Formation kinetics and transition mechanism of CaO·SiO2 in low-calcium system during high-temperature sintering
来源期刊:中南大学学报(英文版)2020年第11期
论文作者:潘晓林 崔维学 张灿 于海燕
文章页码:3269 - 3277
Key words:calcium silicate compounds; formation kinetics; crystal structure; microstructure; sinter process
Abstract: The crystal structure, formation kinetics and micro-morphology of CaO·SiO2 during high-temperature sintering process were studied in low-calcium system by XRD, FT-IR, Raman and SEM-EDS methods. When the molar ratio of CaCO3 to SiO2 is 1.0, β-2CaO·SiO2 forms firstly during the heating process, and then CaO·SiO2 is generated by the transformation reaction of pre-formed 2CaO·SiO2 with SiO2. 3CaO·SiO2 and 3CaO·2SiO2 do not form either in the heating or sintering process. Rising the sintering temperature and prolonging the holding time promote the phase transition of 2CaO·SiO2 to CaO·SiO2, resulting in the sintered products a small blue shift and broadening in Raman spectra. The content of CS can reach 97.4% when sintered at 1400 °C for 1 h. The formation kinetics of CaO·SiO2 follows the second-order chemical reaction model, and the corresponding apparent activation energy and pre-exponential factor are 505.82 kJ/mol and 2.16×1014 s-1 respectively.
Cite this article as: PAN Xiao-lin, CUI Wei-xue, ZHANG Can, YU Hai-yan. Formation kinetics and transition mechanism of CaO·SiO2 in low-calcium system during high-temperature sintering [J]. Journal of Central South University, 2020, 27(11): 3269-3277. DOI: https://doi.org/10.1007/s11771-020-4545-1.

J. Cent. South Univ. (2020) 27: 3269-3277
DOI: https://doi.org/10.1007/s11771-020-4545-1
PAN Xiao-lin(潘晓林)1, 2, CUI Wei-xue(崔维学)2, ZHANG Can(张灿)2, YU Hai-yan(于海燕)1, 2
1. Key Laboratory for Ecological Metallurgy of Multimetallic Mineral, Northeastern University,Shenyang 110819, China;
2. School of Metallurgy, Northeastern University, Shenyang 110819, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: The crystal structure, formation kinetics and micro-morphology of CaO·SiO2 during high-temperature sintering process were studied in low-calcium system by XRD, FT-IR, Raman and SEM-EDS methods. When the molar ratio of CaCO3 to SiO2 is 1.0, β-2CaO·SiO2 forms firstly during the heating process, and then CaO·SiO2 is generated by the transformation reaction of pre-formed 2CaO·SiO2 with SiO2. 3CaO·SiO2 and 3CaO·2SiO2 do not form either in the heating or sintering process. Rising the sintering temperature and prolonging the holding time promote the phase transition of 2CaO·SiO2 to CaO·SiO2, resulting in the sintered products a small blue shift and broadening in Raman spectra. The content of CS can reach 97.4% when sintered at 1400 °C for 1 h. The formation kinetics of CaO·SiO2 follows the second-order chemical reaction model, and the corresponding apparent activation energy and pre-exponential factor are 505.82 kJ/mol and 2.16×1014 s-1 respectively.
Key words: calcium silicate compounds; formation kinetics; crystal structure; microstructure; sinter process
Cite this article as: PAN Xiao-lin, CUI Wei-xue, ZHANG Can, YU Hai-yan. Formation kinetics and transition mechanism of CaO·SiO2 in low-calcium system during high-temperature sintering [J]. Journal of Central South University, 2020, 27(11): 3269-3277. DOI: https://doi.org/10.1007/s11771-020-4545-1.
1 Introduction
The calcium metasilicate compound of CaO·SiO2 (CS) is widely used in ceramics, cement, paint, coating, steel, rubber and plastics industries, due to its high thermal and chemical stability, good dielectric and mechanical properties as well as high whiteness [1]. In the ceramics industry, the addition of CS can reduce the sintering temperature and time, and enhance the mechanical properties [2]. For biomaterials, CS can be used as a potential matrix of bone tissue due to its good bioactivity in body fluids and the formation of bone apatite on its surface [3, 4]. For composite materials, it is very suitable to add as reinforcing filler of polymer- based composite materials due to its good stability, excellent mechanical and electrical properties [5, 6].
