J. Cent. South Univ. Technol. (2008) 15: 479-483
DOI: 10.1007/s11771-008-0090-z
Effect of magnetic field on property and structure of polyaniline doped with multiple sulfonic acids
MA Li(马 利)1, 2, HUANG Ke-long(黄可龙)1, CHEN Chao(陈 超)2,
GAN Meng-yu(甘孟瑜)2, LU Wei(卢 苇)2, CHEN Feng-qiang(陈奉强)2
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. College of Chemistry and Chemical Engineering, Chongqing University, Chongqing 400044, China)
Abstract: Polyaniline(PAn) doped with multiple sulfonic acid system of dodecylbenzenesulfonic acid(DBSA) and sulfosalicylic acid(SSA) was synthesized by emulsion polymerization using ammonium persulfate(APS) as an oxidizing agent in the presence and the absence of a constant magnetic field(MF)of 0.8 T. The structure and properties of the PAn were characterized by X-ray diffractometer(XRD), thermogravimetric apparatus(TGA), FT-IR spectroscope(FT-IR) and four probe digital multimeter. The results indicate that, when the molar ratio of DBSA to SSA is 1/3, that of dopant to An is 3/2, that of APS to An is 4/5 in the synthesizing media, and the doping time is 3 h, the conductivity of the PAn synthesized in the presence of the MF of 0.8 T reaches 5.88 S/cm, which is higher than that of the PAn synthesized in the absence of the MF. The thermal stability, the crystallinity and the doping degree of the PAn synthesized in the presence of the MF are also improved. MF not only enhances the conductivity, but also reduces the doping time, the dosage of the dopant and the oxidizing agent when the conductivity reaches the maximum.
Key words: polyaniline; magnetic field; emulsion polymerization; multiple sulfonic acids
1 Introduction
Polyaniline(PAn) is unique in the family of the conducting polymers and has attracted a great deal of interest because of its low cost, ease of preparation, unique chemical and physical properties controlled by the oxidation and protonation state, as well as excellent environmental stability[1]. Due to its electrical and electronic properties, polyaniline can be applied to rechargeable organic batteries, electrochromic display, electromechanical actuators, anti-corrosion coating, and electromagnetic interference shielding[2-4].
It is well known that all materials respond to a magnetic field(MF). The response may be repulsion (negative magnetic susceptibility) representing diamagnetism, or attraction (positive magnetic susceptibility) representing para- and ferro-magnetism. Most organic and inorganic polymeric materials are diamagnetic. The most important magnetic field effect on purely diamagnetic molecule is magnetic orientation and concentration. This is a result of the anisotropic diamagnetic susceptibility of the molecules in general[5]. A strong correlation between the structure of this kind of polymeric materials and their ability for magnetization may be expected. As a result of MF treatment, changes in supermolecular structure formation and degree of crystallization were observed, as well as improvement of mechanical properties[6].
The radical polymerization is directly dependent on radical presence and is influenced by the existence of an external MF[7-9]. This statement is based on the interpretation of the radical pairs mechanism[10]. Owing to the presence of the MF, changes occur from the singlet to the triplet state in the spin multiplicity of the initiator radicals and the radical life increases. As a result, the initiation efficiency is enhanced due to the decrease in the “cage” effect and the reduction of radical recombination[11]. Recently, few papers concerning the effect of the MF on PAn have been published[12-15]. However, very limited research has been done on the MF effects on the structure and properties of PAn doped with multiple sulfonic acids.
In the present work, PAn doped with multiple sulfonic acid system of dodecylbenzenesulfonic acid (DBSA) and sulfosalicylic acid(SSA) was synthesized by emulsion polymerization method using ammonium persulfate (APS) as an oxidizing agent in the presence and the absence of a constant MF of 0.8 T. And the struc- ture and properties of the as-prepared PAn were studied.
2 Experimental
2.1 Materials
Aniline was distilled twice under reduced pressure. All other materials were analytical grade reagents.
