温度分别为140,170,200,230和260 ℃对水热法氧化铁物相及形貌的变化。图1所示为不同温度下水热反应所得氧化铁的XRD图谱。
由图1(a)可知:当反应温度为140 ℃时,水热产物物相为α-FeOOH和α-Fe2O3,且其结晶较差,说明该温度下Fe(OH)3胶体没有完全脱水转化成Fe2O3晶相;随着反应温度升高,水热产物中α-FeOOH消失;当温度为170,200,230和260 ℃时(见图1(b)~(e)),水热产物均为斜方六面体晶系[空间群为:R-3C(167)] α-Fe2O3晶相,其晶饱参数a=0.503 6 nm,b=0.503 6 nm,c=1.374 9 nm(JCPDS卡片, No.33-0664),无其他杂相;随着反应温度的升高,水热法氧化铁各衍射峰逐渐增强,在此实验条件下温度为260 ℃时所得衍射峰强度最强。由XRD峰与颗粒粒径关系可知:随着温度的增加,所得水热法氧化铁颗粒粒径将逐渐增大,结晶更完整。
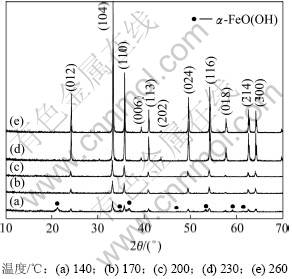
图1 不同温度下水热法所得氧化铁的XRD图谱
Fig.1 XRD patterns of iron oxide hydrothermally prepared at different temperatures
图2所示是反应温度为140,170,200,230和260 ℃时所得水热产物的SEM照片。
由图2可知:当反应温度为140 ℃时(图2(a)),所得的水热产物为球状体α-Fe2O3和针状体α-FeOOH,并伴随大量微晶,且粒子团聚严重。说明此温度下Fe(OH)3没有完全脱水转化为α-Fe2O3,SEM分析与XRD分析结果相吻合;当反应温度为170 ℃时,所得的水热产物为球形氧化铁(图2(b)),该表明Fe(OH)3完全脱水转化为α-Fe2O3;当反应温度为200 ℃,水热产物呈现小圆饼状氧化铁(图2(c)),粒径为0.1~0.3 ?m;当反应温度达到230 ℃时,水热产物为大小均匀的六边形氧化铁片状体(图2(d)),其平均粒径约为1.0 ?m,厚度约为0.1 ?m,径厚比约为10,外观颜色为紫红色,片状粒子间呈现疏松状态;当反应温度达到260 ℃时,水热法所得氧化铁六边形片状体粒径增大,平均粒径约为1.5 ?m,厚度约为0.1 ?m,径厚比达到15(图2(e))。总之,随着反应温度增加,水热法所得氧化铁颗粒形貌由球形向片状转变,粒径由小变大。
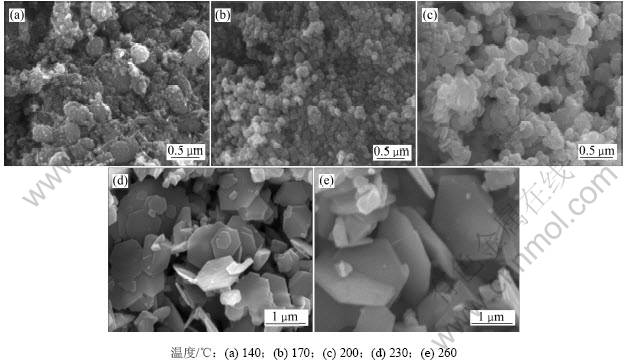
图2 不同温度下水热法所得氧化铁的SEM照片
Fig.2 SEM images of iron oxide hydrothermally prepared at different temperatures
2.2 n(Fe2+)/n(Fe3+)对水热法氧化铁物相及形貌的影响
取200 mL初始液总铁浓度为2.5 mol/L的酸浸液(即水热体系总铁浓度为1.25 mol/L),加氨水中和至pH为7,添加2 g氧化铁晶种后于230 ℃进行水热反应30 min,考察n(Fe2+)/n(Fe3+)分别为0,0.04,0.07,0.10和0.12对水热法氧化铁物相及形貌的影响。
图3所示为不同n(Fe2+)/n(Fe3+)时水热反应所得氧化铁的XRD图谱。由图3可知:当n(Fe2+)/n(Fe3+)≤0.1 (图3(a)~(d)),水热法所得产物的物相是α-Fe2O3;当n(Fe2+)/n(Fe3+)>0.1时(图3(e)),水热产物中出现Fe3O4物相。此外,随着n(Fe2+)/n(Fe3+)的增加,产物外观颜色变化为鲜红色→紫红色→暗红色。这是因为当pH=7时,水热法溶液中的Fe2+主要以Fe2+,FeOH+和Fe(OH)2形式存在,其中FeOH+的浓度达到最大,约为总亚铁的60%[18],所以当Fe2+为一定量时即n(Fe2+)/n(Fe3+)= 0.12,溶液中的Fe(OH)2量增多,而在水热条件下其与Fe(OH)3反应易生成四氧化三铁。