CS has two crystalline forms [7, 8]: α-CS is a high-temperature calcium metasilicate (wollastonite) with a ternary ring structure, while β-CS is a low-temperature calcium metasilicate with a chain structure. The transformation of β-CS to α-CS occurs at 1125 °C. The synthesis of CS mainly includes the hydro-chemical methods and solid- state reaction methods [9, 10]. The hydro-chemical methods are comprised of hydrothermal method, chemical precipitation and sol-gel method, which have the advantages of good chemical homogenization and small particles. However, they also have disadvantages: the precursors required by hydrothermal method are very expensive, which is adverse to industrial production; the particle size of samples synthesized by the chemical precipitation is difficult to control, and the addition of precipitant may result in agglomeration or inhomogeneous composition; the sol-gel process is strictly required. Therefore, the solid-state reaction is the main synthetic method in the manual preparation of CS at present, because of its wide application, simple process and easy industrialization.
Some scholars have studied the synthesis and stability of CS. ARLYUK [11] reported that 2CaO·SiO2 (C2S) is usually generated first in CaO-SiO2 system, and it is difficult to form 3CaO·SiO2 (C3S) and 3CaO·2SiO2 (C3S2). However, ABOUZEID et al [12] found that C2S forms first at 1200 °C, reaches the maximum reaction degree after 0.5 h, and then C3S2 and CS are generated. LIU et al [13] reported that the stability of CS is higher than that of β-C2S both in caustic solution and soda solution. CS is a promising target phase to replace β-C2S in the soda-lime sinter process to produce alumina from low-grade aluminum resources, which can significantly reduce the lime addition and the corresponding residue amount. However, the formation mechanism of CS in low-calcium system (the molar ratio of CaO to SiO2 is around 1.0) during the sintering process is still unclear at present. Meanwhile, the crystal transition and formation kinetics of CS have not been reported so far. In this study, the formation kinetics and transition mechanism of CS during high- temperature heating and sintering processes were systematically studied using CaCO3 and SiO2 as raw materials.
2 Experimental
The analytical reagents of CaCO3 and SiO2 with the molar ratio (C/S) of 1:1 were mixed in a polyethylene tank for 2 h, and pressed into a cylinder with a diameter of 2 cm. The samples were sintered in a MoSi2 furnace (KSL-1700X). Some samples were heated to the preset temperatures without any duration, and the other samples were sintered at different temperatures for 1 h. All the samples were heated at a uniform rate of 5 °C/min, and cooled in the air after sintering. The sintered samples were treated by two ways for tests. The samples for SEM observation were ground with sandpaper step by step and polished carefully without breaking. The other samples were crushed and milled to below 74 mm in particle size for tests.
The phase compositions and crystal structure of sintered samples were analyzed using a Philips X’Pert PW3050/60 X-ray diffractometer with CuKα1 radiation. The Fourier transform infrared spectroscopy (FTIR, SHIMADZU IRPrestige-21) was performed to study the absorption spectra, and KBr was used as the matrix material. The Raman spectra of samples were determined by a laser focusing Raman analyzer (HR800) with the wavelength of 488 nm and the laser power of 0.8 W. The microstructure of sintered samples was obtained by a scanning electron microscope (SEM, SHIMADZU SSX-550) with an energy-dispersive X-ray spectroscope (DX-4).
The semi-quantitative analysis was carried out to calculate the phase compositions of sintered samples according to the XRD spectra [14]. MgO was added to the samples after sintering by 10 wt% as internal standard. The calculation formula is as follows:
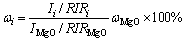 (1)
(1)
where Ii, RIRi and ωi represent the characteristic peak intensity, and the value of reference intensity ratio and mass percentage of phase i, respectively; IMgO, RIRMgO and ωMgO represent the characteristic peak intensity, and the value of reference intensity ratio and mass percentage of MgO, respectively.
3 Results and discussion
3.1 Formation behavior of CS
According to Ref. [15], both CS and β-C2S can form when the C/S molar ratio is 1.0. The XRD spectra of CaCO3 and SiO2 mixture heated to different temperatures without duration are shown in Figure 1, and the corresponding phase compositions calculated from the XRD data are presented in Table 1. The phases mainly include CaO, SiO2, CS and β-C2S. The CS (PDF card 31-0300#) belongs to a cyclic calcium silicate with P-1(2) space groups, and the crystallographic parameters are as follows: a=b=0.6820 nm,c=1.9650 nm, α=β=90.4°, γ=119.3°. The β-C2S (PDF card 33-0302#) belongs to larnite with P21/n(14) space groups, and the crystallographic parameters are as follows: a=0.9310 nm, b=0.6756 nm, c=0.5506 nm, α=γ=90.0°, β=94.46°.