2.2 Preparation of PAn salts
A calculated amount of DBSA and SSA were added to a three-necked round bottomed flask containing 2.325 g aniline monomer, 25 mL xylene and a quantitative volume of deionized water, then the mixture was stirred for 30 min to form uniform milk-white emulsion. A calculated amount of ammonium persulfate(APS) was dissolved into a quantitative volume of deionized water and added slowly into the above emulsion. After completion of the addition (addition time about 0.5 h), stirring was continued for some hours to ensure completion of the reaction. The whole process of the reaction was in the presence or the absence of a constant MF (0.8 T). At the end of the reaction, acetone was added to terminate the polymerization reaction and the mixture was precipitated with ethanol, and washed with distilled water several times and then dried at 65 ℃ to a constant mass.
2.3 Measurements
XRD 6000 with 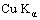 radiation of wavelength 0.154 nm and continuous scanning speed of 0.045 (?)/min. The TGA diagram with a Setaram DSC141 thermal analysis system was recorded up to 600 ℃ at a heating rate of 10 ℃/min under air atmosphere. Conductivity measurement was carried out on a four probe connected to a SX1934 (SZ-82) digital multimeter. The FT-IR spectrum was recorded using a Nicolet 550Ⅱinstrument by the KBr pellet technique.
radiation of wavelength 0.154 nm and continuous scanning speed of 0.045 (?)/min. The TGA diagram with a Setaram DSC141 thermal analysis system was recorded up to 600 ℃ at a heating rate of 10 ℃/min under air atmosphere. Conductivity measurement was carried out on a four probe connected to a SX1934 (SZ-82) digital multimeter. The FT-IR spectrum was recorded using a Nicolet 550Ⅱinstrument by the KBr pellet technique.
3 Results and discussion
3.1 Effect of polymerization conditions on conduc- tivity of PAn
3.1.1 Determination of n(DBSA)/n(SSA)
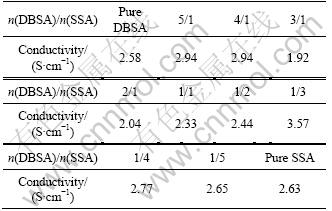
The effect of n(DBSA)/n(SSA) was studied and the conductivity of the PAn is listed in Table 1, in which the molar ratio of APS to An was kept constant (n(APS)/ n(An)=4/5) and the molar ratio of DBSA to SSA was varied. When n(DBSA)/n(SSA)=1/3 in the synthesizing media, the conductivity of the PAn reaches 3.57 S/cm, which is much higher than those of other PAn samples. This result may be due to the co-operation of the differences of the content, molecule size, doping capability and water-solubility between DBSA and SSA.
Table 1 Conductivity data of PAn
3.1.2 Effect of doping time on conductivity of PAn
The effect of doping time on conductivity of PAn was discussed, in which n(DBSA)/n(SSA), n(dopant)/ n(An) and n(APS)/n(An) were kept constant (1/3, 3/2, 4/5, respectively) and only the doping time was changed. Fig.1 shows that under the conditions of 4 h in 0 T and 3 h in 0.8 T, the conductivities of the PAn reach the maximums, which are 4.20 S/cm and 5.56 S/cm, respectively; with the further increase of the doping time, the conductivity of the PAn decreases gradually. This result indicates that the MF can not only enhance the conductivity, but also reduce the doping time when the conductivity reaches the maximum. According to the rule of spin conservation in chemical reaction, the radicals generated in singlet state will recombine only with singlet; the radicals in the triplet state will recombine only with triplets, if they have enough energy. The application of external magnetic field to radical pair determines the splitting of t+ and t- from t0. The levels t+ and t- do not interact with S and do not participate in S-T evolution. The recombination in MF is 1/3 of that in absence of MF. As a result of changes in the radical pair multiplicity, there is a diminution of the cage effect and also a lifetime broadening of the radicals concomitantly with the diminution of the geminate radicals’ recombination. This leads to the increase in the reaction rate, the doping rate and the doping degree in the polymerization process of aniline. Therefore, the MF can not only enhance the conductivity but also reduce the doping time.