图4所示为不同n(Fe2+)/n(Fe3+)时水热法所得氧化铁的SEM照片。由图4可知:水热法氧化铁形貌随n(Fe2+)/n(Fe3+)变化显著;当n(Fe2+)/n(Fe3+)=0时,采用水热法所得氧化铁粒子为球形,颗粒表面突兀不平(见图4(a)),这是因为所形成的氧化铁粒子细小,比表面积很大,表面能高而产生团聚体。根据n(Fe2+)/ n(Fe3+)=0时所得氧化铁的XRD衍射数据如表3所示。由Scherrer公式(式(1))可知:晶粒直径(D)与衍射峰的半高峰宽成反比,可以估算各主要晶面衍射峰所得的晶粒尺寸Dhkl,进而得到平均晶粒尺寸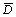 。
。
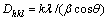 (1)
(1)
式中:θ为布拉格衍射角(°);β为衍射峰的半高峰宽;k=0.89;λ为波长,λ=0.154 056 2 nm。经计算,当 n(Fe2+)/n(Fe3+)=0时水热法所得产物的平均晶粒尺寸为59.15 nm,与SEM分析结果基本一致。当n(Fe2+)/n(Fe3+)=0.04时,水热法所得氧化铁呈均一的球状体(见图4(b)),平均粒径约为0.25 ?m,颗粒之间有较好的分散性;当n(Fe2+)/n(Fe3+)=0.07时,采用水热法所得氧化铁为六边形薄片状体(图4(c)),平均粒径为0.50 ?m,径厚比大于5;而当n(Fe2+)/n(Fe3+)=0.1,采用水热法所得氧化铁呈现六边形片状体,平均粒径约为1.0 ?m,径厚比约为10(图4(d));当n(Fe2+)/n(Fe3+)=0.12时,水热产物变为球形,且晶粒粒径不均一(图4(e))。
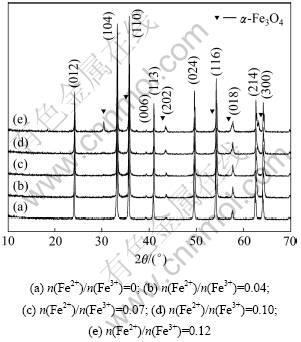
图3 不同n(Fe2+)/n(Fe3+)时水热法所得氧化铁的XRD图谱
Fig.3 XRD patterns of iron oxide hydrothermally prepared at different n(Fe2+)/n(Fe3+)

图4 不同n(Fe2+)/n(Fe3+)时水热法所得氧化铁的SEM照片
Fig.4 SEM images of iron oxide hydrothermally prepared at different n(Fe2+)/n(Fe3+)
表3 n(Fe2+)/n(Fe3+)=0时水热法制得氧化铁的晶粒尺寸
Table 3 Grain size of iron oxide hydrothermally prepared at n(Fe2+)/n(Fe3+)=0
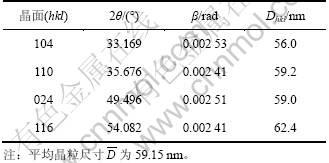
图5(a)所示为n(Fe2+)/n(Fe3+)为0.1时水热法所得氧化铁的TEM照片,图5(b)所示为单个六边形氧化铁片状体的TEM照片。从图5(b)可以看到片状氧化铁为正六边形,其中,插图为单个六边形氧化铁片状体的SAEM 照片,显示水热法产物具有较高的结晶度,与氧化铁(α-Fe2O3)菱形体晶相结构完全匹配,并且每个六边形氧化铁片状体为单晶体。
经上述XRD,SEM和TEM分析,当n(Fe2+)/ n(Fe3+)≤0.1时,随着n(Fe2+)/n(Fe3+)的增加,颗粒粒径逐渐增大,且氧化铁的形貌由球状体向片状体转变。这是因为在短时间内一定量的Fe2+可以催化Fe(OH)3胶体向α-Fe2O3转化[18-19]。研究结果表明:Fe2+的量越大,水热法所得氧化铁粒径越大,其催化作用越强,并在n(Fe2+)/n(Fe3+)=0.1时Fe2+的催化作用最强,所获得氧化铁(α-Fe2O3)粒子粒径最大,而Fe2+过量时水热产物中出现Fe3O4物相,其催化作用不明显。除此之外,由于硫铁矿烧渣酸浸液中存在一定量的Al3+,六边形氧化铁片状体的特殊形貌的形成也可能是由于Al3+在α-Fe2O3(0001)晶面上发生强烈的吸附,从而抑制氧化铁晶核沿c轴方向生长[20-21]。由于体系中相存在形式较复杂,在微量Fe2+存在下六边形片状氧化铁粒子的详细生长机理尚需进一步探讨。
