文章编号:1004-0609(2014)02-0535-07
银在氢氧化钠溶液中的电化学行为
万 晔1, 2, 3,王秀梅1,张 扬1,刘思宇2,孙 红2
(1. 沈阳建筑大学 材料科学与工程学院,沈阳 110168;
2. 沈阳建筑大学 交通与机械学院,沈阳 110168;
3. 中国科学院 金属研究所 金属腐蚀与防护国家重点实验室,沈阳 110016)
摘 要:通过设计三电极电化学系统,采用循环伏安、动电位阳极极化以及恒电位阳极极化方法研究了银在不同氢氧化钠溶液的电化学行为。结果表明:银在NaOH溶液中,循环伏安和动电位阳极极化正向扫描中出现Ag2O和AgO的氧化峰。随着NaOH浓度的增加,在相同电极电位条件下,阳极电流密度逐渐增加,极化后银的腐蚀增质也随之增加;极化后样品表面的腐蚀产物聚集更为严重,出现较多的孔洞和微裂纹,从而为电解质溶液和基体之间的物质传输提供了更多通道,加速了银的电化学腐蚀进程。
关键词:银;氢氧化钠溶液;循环伏安;动电位阳极极化;恒电位阳极极化
中图分类号:O646 文献标志码:A
Electrochemical behavior of silver in NaOH solution
WAN Ye1, 2, 3, WANG Xiu-mei1, ZHANG Yang1, LIU Si-yu2, SUN Hong2
(1. School of Materials Science and Engineering, Shenyang Jianzhu University, Shenyang 110168, China;
2. School of Traffic and Mechanical Engineering, Shenyang Jianzhu University, Shenyang 110168, China;
3. State Key Laboratory for Corrosion and Protection, Institute of Metal Research,
The Chinese Academy of Sciences, Shenyang 110016, China)
Abstract: The electrochemical behaviors of silver in NaOH solution were investigated via cyclic voltammetry, anodic polarization, mass gain and scanning electron microscope. The results reveal that the current density peak of Ag2O forms and follows with the formation of AgO during the anodic polarization. Anodic current density of silver decreases sharply to a stable value, and silver oxide with the same size are formed on silver’s surface under potentiostatic polarization in the three NaOH solutions. The higher the NaOH concentration is, the denser the silver oxides distribute on the surface of silver. At the same potential for the potentiodynamic polarization curves of silver, the anodic current density of