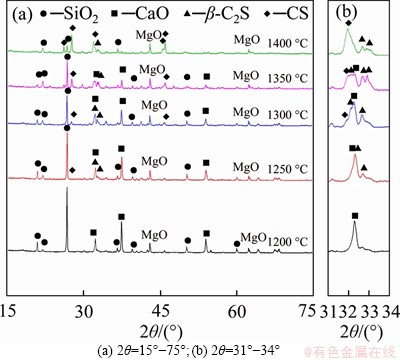
Figure 1 XRD spectra of samples when heated to different temperatures without duration:
Table 1 Phase compositions of samples heated to different temperatures without duration
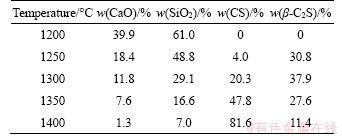
As shown in Figure 1(a), the mixture of CaCO3 and SiO2 does not react when sintered to 1200 °C because of the low diffusion rate during the solid-state reaction. As the reaction temperature rises, the diffraction peak intensity of CaO and SiO2 gradually decreases, while the peak intensity of CS increases significantly. As listed in Table 1, the contents of SiO2 and CaO in the sample after heating to 1200 °C are 61.0% and 39.9%, respectively, but they decrease to 7.0% and 1.3%, respectively, when heated to 1400 °C. It indicates that the increase of sintering temperature is largely beneficial to the solid-state reaction of CaCO3 and SiO2. The phases of C3S2 and C3S were not found in the sintered samples under various sintering temperatures, indicating that C3S2 and C3S are not involved in the reaction process when the C/S ratio is 1.0. Figure 1(b) reflects the changes of peak intensity between CS and β-C2S. The primary phase when heated to 1250 °C is C2S. As the heating temperature rises, the peak intensity of C2S reaches a maximum at 1300 °C, and then decreases. Therefore, the formation of C2S is prior to CS when the C/S ratio is 1.0, and then it transforms to CS as the sintering temperature rises.
The XRD spectra of CaCO3 and SiO2 mixture sintered at different temperatures for 1 h are shown in Figure 2. The peak intensity of CS gradually increases as the sintering temperature rises, and it reaches the strongest peak intensity at 1400 °C, indicating that the sintered sample is mainly composed of CS. On the contrary, the diffraction peak intensity of C2S decreases gradually, and it almost disappears at 1400 °C. As presented in Table 2, the content of CS increases from 6.3% to 97.4% when the sintering temperature rises from 1200 °C to 1400 °C, and the content of β-C2S decreases from 41.0% to 2.5%. It also indicates that CS is generated by the conversion reaction of C2S, which is consistent with the XRD results in Figure 1 and Table 1. Meanwhile, prolonging the holding time is beneficial to promote the transformation of C2S to CS.
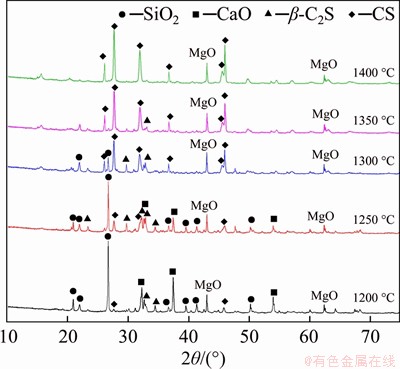
Figure 2 XRD spectra of samples sintered at different temperatures for 1 h
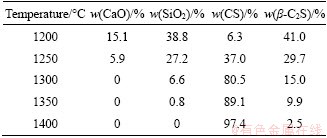
Table 2 Phase compositions of samples sintered at different temperatures for 1 h
The FT-IR spectra of samples heated to different temperatures without duration are shown in Figure 3. The main absorption peaks when heated to 1200 °C are centered at 1152, 1112, 1086, 879, 780, 695, 629 and 477 cm-1. By comparing the infrared spectra of quartz, C2S and CS [16], it can be seen that most of the characteristic peaks correspond to quartz. However, the absorption peaks at 879 and 418 cm-1 were conformed to the typical characteristic peaks of β-C2S, which belong to the asymmetric expansion vibration and the bending vibration of O-Si-O bonds in C2S, respectively. It indicates that a low-symmetry island structure of isolated Si-O tetrahedron initially forms at 1200 °C. When the heating temperature rises to 1300 °C, SiO2 only has the characteristic peaks at 1112 and 780 cm-1, but the corresponding peak intensities decline significantly. Besides the absorption peaks at 879 and 418 cm-1, the characteristic peaks of β-C2S also appear at 998, 890, 858, 629 and 477 cm-1, indicating that the island structure of C2S forms at 1300 °C. Meanwhile, new absorption peaks at 939, 920, 721, 565 and 498 cm-1 exist, which were assigned to the characteristic peaks of high-temperature wollastonite (α-CS). The absorption peak at 721 cm-1 is the typical peak of α-CS with a ternary ring structure. When the heating temperature rises to 1400 oC, most of the characteristic peaks of β-C2S disappear, and only the absorption peaks at 998, 858 and 629 cm-1 exist. The absorption peaks of α-CS are located at 1075, 985, 940, 920, 715, 560, 480 and 431 cm-1, which are consistent with the characteristic peaks of high-temperature wollastonite. As the heating temperature rises, the content of C2S increases first and then decreases,while the content of CS increases gradually, which are consistent with the XRD results.