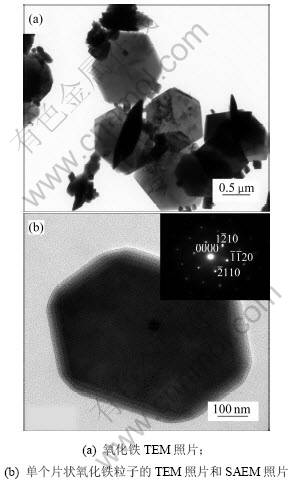
图5 n(Fe2+)/n(Fe3+)为0.1时水热法所得氧化铁的TEM照片和SAEM照片
Fig.5 TEM image and SAED pattern of iron oxide hydrothermally prepared at n(Fe2+)/n(Fe3+)=0.1
2.3 水热体系中总Fe浓度对水热法氧化铁形貌的 影响
在n(Fe2+)/n(Fe3+)=0.1、初始液总铁浓度为2.5 mol/L的酸浸液中加氨水中和至pH为7,添加2 g氧化铁晶种在230 ℃进行水热反应30 min,考察取酸浸液为100,200和300 mL即水热体系中总Fe浓度(c(TFe))分别为0.625,1.25和1.875 mol/L时氧化铁形貌的变化。对不同总Fe浓度水热法所得氧化铁进行SEM分析。其SEM照片如图6所示。
当水热体系中c(TFe)=0.625 mol/L时,氧化铁SEM照片如图6(a)所示,可见:其形貌不一,主要呈现米粒状和团块状,且粒径范围较大。这可能是因为该条件下Fe(OH)3胶体水解生成的初级粒子较少,且在水热条件下初级粒子间有一定距离短时间很难快速聚集。图6(b)所示为水热体系中c(TFe)=1.250 mol/L所得氧化铁的SEM照片,此条件下所得氧化铁为形貌规则的六边形片状体,水热产物结晶较完整,说明该初级氧化铁粒子能在短时间聚集起来并形成一定的规则形貌。图6(c)所示为水热体系中c(TFe)=1.875 mol/L所得氧化铁的SEM照片,可知:该水热产物为均一的球形氧化铁,粒子大小约为0.3 ?m。这是由于水热体系中高浓度的Fe(OH)3胶体经水解后迅速生成大量的初级氧化铁粒子,其具有高比表能,因此,在高温水热条件下能够迅速聚集并均匀生长。
2.4 晶种量对水热法氧化铁形貌的影响
取200 mL n(Fe2+)/n(Fe3+)=0.1、初始液总铁浓度为2.5 mol/L的酸浸液(即水热体系中总Fe浓度(c(TFe))为1.250 mol/L),加氨水中和至pH为7,添加一定量的氧化铁晶种在230 ℃进行水热反应30 min,考察晶体量分别为0,2和6 g时对由水热法所得氧化铁形貌的影响,结果如图7所示。
如图7(a)所示,在不加晶种的情况下,采用水热法所得氧化铁粒子呈椭球状,平均粒径约为0.5 ?m,轴径比约为2:1。该椭球状氧化铁的形成主要是因为该水热体系中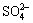 在平行c轴晶面上的特征吸附,从而抑制晶核在该方向上生长[22]。图7(b)所示为加入晶种量为2 g时水热法所得氧化铁的SEM照片,此条件下所得氧化铁粒子为六边形片状体,说明适量的晶种可以促使氧化铁初级粒子快速聚集生长。当晶种量为6 g时,水热法所得氧化铁粒子粒径不一,同时有大的片状体存在,如图7(c)所示,表明晶种量过多,晶核附着生长的点很多,只有少数的晶粒聚集长大。因此,适当的晶种量是促使生成氧化铁片状体的重要条件 之一。
在平行c轴晶面上的特征吸附,从而抑制晶核在该方向上生长[22]。图7(b)所示为加入晶种量为2 g时水热法所得氧化铁的SEM照片,此条件下所得氧化铁粒子为六边形片状体,说明适量的晶种可以促使氧化铁初级粒子快速聚集生长。当晶种量为6 g时,水热法所得氧化铁粒子粒径不一,同时有大的片状体存在,如图7(c)所示,表明晶种量过多,晶核附着生长的点很多,只有少数的晶粒聚集长大。因此,适当的晶种量是促使生成氧化铁片状体的重要条件 之一。