silver increases with NaOH concentration increasing. Mass gain of the potentiodynamic anodic polarized silver increases with NaOH concentration as well. The morphologies of the potentiodynamic anodic polarized silver shows that the corrosion products accumulate more severe and there are more micro-cracks on the surface of silver polarized with higher NaOH concentration than with lower NaOH concentration. The micro-cracks provide channels for ion transport, which is essential for further corrosion of silver in NaOH solution, through the corrosion products.
Key words: silver; NaOH solution; cyclic voltammetry; potentiodynamic polarization; potentiostatic polarization
由于具有较低的剪切强度和优良的导热、导电、延展性,银被广泛地应用在交通、电子、通讯、能源、生物卫生、航空航天、核工业等领域[1-2]。然而银在使用过程中容易发生腐蚀,甚至失效,因此银的极化行为是关系到该领域的结构和功能可靠性的关键问题[3],并使银的腐蚀机制研究成为是一个重要的研究方向[4]。碱性环境对银的电化学腐蚀行为有着重要的影响[5-9],在碱性溶液中,银发生电化学腐蚀后,其表面的氧化物的结构和状态发生变化,并直接影响其使用性能。MUELLER等[10]通过研究发现银在人造唾液中的电化学腐蚀规律。 研究组[9]揭示了银 在碱性溶液中经过多次循环伏安扫描后,由于其表面积的变化导致其充放电能力的变化。LEE等[11]研究了不同晶体结构银的电化学腐蚀特点,发现在Na2S溶液中(200)晶面比(111)晶面更容易发生腐蚀。我国在银的腐蚀方面的研究较少,仅有的一些研究主要集中在污染气体腐蚀或硫化腐蚀等方面[12-13],目前还没有发现有关于其电化学腐蚀的研究。纵观这些国内外关于银腐蚀的研究结果,虽然提出了银在不同条件下的腐蚀规律,但是缺乏银在NaOH溶液中的阳极腐蚀规律和机制的详细研究。
研究组[9]揭示了银 在碱性溶液中经过多次循环伏安扫描后,由于其表面积的变化导致其充放电能力的变化。LEE等[11]研究了不同晶体结构银的电化学腐蚀特点,发现在Na2S溶液中(200)晶面比(111)晶面更容易发生腐蚀。我国在银的腐蚀方面的研究较少,仅有的一些研究主要集中在污染气体腐蚀或硫化腐蚀等方面[12-13],目前还没有发现有关于其电化学腐蚀的研究。纵观这些国内外关于银腐蚀的研究结果,虽然提出了银在不同条件下的腐蚀规律,但是缺乏银在NaOH溶液中的阳极腐蚀规律和机制的详细研究。
本文作者利用电化学工作站对银进行电化学测试,对阳极极化后银样品的质量变化进行分析,并结合扫描电镜结果系统地研究了银在NaOH溶液中的电化学腐蚀行为和机制,为银在碱性环境中的可靠应用提供参考。
1 实验
1.1 样品
试验选用纯度为99.99%的银片,样品尺寸为10 mm×10 mm×1.5 mm。样品靠近顶端钻一个小孔,用于连接细导线。将样品采用800号砂纸打磨,随后用去离子水和无水乙醇清洗,干燥后备用。
1.2 电化学试验