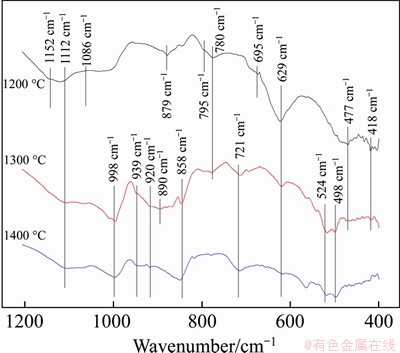
Figure 3 FT-IR spectra of samples heated to different temperatures without duration
Figure 4 shows the Raman spectra of samples sintered under different conditions. The Raman spectra of CS was reported as follows [17]: the bands lower than 400 cm-1 belong to the external vibration; the bands in 400-700 cm-1 mainly correspond to the bending vibration of Si-O tetrahedron; the bands above 700 cm-1 refer to the stretching vibration of Si-O tetrahedron. The vibrational peaks centered at 139, 371, 576, 893, 983, 1072 cm-1 belong to the characteristic peaks of wollastonite, among which 371, 576 and 983 cm-1 are the typical characteristic peaks of α-CS [18]. It indicates that the crystal structure of α-CS formed at high temperature can be kept by the rapid cooling to room temperature. As the sintering temperature and holding time increase, the peak intensity of CS gradually increases, indicating that the content of CS increases gradually. It is consistent with the XRD and FT-IR results. The bands at 825 and 858 cm-1 are the characteristic peaks of β-C2S, which were appointed to the symmetric stretching vibration and asymmetric stretching vibration of Si-O tetrahedron, respectively. The intensity of β-C2S gradually enhances at 1250-1300 °C and decreases at 1300-1400 °C, indicating that the content of β-C2S increases firstly and then decreases as the sintering temperature rises. As presented in Figure 4, when the sintering temperature rises and the holding time prolongs, there is a small blue shift and broadening in the stretching vibration, bending vibration and lattice vibration, which is mainly due to the changes of bond length and bond angle caused by the deformation correction of Si-O tetrahedron around the bridging oxygen bonds [19].
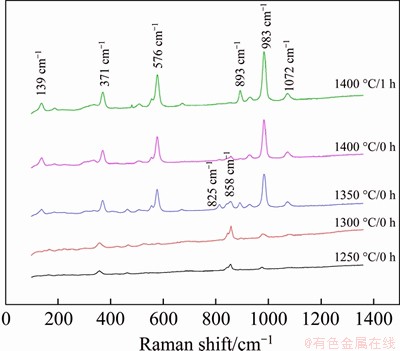
Figure 4 Raman spectra of samples sintered under different conditions
3.2 Formation kinetics of CS
According to the above analyses, the formation process of CS using CaCO3 and SiO2 as raw materials can be concluded as Eqs. (2)-(4). It involves the decomposition reaction of calcium carbonate, the direct combination reaction of C2S and the conversion reaction of C2S to CS.