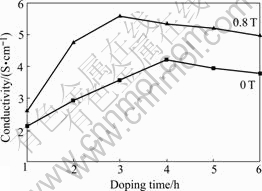
Fig.1 Effect of doping time on conductivity of PAn (n(DBSA)/ n(SSA)=1/3, n(dopant)/n(An)=3/2, n(APS)/n(An)=4/5)
3.1.3 Effect of n(dopant)/n(An) on conductivity of PAn
The effect of n(dopant)/n(An) on the conductivity of the PAn is shown in Fig.2. When n(dopant)/n(An) is 3/2 in 0 T and 5/4 in 0.8 T, the conductivities of the PAn reach the maximums, which are 3.57 and 5.88 S/cm, respectively. Obviously, the MF cannot only enhance the conductivity, but also reduce the dosage of the dopant at which the conductivity reaches the maximum. With further increase of n(dopant)/n(An), the conductivity of the PAn decreases gradually. The possible reason is that with the increase in the concentration of dopant, the pH of the synthesizing media reduces gradually, which is in favor of enhancing the conductivity of PAn; when the breakthrough achieves, with further increase of the concentration of dopant, it is difficult to remove the excessive dopant from the PAn product, which is negative for increasing the conductivity of PAn.
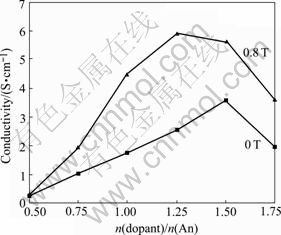
Fig.2 Effect of n(dopant)/n(An) on conductivity of PAn (n(DBSA)/n(SSA)=1/3, n(APS)/n(An)=4/5, doping time is 3 h)
3.1.4 Effect of n(APS)/n(An) on conductivity of PAn
The effect of n(APS)/n(An) on the conductivity of the PAn is shown in Fig.3. When the n(APS)/n(An) is 1/1 in 0 T and 4/5 in 0.8 T, the conductivities of the PAn reach to the maximums, which are 3.84 S/cm and 5.57 S/cm, respectively. Obviously, the magnetic field can not only enhance the conductivity but also reduce the dosage of the oxidizing agent that the conductivity reaches the maximum. With further increase of the n(APS)/n(An), the conductivity of the PAn decreases gradually. The possible reason is that the excessive oxidation can destroy the conjugated π system of the PAn molecule, which results in the decrease in conductivity of the PAn.
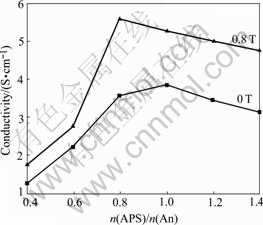
Fig.3 Effect of n(APS)/n(An) on conductivity of PAn (n(DBSA)/n(SSA)=1/3, n(dopant)/n(An)=5/4, doping time is 3 h)
3.2 Characterization
The optimum polymerization conditions of the PAn in the presence and the absence of the MF were obtained by orthogonal experiments. The results are listed in Table 2. The thermal stability and the structure of the products are characterized by XRD, TGA and FT-IR spectroscope.
Table 2 Optimum conditions of synthesizing PAn

3.2.1 XRD analysis
XRD patterns of the PAn are shown in Fig.4. The result indicates that the PAn products are partially crystalline. Generally, a similar X-ray diffraction pattern is observed with difference in intensities for synthesized products under 0.8 T and 0 T. X-ray diffraction pattern of the PAn products show peaks at 2θ=20? and 25? which arises from scattering with momentum transfer approximately perpendicular to the PAn chains. These positions are in accordance with the earlier reports[16-17]. The intensities of peaks observed in synthesized products without MF are much less compared with those under 0.8 T, showing that the PAn synthesized in the presence of the MF is in better order and has higher crystallinity than the PAn synthesized in the absence of the MF, which indicates MF can enhance polymer sub-chain alignment.