综上所述,在不同实验条件下硫铁矿烧渣水热法合成的氧化铁的物相组成和形貌特征各不同。表4列出了不同实验条件下所得氧化铁的物相组成和形貌 特征。
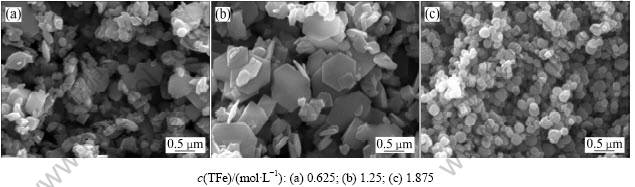
图6 不同总Fe浓度下水热法所得氧化铁的SEM照片
Fig.6 SEM images of iron oxide hydrothermally prepared with different total iron concentrations
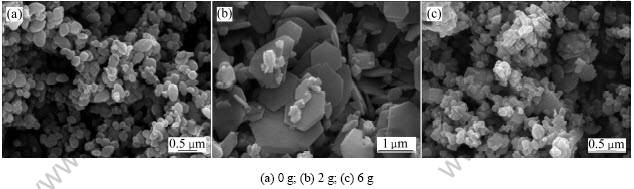
图7 不同晶种量水热法所得氧化铁的SEM照片
Fig.7 SEM images of iron oxide hydrothermally prepared with different amounts of crystal seed
表4 不同实验条件下所得水热法氧化铁的物相组成和形貌特征
Table 4 Phase composition and morphologies of iron oxide hydrothermally synthesized under different experimental conditions
3 结论
(1) 随着反应温度的增加,采用水热法所得氧化铁由α-FeOOH相向α-Fe2O3相转变,粒径由小变大,形貌由球形逐渐向片状转变。当反应温度为140 ℃时,氧化铁呈现α-FeOOH和α-Fe2O3两相,形貌为棒状和球状体;当反应温度≥170 ℃时,氧化铁物相为α-Fe2O3,形貌由球形向小圆饼状和片状依次转变。
(2) 随着n(Fe2+)/n(Fe3+)的增加,采用水热法所得氧化铁由α-Fe2O3相向α-Fe2O3和Fe3O4两相转变,粒径由小变大,形貌由球形逐渐向片状转变。即当n(Fe2+)/n(Fe3+)≤0.10时,氧化铁的物相为α-Fe2O3,形貌随n(Fe2+)/n(Fe3+)的增加由球状转化为片状,在n(Fe2+)/n(Fe3+)=0.10时其六边形片状体粒径最大即 1 ?m;当n(Fe2+)/n(Fe3+)=0.12时,氧化铁物相为α-Fe2O3和Fe3O4。
(3) 随着水热体系中总Fe浓度增加,氧化铁由不均一形貌依次转变为片状和球形。当水热体系总Fe浓度为1.250 mol/L时,氧化铁为六边形片状体;当水热体系总Fe浓度为1.875 mol/L时,氧化铁为球状体。
(4) 随着氧化铁晶种量的增加,氧化铁由椭球状向片状和不均一形貌依次转变。当没有加入晶种、晶种量为2 g和6 g时,氧化铁分别为椭球状、六边形片状和不均一形貌。
参考文献:
[1] Giuntim M, Baroni D, Bacci E. Hazard assessment to workers of trace metal content in pyrite cinders[J]. Bulletin of Environmental Contamination and Toxicology, 2004, 72(2): 352-357.
[2] ?lvarez-Valero A M, Sáez R, Pérez-López R, et al. Evaluation of heavy metal bio-availability from Almagrera pyrite-rich tailings dam (Iberian Pyrite Belt, SW Spain) based on a sequential extraction procedure[J]. Journal of Geochemical Exploration, 2009, 102(2): 87-94.
[3] Tugrul N, Derun E M, Piskin M. Utilization of pyrite ash wastes by pelletization process[J]. Powder Technology, 2007, 176(2): 72-76.