CaCO3(s)=CaO(s)+CO2(g) (2)
2CaO(s)+SiO2(s)=2CaO·SiO2(s) (3)
2CaO·SiO2(s)+SiO2(s)=2(CaO·SiO2)(s) (4)
The Coats-Redfern method is one of the simplest and widely applied kinetic models for studying the solid-state reactions, especially for those with decomposition [20-22]. The authors successfully used the Coats-Redfern method to study the formation kinetics of calcium aluminate compounds when using CaCO3, Al2O3 and Na2CO3 [23]. In this work, the Coats-Redfern method was also used to determine the reaction model and the corresponding equation of CS, as presented in Eq. (5). The data of CS formation from CaCO3 and SiO2 mixture when heated to different temperatures without duration were adopted. Considering the simple solid-state reactions of CS based on the CaO-SiO2 binary system, only the nucleation model, diffusion model and geometric contraction model were selected to fit the kinetic model of CS formation, as shown in Table 3.
ln[g(α)/T2]=ln[RA/(βE)(1-2RT/E)]-E/(RT) (5)
where α is the formation percentage of CS, %; g(α) is the function of α as listed in Table 3; T is the reaction temperature, K; R is the gas constant, J/(K·mol); A is the pre-exponential factor, s-1; β is the heating rate, K/min; E is the apparent activation energy, kJ/mol.
According to the relevant research results in Ref. [24], the value of 2RT/E is very close to 0 during the high-temperature sintering reaction, thus the value of (1-2RT/E) is approximately equal to 1. Then the formula ln[(RA/(βE))(1-2RT/E)] can be regarded as a constant, and ln[g(α)/T2] is approximately linear to E/(RT). Finally, the reaction activation energy and the pre-exponential factor can be obtained by the slope and the intercept of corresponding curves. The calculated results of CS formation with different solid-state reaction rate models are presented in Table 3. The F2 model is the best to represent the formation of CS during high-temperature sintering, and the corresponding fitting r and R2 are much higher than other models. The fitting curve according to the F2 model is shown in Figure 5. Meanwhile, the apparent activation energies and pre-exponential factors of different models with the fitting R2 values higher than 0.95 were calculated as listed in Table 3. As the theoretical range of pre-exponential factor based on the solid-state reaction is usually between 106 and 1018 [25], it proves that the formation of CS follows the F2 model, which is also in good agreement with the formation model of calcium aluminate compounds [23].
Table 3 Calculated results of CS formation with different solid-state reaction rate models
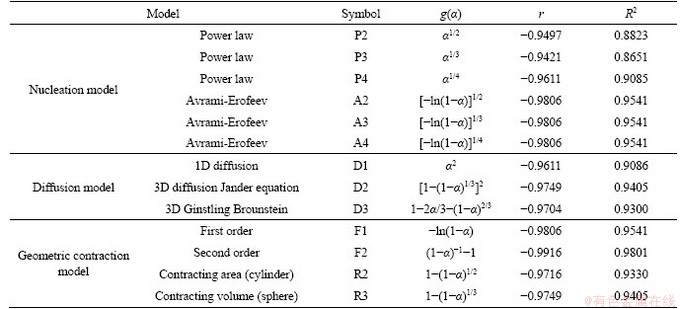
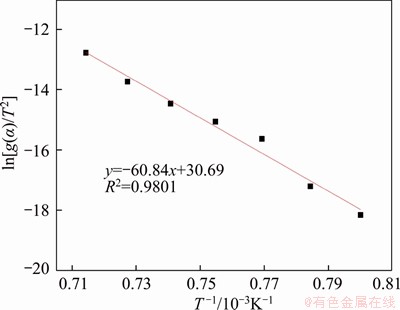
Figure 5 Linear relationship for formation of CS according to F2 model
Table 4 Calculation results of activation energy and pre-exponential factor of CS formation
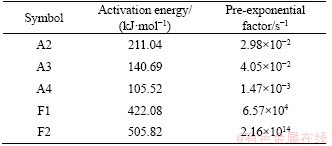
The apparent activation energy and pre- exponential factor were calculated to be 505.82 kJ/mol and 2.16×1014 s-1, respectively. The formation of CS belongs to the second-order reaction model, and the formation kinetic equation is:
(1-α)-1-1=2.16×1014exp(-505820/(RT))t (6)
3.3 Morphology of CS
The SEM morphology of sample heated to 1250 °C without duration and its map scanning results are shown in Figure 6, and the EDS results are presented in Table 5. It is mainly composed of blocky particles and pores with different sizes (Figure 6(a)). As shown in Figures 6(b)-(d), Si element is mainly concentrated in large-size blocky particles, Ca element is evenly distributed except the large-size blocky particles, and O element is mainly concentrated on the massive particles with large grain size. The large-size blocky particles in highlight regions of Figure 6(b) (A) correspond to SiO2, and the small-size particles with a uniform distribution (B) correspond to CaO or undecomposed CaCO3. The medium-size particles (C) correspond to C2S, which are mostly distributed near the large-size particles. It can be concluded that C2S is generated by the diffusion of CaO to blocky SiO2 according to their locations.