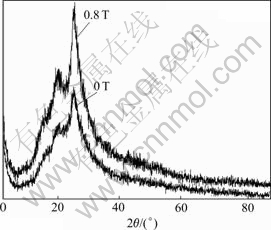
Fig.4 X-ray diffraction patterns of PAn
3.2.2 TGA analysis
The TGA curves (Fig.5) of the PAn shows a three-step mass loss. The mass loss of about 6%-9% is associated with the loss of moisture. The second step that commences after 220 ℃, the mass loss in the range of 20%-36%, is due to the removal of the dopant and some oligomers. A slow and gradual mass loss profile is observed for the PAn about 440 ℃, which is attributed to the degradation of the salts[2]. As a comparison, sample synthesized in the presence of the MF of 0.8 T seems to have a better thermal stability than sample prepared in the absence of the MF, which is in accordance with the above XRD result.
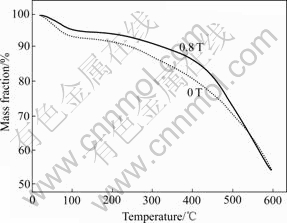
Fig.5 TGA curves of PAn
3.2.3 FT-IR analysis
The FT-IR spectra of the PAn products are shown in Fig.6. Doping PAn leads to the formation of —Q=N+H— group (Q means quinoid ring). The positive charge on the polymer chain may lead to an increase in the dipole moment of the molecule and consequently results in an increase in the intensity of the IR bands. For the PAn, the bands around 1 306 and 1 250 cm-1 correspond to N—H bending and asymmetric C—N stretching modes of the benzenoid ring, respectively. The band in 1 480-1 496 cm-1 is assigned to C—N stretching of the quinoid ring and the band in 1 562-1 572 cm-1 is assigned to C=C stretching defor- mation of quinoid, which is due to the protonation of PAn, by the dopants. In the IR spectrum of the PAn, a fairly intense band appears in 805-835 cm-1, which is assigned to 1, 4-disubstituted benzene[12]. The band in 1 142-1 152 cm-1 is doing-mode of N=Q=N. The presence of SO32- group is confirmed by the appearance of a band around 518 cm-1 in the spectrum of the PAn for degenerating bending mode of the SO32- group. The spectral intensity of these bands is a typical of the highly doped emeraldine salt form of polymer[13]. Slight shifts appear in certain IR bands of the PAn products. The bands at 1 572, 1 496 and 1 152 cm-1 in the spectrum of the sample synthesized without MF are observed, but these bands shift to lower frequencies for sample synthesized in 0.8 T by 10, 16 and 12 cm-1, showing that a highly conjugated π system might be formed in the PAn molecular chains synthesized in the presence of the MF. As a result, the PAn prepared in the presence of the MF has higher doping degree than the PAn prepared in the absence of the MF.
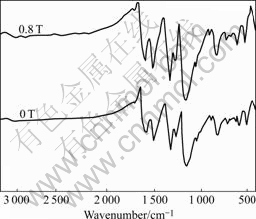
Fig.6 FT-IR spectra of PAn
4 Conclusions
1) When n(DBSA)/n(SSA)=1/3, n(dopant)/n(An)= 5/4, n(APS)/n(An)=4/5 in the synthesizing media, and the doping time is 3 h, the conductivity of the PAn synthesized in the presence of the MF reaches 5.88 S/cm, which is higher than the PAn synthesized in the absence of the MF.
2) The conductivity, the thermal stability, the crystallinity and the doping degree of the PAn synthesized in the presence of the MF are improved.
3) MF can not only enhance the conductivity, but also reduce the doping time, the dosage of the dopant and the oxidizing agent that the conductivity reaches the maximum.
References
[1] ZHANG Li-xia, ZHANG Li-juan, WAN Mei-xiang. Polyaniline micro/nanofibers doped with saturation fatty acids [J]. Synthetic Metals, 2006, 156(5/6): 454-458.