电化学仪器采用美国AMETEK公司的PARSTAT 2273电化学工作站进行测试。电化学测试软件为PowerSuite 。电解池为三电极系统,参比电极为饱和甘汞电极(SCE,如无特殊说明,本试验中所有电位均为相对于饱和甘汞电极而言的电位),工作电极为银片(暴露于电解液的工作电极面积为1 cm2),辅助电极为铂网。电化学试验所用的电解质为不同浓度的NaOH溶液,每次试验所用的溶液体积均为0.25 L。试验开始时首先将工作电极在-1.2 V(vs SCE,下同)电位下极化1 min以去除表面可能存在的氧化膜。采用15 mV/s的扫描速度进行动电位极化曲线测试。恒电位极化试验是在电位为0.28 V条件下进行的。电化学试验完成后,取出银样品,立即用去离子水冲洗干净,用冷风吹干,进行形貌测试。所有电化学试验均在25 ℃条件下进行,每个电化学试验均取3个平行样品进行重复试验。
。电解池为三电极系统,参比电极为饱和甘汞电极(SCE,如无特殊说明,本试验中所有电位均为相对于饱和甘汞电极而言的电位),工作电极为银片(暴露于电解液的工作电极面积为1 cm2),辅助电极为铂网。电化学试验所用的电解质为不同浓度的NaOH溶液,每次试验所用的溶液体积均为0.25 L。试验开始时首先将工作电极在-1.2 V(vs SCE,下同)电位下极化1 min以去除表面可能存在的氧化膜。采用15 mV/s的扫描速度进行动电位极化曲线测试。恒电位极化试验是在电位为0.28 V条件下进行的。电化学试验完成后,取出银样品,立即用去离子水冲洗干净,用冷风吹干,进行形貌测试。所有电化学试验均在25 ℃条件下进行,每个电化学试验均取3个平行样品进行重复试验。
1.3 腐蚀增质
在动电位扫描之前对银片称量,记录其质量,并在扫描结束后马上用蒸馏水冲洗银片,氮气吹干后称量,用于比较电化学极化实验前后质量的变化。采用瑞士Mettler-Toledo型号的天平称量,每组试验均选取3个平行样品进行试验。
1.4 形貌观察
采用日本JEOL公司JSM-6700型号的扫描电镜(SEM)对部分电化学实验结束后的样品表面形貌进行观察。
2 结果与讨论
2.1 循环伏安曲线
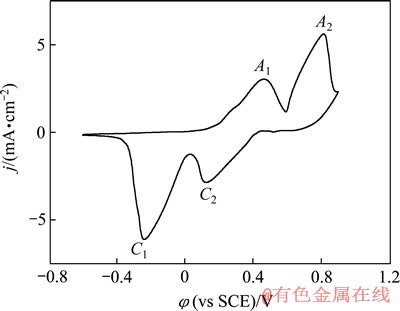
图1 银在0.1 mol/L NaOH溶液中的循环伏安曲线
Fig. 1 Cyclic voltammetric curve of silver in 0.1 mol/L NaOH solution
为考察银在碱性溶液中的电化学行为,对抛光后的银样品在0.1 mol/L NaOH溶液中进行循环伏安扫描,实验结果如图1所示。由图1可以明显看到,大约在0.20 V处产生了一个缓慢上升的阳极电流密度,在0.462 V处有一个阳极氧化峰A1。一般认为该峰对应单质银氧化为一价银的过程[6-9]。在0.816 V处出现第二个阳极氧化峰A2,该峰对应于二价银的出现。一般认为,第一个阳极氧化峰形成大约3个原子层厚度的Ag2O层[9],但是很快就增加到第二个阳极氧化峰,该峰对应于AgO的形成[6-9, 14]。
在循环伏安的回扫过程中,阴极还原峰C2代表二价银还原为一价的银,阴极还原峰C1代表一价的银还原为单质银[6-9]。银在该循环伏安过程中氧化和还原 对应的电化学反应如下列式(1)~(4)所示:
A1: 2Ag+2OH--2e→Ag2O+H2O (1)
A2: Ag2O+2OH--2e→AgO+H2O (2)
C2: 2AgO+H2O+2e→Ag2O+2OH- (3)
C1: Ag2O+H2O+2e→2Ag+2OH- (4)
2.2 动电位阳极极化