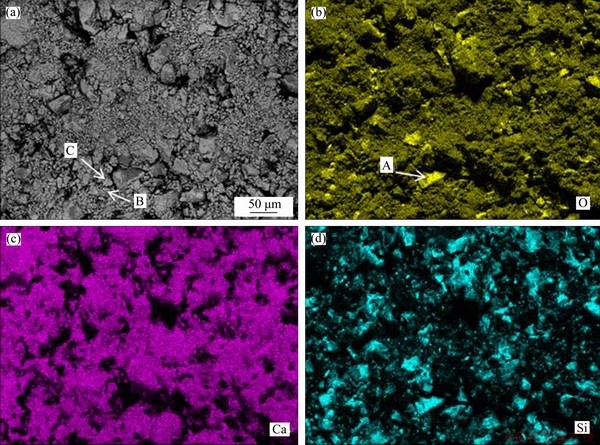
Figure 6 SEM image of sample heated to 1250 °C without duration (a) and its map scanning results (b-d)
Table 5 EDS results of sample heated to 1250 °C without duration in mole fraction
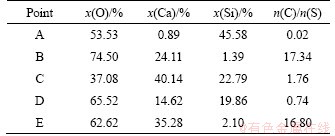
As seen in Figure 7 and Table 5, some blocky particles (D) corresponding to CS form during the heating process, and the CaO to SiO2 molar ratio is below 1 because of the low diffusion rate and short reaction time. Meanwhile, a large quantity of CaO (E) exists on the surface of CS.

Figure 7 Morphology of initial formed CS in sample heated to 1250 °C without duration (a) and amplification of rectangular area (b)
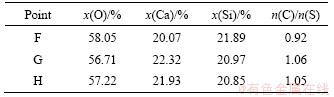
The SEM images of sample sintered at 1400 °C for 1 h are shown in Figure 8, and the EDS results are listed in Table 6. It melts partly at 1400 °C. The melting areas are distributed in grid morphology (Figure 8(b)) and the non-melting areas are distributed in dispersed particles with much smaller size (Figure 8(c)). The CaO to SiO2 molar ratios in different regions are about 1.0, indicating that the formation of CS is uniform and complete.
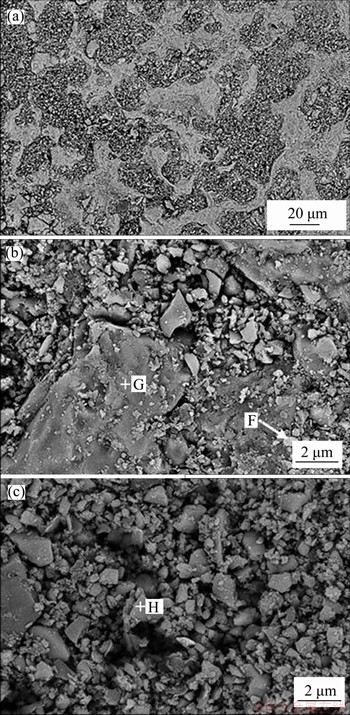
Figure 8 SEM images of sample sintered at 1400 °C for 1 h (a) and amplification of melting area (b) and non-melting area (c)
Table 6 EDS results corresponding to Figure 8 in molar fraction
4 Conclusions
1) β-C2S forms firstly during the heating process of CaCO3 and SiO2 mixture with the equal molar ratio, and then CS is generated by the transformation reaction of pre-formed β-C2S with SiO2.
2) Increasing the sintering temperature and prolonging the holding time are helpful to promote the phase transition of C2S to CS, and the content of CS can reach 97.4% when sintered at 1400 °C for 1 h.
3) The formation process of CS follows the second-order chemical reaction model, and the corresponding apparent activation energy and pre-exponential factor are 505.82 kJ/mol and 2.16×1014 s-1, respectively. The formation kinetic equation of CS is as follows: (1-α)-1-1=2.16×1014 exp(-505820/(RT))t.
Contributors
The overarching research goals were developed by PAN Xiao-lin and YU Hai-yan. CUI Wei-xue and ZHANG Can provided and analyzed the measured data. The initial draft of the manuscript was written by PAN Xiao-lin and CUI Wei-xue. All authors replied to reviewers’ comments and revised the final version.
Conflict of interest
PAN Xiao-lin, CUI Wei-xue, ZHANG Can, and YU Hai-yan declare that they have no conflict of interest.
