氮川三乙酸的脱水反应机理
唐新村1,陈静波1,贾殿赠2,罗卓1,李连兴1,曾智文1,肖元化1
(1. 中南大学 化学化工学院,湖南 长沙,410083;
2. 新疆大学 化学化工学院,新疆 乌鲁木齐,830046)
摘 要:运用量子化学密度泛函理论(DFT),在B3LYP/6-311++G(d,p)的计算水平上研究氮川三乙酸(NTA)的脱水反应机理。通过对反应物、过渡态和产物进行优化,对频率和自然键轨道进行分析,得到对应的稳定构型;频率分析结果表明过渡态只存在1个虚频,并通过振动分析和内禀反应坐标对过渡态进行确认。运用Gaussian03程序进行计算。研究结果表明:氮川三乙酸脱水反应的微观途径为NTA→TS→P+H2O,其所需活化能为163 kJ/mol,反应物和产物的能量差为69 kJ/mol,该反应为吸热反应;采用经典过渡态理论计算得到反应的速率常数为5.825×10-16 s-1。
关键词:氮川三乙酸;反应机理;密度泛函理论;过渡态
中图分类号:O641.12+1 文献标志码:A 文章编号:1672-7207(2010)02-0446-04
Process of dehydration of nitrilotriacetic acid
TANG Xin-cun1, CHEN Jing-bo1, JIA Dian-zeng2, LUO Zhuo1, LI Lian-xing1,
ZENG Zhi-wen1, XIAO Yuan-hua1
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083 China;
2. School of Chemistry and Chemical Engineering, Xinjiang University, Urumuqi 830046, China)
Abstract: Quantum chemistry density functional theory (DFT) on the level of B3LYP/6-311++G (d, p) calculations is used to study the dehydration reaction mechanism of nitrilotriacetic acid (NTA). The corresponding stable configuration was gotten by optimizing the reactants, the transition state and the product, and analyzing the frequency and the natural bond orbital. Frequency analysis results of the transition state show that there is only one virtual band, and it is confirmed by analysis of the vibration and the intrinsic reaction coordinate. The results from the Gaussian03 calculation show that: NTA dehydration reaction as a means of microcosm is NTA→TS→P+H2O. The activation energy required and the energy of the reaction product are 160.206 0 and 68.961 3 kJ/mol, respectively. The reaction is endothermic reaction. The reaction rate constant is calculated to be 5.825×10-16 s-1 using classical transition state theory.
Key words: nitrilotriacetic acid; reaction mechanism; density functional theory; transition states