[4] Abdrakhimov1 A V, Abdrakhimova1 E S, Abdrakhimovl V Z. Technical properties of roof tiles made of technogenic material with pyrite cinder[J]. Environmental Protection, 2006, 63(4): 26-28.
[5] Alp I, Deveci H, Yazici E Y, et al. Potential use of pyrite cinders as raw material in cement production: Results of industrial scale trial operations[J]. Journal of Hazardous Materials, 2009, 166(1): 144-149.
[6] Hojamberdiev M, Muhamedbaeva Z, Madhusoodana C D. Use of natural and thermally activated porphyrite in cement production[J]. Construction and Building Materials, 2009, 23(8): 2757-2762.
[7] ZHENG Ya-jie, GONG Zhu-qing, LIU Li-hua, et al. Comparisons of species and coagulation effects of PFS solution and solid PFS from pyrite cinders[J]. Transactions of Nonferrous Metals Society of China, 2002, 12(5): 983-986.
[8] 郑雅杰, 陈梦君, 黄桂林. 硫铁矿烧渣制备钾铁蓝[J]. 中南大学学报: 自然科学版, 2006, 37(2): 252-256.
ZHENG Ya-jie, CHEN Meng-jun, HUANG, Gui-lin. Preparation of potassium iron blue from pyrite cinders[J]. Journal of Central South University: Science and Technology, 2006, 37(2): 252-256.
[9] 杨喜云, 龚竹青, 郑雅杰. 硫铁矿烧渣制备静电复印显影剂用Fe3O4 [J]. 功能材料, 2005, 36(5): 667-670.
YANG Xi-yun, GONG Zhu-qing, ZHENG Ya-jie. Preparation of magnetite for electrostatic copying toner from pyrite cinders[J]. Journal of Functional Materials, 2005, 36(5): 667-670.
[10] 阳征会, 龚竹青, 陈文汨, 等. 铁渣制备高纯α-Fe2O3[J]. 矿冶工程, 2005, 25(6): 70-73.
YANG Zheng-hui, GONG Zhu-qing, CHEN Wen-mi, et al. Preparation of high-purity α-Fe2O3 particles from iron cinder[J]. Ming and Metallurgical Engineering, 2005, 25(6): 70-73.
[11] 陈吉春, 梁海霞. 硫铁矿烧渣制取铁红[J]. 化工环保, 2004, 24(3): 210-212.