References
[1] LONG L H, CHEN L D, BAI S Q, CHANG J, LIN K L. Preparation of dense beta-CaSiO3 ceramic with high mechanical strength and HAp formation ability in simulated body fluid [J]. Journal of the European Ceramic Society, 2006, 26(9): 1701-1706. DOI: 10.1016/j.jeurceramsoc.2005. 03.247.
[2] DAI B, ZHU H K, ZHOU H Q, YUE Z X. Sintering, crystallization and dielectric properties of CaO-B2O3-SiO2 system glass ceramics [J]. Journal Central South University, 2012, 19(8): 2101-2106. DOI: 10.1007/s11771-012-1251-7.
[3] CHEN Z, ZHAI J, WANG D, CHEN C Z. Bioactivity of hydroxyapatite/wollastonite composite films deposited by pulsed laser [J]. Ceramics International, 2018, 44(9): 10204-10209. DOI: 10.1016/j.ceramint.2018.03.013.
[4] SHIRAZI F S, MOGHADDAM E, MEHRALI M, OSHKOUR M M, METSELAAR H S C, KADRI N A, ZANDI K, ABU N A. In vitro characterization and mechanical properties of β-calcium silicate/POC composite as a bone fixation device [J]. Journal of Biomedical Materials Research Part A, 2014, 102(11): 3973-3985. DOI: 10.1002/jbm.a.35074.
[5] YAACOB M M, KAMARUDDIN N, MAZLAN N A, NORAMAT N F, ALSAEDI M A, AMAN A. Dielectric properties of polyvinyl chloride with wollastonite filler for the application of high-voltage outdoor insulation material [J]. Arabian Journal for Science and Engineering, 2014, 39(5): 3999-4012. DOI: 10.1007/s13369-014-0996-8.
[6] LIANG J Z, LI B, RUAN J Q. Crystallization properties and thermal stability of polypropylene composites filled with wollastonite [J]. Polymer Testing, 2015, 42(4): 185-191. DOI: 10.1016/j.polymertesting.2015.01.017.
[7] WANG H P, ZHANG Q L, YANG H, SUN H P. Synthesis and microwave dielectric properties of CaSiO3 nanopowder by the sol-gel process [J]. Ceramics International, 2008, 34(4): 1405-1408. DOI: 10.1016/j.ceramint.2007.05.001.
[8] LAPCIK L, MANAS D, LAPCIKOVA B, MARTIN V, Michal S, KLARA C, JAKUB V, KRISTIAN W, RICHARD G, NEIL R. Effect of filler particle shape on plastic-elastic mechanical behavior of high density poly(ethylene)/mica and poly(ethylene)/wollastonite composites [J]. Composites Part B: Engineering, 2018, 141(5): 92-99. DOI: 10.1016/ j.compositesb.2017.12.035.
[9] LONG L H, CHEN L D, CHANG J. Low temperature fabrication and characterizations of β-CaSiO3 ceramics [J]. Ceramics International, 2006, 32(4): 457-460. DOI: 10.1016/j.ceramint.2005.03.023.
[10] CAO Z, CAO Y D, ZHANG J S, SUN B, LI X L. Preparation and characterization of high-strength calcium silicate boards from coal-fired industrial solid wastes [J]. International Journal of Minerals, Metallurgy and Materials, 2015, 22(8): 892-900. DOI: 10.1007/s12613-0.15-1147-2.
[11] ARLYUK B I. Study of an effect of a nepheline raw material composition on process parameters of alumina production by the sintering methods [J]. Light Metals, 1994: 59-66.
[12] ABOUZEID A Z M. Primary crushing plant design [J]. International Journal of Mineral Processing, 1979, 6(2): 169-170. DOI: 10.1016/0301-7516(79)90024-3.
[13] LIU G H, LI X B, PENG Z H, ZHOU Q S. Stability of calcium silicate in basic solution [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(5): 1235- 1238. DOI: 1003-6326(2003)05-1235-04.
[14] PAN X L, ZHANG D, WU Y, YU H Y. Synthesis and characterization of calcium aluminate compounds from gehlenite by high-temperature solid-state reaction [J]. Ceramics International, 2018, 44(12): 13544-13550. DOI: 10.1016/j.ceramint.2018.04.186.
[15] LI X B, LU W J, PENG Z H, LIU G H, ZHOU Q S. Researches on the sinter of calcium silicate and its digestion properties in alkaline solutions [J]. Mining and Metallurgical Engineering, 2005, 25(1): 47-49. (in Chinese)
[16] ZHANG D, HU S S, SUN H L, ZHANG W, WANG B. Mineral transition of high-temperature sintering confirmed in CaAl2O4-Ca2SiO4 non-equilibrium binary system [J]. Construction and Building Materials, 2020, 234: 117402. DOI: 10.1016/j.conbuildmat.2019.117402.