氮川三乙酸又叫氨三乙酸,分子式为N(CH2COOH)3,简称NTA,具有很强的生物可分解 性[1-3],其作为氨羧络合剂广泛应用于洗涤、阻垢和除垢、无氰电镀、聚氨酯泡沫发泡催化等精细化工领 域[4-13],但人们对其在有机合成中的应用研究较少。本文作者试图在溶液态下对NTA的3个羧基基团进行酯化和酰胺化反应。NTA在水和常规中性或酸性有机溶剂中的溶解性能非常差,在碱性溶剂(如乙二胺)中虽然可以解决溶解度问题,但由于NTA与碱性溶剂形成稳定的酸根离子,无法得到目标产物。采用有机固相合成的方法虽然从根本上避开了由溶解度较差带来的系列问题,但NTA在受热过程中存在脱水现象,脱水后的中间体阻止反应进一步进行,而且对反应的微观理论研究未见相关报道。在此,本文作者拟用量子化学密度泛函理论(DFT)对该反应机理进行理论计算,讨论反应过程中的过渡态、反应可行性以及反应途径和机理,以便深入了解该反应的微观过程和反应特征。
1 计算方法
采用量子化学密度泛函理论(DFT)在B3LYP/6-311++G(d,p)计算水平[14-15]上对反应物、过渡态和产物的结构进行优化,得到氨三乙酸分子的稳定构型,在同一水平上计算反应过程中所有驻点的频率,得过渡态有唯一虚频,并用内禀反应坐标(IRC)对过渡态进行进一步确认。在同一理论水平上计算其能量,并对零点能进行校正,得到正、逆反应的活化能,采用经典过渡态理论计算反应速率常数。所有的量子化学计算都通过Gaussian03程序[16]完成。
2 结果与讨论
2.1 反应机理分析
反应物、中间体和过渡态的结构示意图如图1所示。其中:IM1是优化得到的氨三乙酸的稳定分子构型,但它不能直接脱水生成氨三乙酸酐,需经由 C(6)—N(1)—C(10)—C(11)旋转得到IM2,再由 N(1)—C(10)—C(11)—C(19)旋转从IM2得到IM3,由IM3可以得到过渡态(TS)。过渡态的振动模式为:C(7),O(19)在成环方向上振动;H(20)和O(18)分别向远离O(19)和C(7)方向振动。因而,本文拟研究的反应微观途径为NTA→TS→P+H2O。IM3和过渡态的结构优化参数见表1和表2。
图1中,从IM1到IM2再到IM3,原子间的键长几乎没有变化,但其中二面角的变化比较大;从IM1到IM2,C(6)—N(1)—C(10)—C(11)二面角由162.024 5?变为59.593 3?,从IM2到IM3,N(1)—C(10)—C(11)—C(19)二面角由-167.870 1?变为-7.629 3?。因而,在此过程中氨三乙酸分子中C(6)—N(1)—C(10)—C(11)—C(19)发生旋转,2个羧基有相互靠近的趋势,由于氧原子的强电负性,IM3中,O(18)和H(20)形成分子内氢键。

图1 反应物、中间体和过渡态的结构示意图
Fig.1 Schematic map of reactant, intermediates and transition state
表1 IM3和TS的键长
Table 1 Bond length of IM3 and transition state 键长/nm

表2 IM3和TS的键角
Table 2 Bond angle of IM3 and transition state 键角/(?)
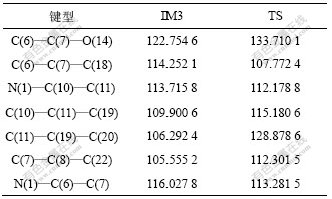
IM3最后经过过渡态生成氨三乙酸酐和水,从表1和表2所示构型参数可以看出:C(7)—O(18)的键长从0.135 0 nm增大到0.165 3 nm。从自然键轨道(NBO)分析可知:随着反应的进行,O(14)上的电子自旋密度逐渐增大(由-0.599变化至-0.471),而C(7)上的电子自旋密度逐渐减小(由0.797变化至0.774)。由反应的键长和键级可知:O(18)和C(7)断裂后,部分单电子逐渐由C(7)转移到O(14)上,C(7)—O(14)的键长则由0.121 1 nm缩短到0.114 0 nm,因而,O(14)上的电子自旋密度增大,C(7)上的电子自旋密度减小。从图1可以看出:C(7)和O(14)形成三键结构。另外,O(19)—H(20)键长从0.097 2 nm增大到0.191 8 nm,而O(18)和H(20)在IM3已经形成分子内氢键,随着O(18)与C(7)断裂,而夺得H(20),O(18)—H(20)的键长进一步减小,直至接近形成过渡态时,键长为0.095 8 nm,而正常的水分子氢氧键键长为0.097 4 nm,两者十分接近。同时,随着H(20)与O(19)的逐渐分离,O(19)上的自由电荷密度从-0.691减小到-0.775,O(19)和C(11)形成双键结构,O(19)上的部分单电子逐渐转移到C(11)上,因而,C(11)上的自由电荷密度从0.784增大到0.805。