CHEN Ji-chun, LIANG Hai-xia. Preparation of iron oxide red pigment from pyrite cinder[J]. Environmental Protection of Chemical Industry, 2004, 24(3): 210-212.
[12] 徐旺生, 占寿祥, 宣爱国, 等. 利用硫铁矿烧渣制备高纯氧化铁工艺研究[J]. 无机盐工业, 2002, 34(2): 37-39.
XU Wang-sheng, ZHAN Shou-xiang, XUAN Ai-guo, et al. Study of process for preparing high purity Fe2O3 from pyrite dregs[J]. Inorganic Chemicals Industry, 2002, 34(2): 37-39.
[13] CHEN Jin-fang, LUO Ye, XU Jun-hui. Visualization study on sedimentation of micron iron oxide particles[J]. Journal of Colloid and Interface Science, 2006, 301(2): 549-553.
[14] 郑雅杰, 符丽纯. 硫铁矿烧渣水热法制备氧化铁[J]. 中南大学学报: 自然科学版, 2007, 38(4): 674-680.
ZHENG Ya-jie, FU Li-chun. Preparation of ferric oxide from pyrite cinders by hydrothermal method[J]. Journal of Central South University: Science and Technology, 2007, 38(4): 674-680.
[15] 郑雅杰, 刘兴瑜, 符丽纯, 等. 含铁废渣制备氧化铁的方法: 中国, CN100537435[P]. 2009-09-09.
ZHENG Ya-jie, LIU Xing-yu, FU Li-chun, et al. Method for manufacturing iron oxide with waste slag containing iron: China, CN 100537435[P]. 2009-09-09.
[16] LIU Zhao-cheng, ZHENG Ya-jie. Preparation of iron oxide red powders from pyrite cinders by hydrothermal method[C]//EPD Congress 2009 Proceedings. Warrendale, PA: The Minerals, Metals & Materials Society, 2009:943-947.
[17] 郑雅杰, 龚竹青, 刘兴瑜, 等. 部分氧化法制备聚合硫酸铁的方法: 中国, CN1446752[P]. 2003-10-08.
ZHENG Ya-jie, GONG Zhu-qing, LIU Xing-yu, et al. Preparation of PFS by partial oxidation: China, CN 1446752[P]. 2003-10-08.
[18] LIU Hui, WEI Yu, SUN Yu-han. The formation of hematite from ferrihydrite using Fe(Ⅱ) as a catalyst [J]. Journal of Molecular Catalysis A: Chemical, 2005, 226(1): 135-140.
[19] LIU Hui, WEI Yu, LI Ping, et al. Catalytic synthesis of nanosized hematite particles in solution[J]. Materials Chemistry and Physics, 2007, 102(1): 1-6.
[20] 曹付玲, 吴育飞, 刘辉, 等. 掺铝铁饼状α-Fe2O3微粒的制备及性能[J]. 化学学报, 2008, 66(12): 1405-1410.
CAO Fu-ling, WU Yu-fei, LIU Hui, et al. Preparation and property of discoid aluminum-doped α-Fe2O3 particles[J]. Acta Chimica Sinica, 2008, 66(12): 1405-1410.
[21] Liu Q Y, Osseo-Asare K. Synthesis of monodisperse Al-substituted hematite particles from highly condensed metal hydroxide gels[J]. Journal of Colloid and Interface Science, 2000, 231(2): 401-403.
[22] Sugimoto T, Wang Y. Mechanism of the shape and structure control of monodispersed α-Fe2O3 particles by sulfate ions[J]. Journal of Colloid and Interface Science, 1998, 207(1): 137-149.
(编辑 杨幼平)
收稿日期:2010-08-22;修回日期:2010-11-31
基金项目:广东省重大科技资助专项(2008A090300016);中南大学贵重仪器开放共享基金资助项目(ZKL2010022)
通信作者:郑雅杰(1959-),男,湖南常德人,教授,从事有色金属冶金、功能材料及污染控制研究;电话:0731-88836285;E-mail: zzyyjj01@yahoo.com.cn