为考察银在NaOH溶液中的阳极极化特征,对处理后的银片在不同浓度的NaOH溶液中进行动电位阳极极化扫描,如图2所示。由图2可以看出,随着NaOH浓度的增加,银的阳极极化发生了显著的变化。如图2中红色曲线为银在1 mol/L NaOH溶液中的极化曲线;由该曲线可以看出,当阳极电位低于0.391 V(银在1 mol/L NaOH溶液中阳极极化过程中第一个阳极峰对应的电极电位)时,为活性溶解区。在此区间银进行正常的阳极溶解,其溶解速度受活化极化控制。从图2还可以看出,随着极化过程的进行,阳极电位逐渐增加,极化曲线出现两个极化电流密度峰。以0.1 mol/L氢氧化钠溶液为例,两个阳极氧化峰值分别位于A点(0.524 V)和B点(0.740 V)。对比图1中的循环伏安曲线可知,第一个阳极氧化峰A点所发生的电化学反应对应于单质银向一价银的氧化物的转换,如式(1)所示。第二个阳极氧化峰B点对应于电极上发生由一价银的氧化物向二价银的氧化物转化的电化学反应,如式(2)所示。
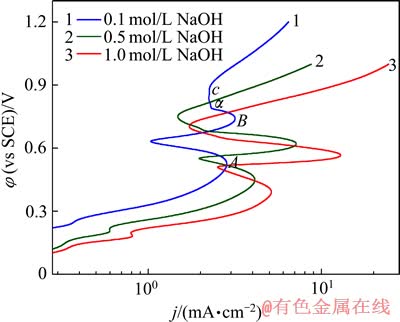
图2 银在不同浓度NaOH溶液中的动电位阳极极化曲线
Fig.2 Potentiodynamic polarization curves of silver in solutions with various NaOH concentrations
值得注意的是,当银在0.1 mol/L NaOH溶液中进行阳极极化时,当曲线上的第二个阳极氧化峰消失时,出现了一段非典型的钝化区α,在此区间,阳极电位持续增加,而阳极电流密度保持在较为稳定的范围,即达到了极限扩散电流DA,要使电流密度超过DA,必须施加较大的不可逆电位。
分析图2中不同NaOH溶液条件下银的阳极极化曲线,并将其阳极氧化峰所对应的阳极电流密度(jA、jB)和阳极电位(φA、φB)作于图3中。从图3可以看出,银在0.5 mol/L NaOH溶液中的电极电位比在1 mol/L NaOH溶液中的电极电位要高,而且在0.1 mol/L NaOH溶液中的电极电位远大于1 mol/L NaOH溶液中的电极电位。
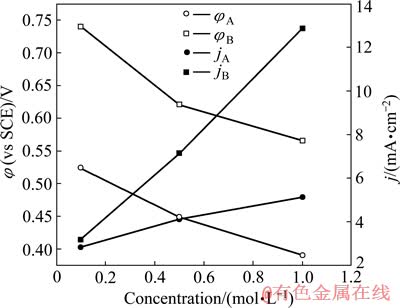
图3 在图2的阳极极化曲线中阳极氧化峰对应的电流密度和电极电位
Fig. 3 Current density and potential of current density peak vs NaOH concentration in Fig. 2
由图2中可以看出,在低于0.391 V的相同电极电位条件下,随着NaOH浓度的增加,银电极上的阳极电流密度逐渐增加,此阶段的阳极反应均受活化极化所控制。在高于0.888 V(图2中曲线1中c点对应的电位值,即银在0.1 mol/L NaOH溶液中阳极极化过钝化区结束时的电极电位)随着NaOH浓度的增加,阳极电流密度逐渐增加。银在1 mol/L NaOH溶液中的阳极电流密度比在0.5 mol/L NaOH溶液中的阳极电流密度大,银在0.1 mol/L NaOH溶液中的阳极电流密度不仅低于0.5 mol/L NaOH溶液中的阳极电流密度,而且远远低于1 mol/L NaOH溶液中的阳极电流密度。由此可见,银在低浓度NaOH溶液中的稳定性比在高浓度NaOH溶液中的稳定性强。当阳极电位达到电解液的分解电压以上时,阳极开始发生如式(5)所示的电化学反应,并析出氧气。
4OH--4e→2H2O+O2 (5)
2.3 质量变化
对在3种不同浓度NaOH溶液中阳极极化前后的银样品(极化暴露的工作面积均为1 cm2)进行质量分析,结果如图4所示。由图4可以看出,随着NaOH浓度的增加,银片的质量增量随之逐渐增加。说明电解液中NaOH浓度越大,银片极化后氧化膜的厚度也越大,银的腐蚀就越严重。从1 mol/L NaOH溶液到0.5 mol/L NaOH溶液,银片的质量增量大于从0.5 mol/L NaOH溶液到0.1 mol/L NaOH溶液中银的质量增量。这一结果与图2和图3中银的第二个阳极氧化峰的电流密度随NaOH浓度的增加而增大的趋势较为吻合。