由以上分析可知:从反应物到过渡态经过了 N(1)—C(10)—C(11)—C(19)—C(6)旋转;O(18)逐渐与C(7)断裂,同与O(19)断裂的H(20)结合生成水分子;而C(7)和O(19)也逐渐靠近,它们的距离为0.199 8 nm,产物中C(7)和O(19)的键长为0.139 0 nm,说明它们有成键的趋势,这与过渡态的振动模式一致。由此可以看出:在过渡态中存在以C(7),O(18),H(20)和O(19)为骨架的四元环和以N(1),C(10),C(11),O(19),C(7)和C(6)为骨架的六元环。
2.2 能量分析
反应物、过渡态和产物的分子能量以及零点能如表3所示。其中:E0为分子的能量;EZP为零点能;E0+EZP为进行零点能校正后的分子总能量。由表3可知:产物的能量低于反应物的能量。因此,该反应为吸热反应,与热重差热实验结果一致。
表3 反应物、过渡态和产物的能量
Table 3 Energies of reactant, transition states and products

设Er,ETS和Ep分别为反应物、过渡态和产物的能量。根据正、逆反应活化能定义,经零点能较正,可以得到正反应活化能为:Ef=ETS-Er=163 kJ/mol,逆反应活化能为: Er=ETS-EP=94 kJ/mol。在优化出的过渡态结构和频率计算基础上,对过渡态结构进行内禀反应坐标(IRC)反应路径解析,得到反应过程中能量以及过渡态构型的变化,能量曲线如图2所示。
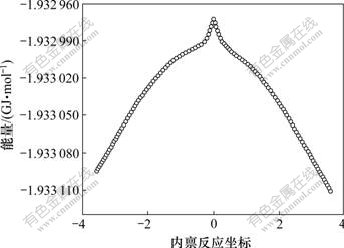
图2 过渡态的内禀反应坐标与能量的关系
Fig.2 Relationship between IRC of transition state and energy
2.3 反应速率常数计算
根据过渡态理论,计算该反应的速率常数公式为:
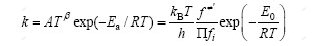
其中:A为指前因子;β为温度指数(一般情况下为 0);Ea为活化能;kB为玻尔兹曼常数;h为普朗特常数;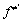 为过渡态的配分函数;
为过渡态的配分函数;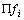 为所有反应物配分函数的乘积;T为热力学温度(298 K);R为普适气体 常数。
为所有反应物配分函数的乘积;T为热力学温度(298 K);R为普适气体 常数。
由计算所得该反应的活化能,利用传统过渡态理论,得到反应
N(CH2COOH)3→CH2COOHN(CH2CO)2O+H2O
的反应速率常数k=5.825×10-16 s-1 (T=298 K时)。
3 结论
(1) 得到氨三乙酸脱水生成酸酐的微观途径为NTA→TS→P+H2O;最稳定的氨三乙酸结构(IM1)不能直接转化为酸酐,需经过C(6)—N(1)—C(10)—C(11)—C(19)旋转得到IM3,才有利于酸酐的生成。
(2) 氨三乙酸的脱水过程是吸热反应,其所需的活化能为163 kJ/mol,在由氨三乙酸制备酸酐的过程中,在脱水剂存在的条件下,加热反应有利于酸酐的生成。
参考文献:
[1] Nancharaiah Y V, Schwarzenbeck N, Mohan T V K, et al. Biodegradation of nitrilotriacetic acid (NTA) and ferric–NTA complex by aerobic microbial granules[J]. Water Research, 2006, 40(8): 1539-1546.
[2] HU Qing-hao, LI Xiu-fen, DU Guo-cheng, et al. Effect of nitrilotriacetic acid on bioavailability of nickel during methane fermentation[J]. Chemical Engineering Journal, 2008, 143(1/3): 111-116.
[3] HU Qing-hao, LI Xiu-fen, DU Guo-cheng, et al. Effect of nitrilotriacetic acid on batch methane fermentation of sulfate-containing wastewater[J]. Process Biochemistry, 2008, 43(5): 553-558.
[4] 王蕾, 陈克宁, 郭翔. 氨三乙酸钠无磷催化剂在BTCA防皱整理中的应用[J]. 天津工业大学学报, 2003, 22(1): 29-32.