[17] MYSEN B O, VIRGO D, SCARFE C M. Relations between the anionic structure and viscosity of silicate melt-a Raman spectroscopic study [J]. American Mineralogist, 1980, 65(8): 690-710. DOI: 10.0000/PMID15469.
[18] LI L, WENTZCOVITCH R M, WEIDNER D J, SILVA C R S D. Vibrational and thermodynamic properties of forsterite at mantle conditions [J]. Journal of Geophysical Research Atmospheres, 2007, 5(9): 1-8. DOI: 10.1029/2007JB005 532.
[19] SWAMY V, DUBROVINSKY L S, F. TUTTI F. High- temperature Raman spectra and thermal expansion of wollastonite [J]. Journal of the American Ceramic Society, 1997, 80(9): 2237-2247. DOI: 10.1111/j.1151-2916.1997. tb03113.x.
[20] FRADE J R. Reexamination of the basic theoretical model or the kinetics of solid-state reactions [J]. Journal of the American Ceramic Society, 1992, 75(7): 1949-1957. DOI: 10.1111/j.1151-2916.1992.tb07222.x.
[21] STRASZKO J, OLSZAKHUMIENIK M, MOZEJKO J. Kinetics of thermal-decomposition of solids [J]. Inzynieria Chemiczna I Procesowa, 1995, 16(1): 45-61. DOI: 10.1007/ BF01131049.
[22] ZUO Z L, YU Q B, LUO S Y, ZHANG J K, ZHOU E Z. Effects of CaO on two-step reduction characteristics of copper slag using biochar as reducer: thermodynamic and kinetics [J]. Energy & Fuels, 2020, 34(1): 491-500. DOI: 10.1021/ acs.energyfuels.9b03274.
[23] YU H Y, PAN X L, TIAN Y P, TU G F. Mineral transition and formation mechanism of calcium aluminate compounds in CaO-Al2O3-Na2O system during high-temperature sintering [J]. International Journal of Minerals, Metallurgy and Materials, 2020, 27(7): 924-932. DOI: 10.1007/ s12613-019-1951-1.
[24] URBANOVICI E, POPESCU C, SEGAL E. Improved iterative version of the Coats-Redfern method to evaluate non-isothermal kinetic parameters [J]. Journal of Thermal Analysis and Calorimetry, 1999, 58(3): 683-700. DOI: 10.1023/A:1010125132669.
[25] TAZUDDIN, AIYER H N, CHATTERJEE A. Phase equilibria studies of CaO-SiO2-Al2O3-Fe2O3-MgO system using CALPHAD [J]. Calphad, 2018, 60(5): 116-125. DOI: 10.1016/j.calphad.2017.12.003
(Edited by ZHENG Yu-tong)
中文导读
CaO·SiO2在低钙高温烧结过程的生成动力学与转变机制
摘要:利用XRD、FT-IR、Raman、SEM-EDS等方法研究了CaO·SiO2在低钙高温烧结过程的晶体结构、生成动力学和微观组织。当反应物CaCO3和SiO2摩尔比为1.0时,加热过程中优先生成2CaO·SiO2,然后2CaO·SiO2进一步与SiO2反应生成CaO·SiO2;烧结过程中不会生成3CaO·2SiO2和3CaO·SiO2。升高反应温度和延长保温时间有助于2CaO·SiO2向CaO·SiO2转化,并使烧结产物的拉曼光谱特征峰发生一定的蓝移和宽化。在1400 °C烧结1 h时CaO·SiO2在烧结产物的含量能够达到97.4%。CaO·SiO2的生成动力学遵循二级化学反应模型,相应的反应表观活化能和指前因子分别为505.82 kJ/mol和2.16×1014s-1。
关键词:硅酸钙化合物;生成动力学;晶体结构;微观组织;烧结法
Foundation item: Projects(51674075, 51774079) supported by the National Natural Science Foundation of China; Project(2018YFC1901903) supported by the National Key R&D Program of China; Project(N182508026) supported by the Fundamental Research Funds for the Central Universities of China
Received date: 2020-03-25; Accepted date: 2020-09-08
Corresponding author: PAN Xiao-lin, PhD, Associate Professor; Tel: +86-24-83686460; E-mail: panxl@smm.neu.edu.cn; ORCID: https://orcid.org/0000-0002-9814-0882