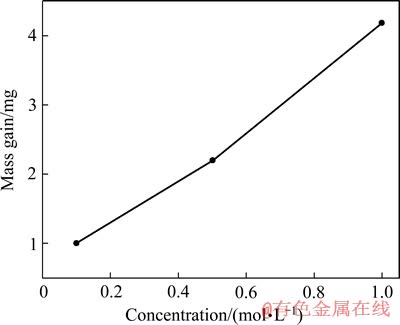
图4 经阳极极化后的银样品质量随NaOH浓度的变化
Fig. 4 Mass gain of silver in solution with various NaOH concentrations
2.4 恒电位阳极极化
银进行恒电位(φ=0.28 V)阳极极化时,电流密度随时间的变化曲线如图5所示。由图5可以看出,在银开始极化时,电流值较大,但随着极化时间的延长,电流值急剧下降,并且很快(大约30 s)达到大约0.05 mA/cm2的一个相对稳定的电流值;另外,虽然NaOH浓度各不相同,但是随着极化时间的延长,最终的稳定电流基本相同。NaOH浓度越高,电流值下降就越快,极化电流达到平衡值所需时间就越长。
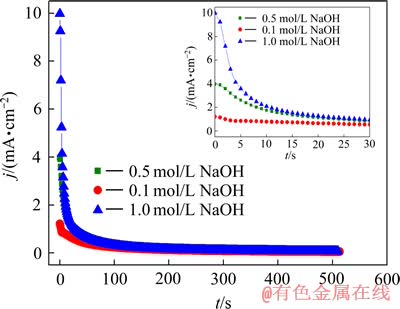
图5 银在不同浓度NaOH溶液中恒电位阳极极化的电流密度随时间的变化
Fig. 5 Variation of polarization current with time during anodic oxidization of silver under potentiostatic control in various NaOH solution (φ=0.28 V)
2.5 极化后的表面形貌
经过动电位极化扫描后,银片表面均由原来的亮白色变为深褐色。将极化后的银片在扫描电子显微镜下进行观察,得到如图6所示的显微组织。由图6可知,经阳极极化后的银片表面腐蚀产物呈现粒状。随着NaOH浓度的不同,颗粒直径尺寸出现明显的差异。在0.1 mol/L NaOH溶液中,银片表面腐蚀产物颗粒形状比较规整,尺寸较为均匀,粒径大约为300 nm左右。在0.5 mol/L NaOH溶液中,银片表面腐蚀产物颗粒尺寸不均,并且出现团聚现象。在1 mol/L NaOH溶液中,银片表面腐蚀产物颗粒的团聚现象十分明显,腐蚀产物层出现较多的孔洞和微裂纹。
在恒电位条件下对银片在不同浓度NaOH溶液中进行极化后,银片表面的形貌如图7所示。在3种不同浓度NaOH溶液中,银片表面的氧化物粒径大小比较一致,粒径约为150 nm。但是也要看到,银的氧化物颗粒分布与溶液中NaOH浓度和银片表面状态有关,溶液中NaOH浓度越大,银片表面的氧化物分布就越致密,尤其是在1 mol/L NaOH溶液中,氧化物几乎布满银片表面。另外,在0.5 mol/L NaOH溶液中恒电位极化后的银片上,选取研磨条纹区进行形貌观察,结果见图7(b)。可以看到,沿着样品研磨条纹区域,银片的腐蚀更为严重,银的氧化物积聚在一起,并有较多粒径约越为10 nm的氧化物颗粒出现。说明银片表面越粗糙,银的腐蚀现象就越严重。
回顾图3的结果可以发现,随着NaOH浓度的增高,银的两个阳极氧化峰的电极电位减缓趋势相同。
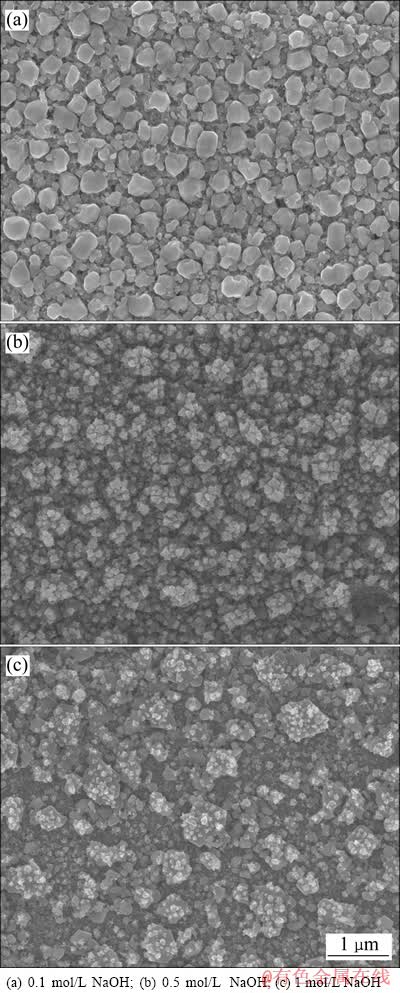
图6 银在不同浓度NaOH溶液中动电位极化后表面的SEM像
Fig. 6 SEM images of potentiodynamic polarized silver in different NaOH solution
在相同的电极电位下,随着NaOH浓度的增加,银电极上的电流密度逐渐增加,而且银的第二个阳极氧化峰的电流密度的增加程度远大于第一个阳极氧化峰电流密度的增加程度。
由前面的式(1)和(2)可知,银在NaOH溶液中动电位阳极极化的两个阳极氧化峰分别形成对应的氧化物Ag2O和AgO。在给定的NaOH溶液中,在浓度误差范围之内,溶液的pH值基本为定值。根据能斯特方程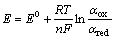 ,可以推断影响体系Ag2O和AgO的半电池电位的主要因素为电解液中两种氧化物的活度,也即银在阳极极化过程中这两种氧化物在不同浓度NaOH溶液中的浓度。由于该氧化物在水溶液中的溶解度很微小,所以