WANG Lei, CHEN Ke-ning, GUO Xiang. Application of sodium ammonium triacetate as a non2phosphorus catalyst in crease-resist finishing of cotton with BTCA[J]. Journal of Tianjin Polytechnic University, 2003, 22(1): 29-32.
[5] 任吉民, 王文韵, 景凤英. 氨三乙酸稀土络合物配体交换反应的NMR研究[J]. 物理化学学报, 1994, 10(9): 785-789.
REN Ji-min, WANG Wen-yun, JING Feng-ying. Ligand exchange reactions of lanthanide complexes with nitrilotriacetate Ln(NTA)2 by 13C and 1H NMR[J]. Acta Physico-Chimica Sinica, 1994, 10(9): 785-789.
[6] 杨德俊. 络合滴定的理论和应用[M]. 北京: 国防工业出版社, 1966: 20.
YANG De-jun. The theory and application of titration of complex compound[M]. Beijing: National Defence Industry Press, 1966: 20.
[7] Khallaf H, O.Oladeji I, Chow L. Optimization of chemical bath deposited CdS thin films using nitrilotriacetic acid as a complexing agent[J]. Thin Solid Films, 2008, 516(18): 5967-5973.
[8] 王君, 张向东, 马睿, 等. 九配位钇Ⅲ氨三乙酸配合物K3[Y(NTA)2(H2O)]·6H2O的合成及分子结构[J]. 无机化学学报, 2001, 17(1): 119-123.
WANG Jun, ZHANG Xiang-dong, MA Rui, et al. Synthesis and molecular structure of nine-coordinate complex K3[Y(NTA)2(H2O)]·6H2O[J]. Chinese Journal of Inorganic Chemistry, 2001, 17(1): 119-123.
[9] Elhabiri M, Carr?r C, Marmolle F, et al. Complexation of iron(Ⅲ) by catecholate-type polyphenols[J]. Inorganica Chimica Acta, 2007, 360(1): 353-359.
[10] Gharib F, Zare K, Mohammadi B. Solvent effects on complexation of molybdenum(Ⅵ) with nitrilotriacetic acid in different aqueous solutions of methanol[J]. Journal of Molecular Liquids, 2006, 124(1/3): 63-67.
[11] Krishnan K A. Adsorption of nitrilotriacetic acid onto activated carbon prepared by steam pyrolysis of sawdust: Kinetic and isotherm studies[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008, 317(1/3): 344-351
[12] Demmink J F, Mehra A, Beenackers A A C M. Absorption of hydrogen sulfide into aqueous solutions of ferric nitrilotriacetic acid:local auto-catalytic effects[J]. Chemical Engineering Science, 2002, 57(10): 1723-1734.
[13] Starynowicz P. Europium(Ⅱ)complexes with nitrilotriacetic acid(NTA)[J]. Polyhedron, 2003, 22(20): 2761-2765.
[14] Becke A D. Density-functional thermochemistry. Ⅲ. The role of exact exchange[J]. Chemical Physics, 1993, 98(7): 5648-5252.
[15] 李来才, 查东, 田安民. 丁二酸脱水制备丁二酸酐的微观反应机理研究[J]. 化学学报, 2005, 63(16): 1545-1550.
LI Lai-cai, ZHA Dong, TIAN An-min. Theoretical study on the microcosmic mechanism of succinic acid dehydration to produce succinic anhydride[J]. Acta Chimica Sinica, 2005, 63(16): 1545-1550.
[16] Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 03 Revision B09[R]. Pittsburgh PA: Gaussian Inc, 2003.
收稿日期:2008-10-22;修回日期:2009-03-18
基金项目:国家自然科学基金资助项目(20676152)
通信作者:唐新村(1972-),男,湖南郴州人,博士,副教授,从事电化学、固相反应和材料化学研究;电话:0731-85117774;E-mail: tangxincun@163.com
(编辑 刘华森)