[2] PALANIAPPAN S, A.AMARNATH C. Polyaniline- dodecylhydrogensulfate-acid salt: Synthesis and characterization [J]. Materials Chemistry and Physics, 2005, 92(1): 82-88.
[3] QIU Jian-hui, FENG Hui-xia. Preparation and properties of PAn/ATTP/PE conductive composites [J]. Trans Nonferrous Met Soc China, 2006, 16(S2): s444-s448.
[4] LEI Ting. Preparation of novel core-shell nanoparticles by electrochemical synthesis [J]. Trans Nonferrous Met Soc China, 2007, 16(6): 1343-1346.
[5] CHIRIAC A P, SIMIONESCU C I. Magnetic field polymerization [J]. Progress in Polymer Science, 2000, 25(2): 219-258.
[6] PETROVA I, DISHOVSKY N, DIMITROV R. Investigation of influence of constant magnetic fields on the structure and properties of chloroprene latex based films [J]. Polymer Testing, 2005, 24(8): 1036-1040.
[7] STEP E N, BUCHACHENKO A L, TURRO N J. Magnetic effects in the photolysis of micellar solutions of phenacylphenylsulfone [J]. Chemical Physics, 1996, 162(1): 189-204.
[8] CHIRIAC A P, SIMIONESCU C I. Aspects regarding the characteristics of some acrylic and methacrylic polyesters synthesized in a magnetic field [J]. Polymer Testing,1996, 15(6): 537-548.
[9] SIMIONESCU C I, CHIRIAC A P, CHIRIAC M V. Polymerization in a magnetic field (1): Influence of ester chain length on the synthesis of various poly(methacrylate)s [J]. Polymer, 1993, 34(18): 3917-3920.
[10] SALIKHOV K M. Theory of magnetic effects in radical reactions at zero field [J]. Chemical Physics, 1983, 82(1/2): 145-162.
[11] NEAMTU I, CHIRIAC A P. Some properties in solution of poly(acrylamide) synthesized in a magnetic field [J]. Polymer Testing, 2001, 20(5): 585-589.
[12] CRUZ-SILVA R, ROMERO-GARCíA J, ANGULO-S?NCHEZ J L. Comparative study of polyaniline cast films prepared from enzymatically and chemically synthesized polyaniline [J]. Polymer, 2004, 45(14): 4711-4717.
[13] ATHAWALE A A, KULKARNI M V, CHABUKSWAR V V. Studies on chemically synthesized soluble acrylic acid doped polyaniline [J]. Materials Chemistry and Physics, 2002, 73(1): 106-110.
[14] DUAN Yu-ping, LIU Shun-hua, GUAN Hong-tao. Orientation effects of high magnetic field on grain shape of doped polyaniline [J]. Function Materials, 2005, 36(9): 1455-1458. (in Chinese)
[15] TORBET J, NICOLAU Y F, DJURADO D. Orientation of CSA-protonated polyaniline chains in solution in m-cresol and in films induced by a high magnetic field [J]. Synthetic Metals, 1996, 101(1/3): 825-826.
[16] MOOM Y B, RUGHOOPUTH S S D V, HEEGER A J, PATIL A O, WUDL F. X-ray scattering study of the conversion of poly(p- phenylene vinylene precursor to the conjugated polymer [J]. Synthetic Metals, 1989, 29(1): 79-84.
[17] SWAPNA RAO P, SUBRAHMANYA S, SATHYANARAYANA D N. Inverse emulsion polymerization: A new route for the synthesis of conducting polyaniline [J]. Synthetic Metals, 2002, 128(3): 311-316.
Foundation item: Project(20176066) supported by the National Natural Science Foundation of China
Received date: 2007-12-26; Accepted date: 2008-03-19
Corresponding author: MA Li, Doctoral candidate, Associate professor; Tel: +86-13983639516; E-mail: mlsys607@126.com
(Edited by YANG Hua)