,可以推断影响体系Ag2O和AgO的半电池电位的主要因素为电解液中两种氧化物的活度,也即银在阳极极化过程中这两种氧化物在不同浓度NaOH溶液中的浓度。由于该氧化物在水溶液中的溶解度很微小,所以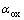 和
和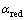 可以认为是1。由此在图2和3中,银在NaOH溶液中的阳极电位主要由体系中溶液的pH值决定,因此在不同浓度NaOH溶液中银的阳极电位减缓趋势相同。
可以认为是1。由此在图2和3中,银在NaOH溶液中的阳极电位主要由体系中溶液的pH值决定,因此在不同浓度NaOH溶液中银的阳极电位减缓趋势相同。

图7 银在不同浓度NaOH溶液中恒电位极化510 s后表面的SEM像
Fig. 7 SEM images of anodic polarized silver under potentiostatic control in various NaOH solution
对于体系的电流密度来说,影响的因素较为复杂。电流密度对阳极和阴极上的电极电位的微小变化都极为敏感。一方面,银的极化过程中阳极电位的微小变化对阳极电流密度产生影响。另一方面,银在NaOH溶液中发生电化学极化时,对应阴极上的电池反应为O2+2H2O+4e→4OH-。根据能斯特方程可知,随着电解液pH值的变化,阴极电极电位变化较大,从而严重影响体系的电流密度。
在不同浓度NaOH条件下,从银的电化学腐蚀后的形貌特征可以估计第二个阳极峰的阳极电流密度大小。在0.1 mol/L NaOH溶液中的动电位极化过程中,由于银的氧化物的成核速率较小,成为控制银的电化学腐蚀的速控步,因此,晶粒有足够的时间在晶核上均匀生长,形成了一层较均匀、致密的腐蚀产物层。该腐蚀产物层能够在一定程度上阻止电解液中的离子向基体的传输,这也是在图2中曲线1出现钝化区域的原因。在1 mol/L NaOH溶液中的动电位阳极极化过程中,由于体系中含有较多的OH-,造成银的氧化物的成核速率大于晶粒的生长速率,颗粒来不及长大就被新形成的晶粒包裹,产生团聚现象。这一现象在图5中直观地得到了体现。因此,相对于在较低浓度NaOH溶液中的电化学腐蚀行为,银在1 mol/L NaOH溶液中阳极极化后,银片表面腐蚀产物颗粒较小,形状不均匀,存在更多的孔洞和微裂纹。一方面,这些孔洞和微裂纹为电解液和基体之间的物质传输提供了更多通道,为进一步的腐蚀提供了前提条件[15-16];另一方面,由此增加的表面积使其表面的功函上升[17];再者,随着NaOH溶液中离子浓度的增加,溶液的导电性增强,所以银在较高浓度NaOH溶液中的腐蚀程度增加[18],导致在1 mol/L NaOH中银片表面更容易发生电化学腐蚀,这也是银在较高浓度NaOH溶液中电化学腐蚀后的质量增量大于在较低浓度NaOH溶液中的质量增量的原因。
在银的第二个阳极氧化峰形成的过程中,阳极上除了有式(2)所示的电极反应,还有腐蚀后的孔洞和微裂纹中新暴露出来的单质银氧化为Ag2O的电极反应,如式(1)所示,从而使该极化过程中的阳极电流密度远大于第一个阳极氧化峰形成时的电流密度。
在银的恒电位极化过程中,在对阳极加载恒定电压的瞬间,通过的阳极电流使得银片表面生成氧化物,但是由于氧化层对电流的阻挡作用,电流迅速下降,并达到一个基本稳定的值,这时氧化膜层下的银基体的腐蚀受到抑制,银的氧化物生长达到一个稳态的状态。在最初的腐蚀过程中形成Ag2O,随着腐蚀过程的持续进行,Ag2O逐渐转化为二价的AgO,在稳定电流阶段,阳极上一直在进行着氧化物不断生成和溶解的动态平衡过程。这一现象与不同浓度NaOH条件下银阳极氧化物的形成机理及银片表面氧化物的状态密切相关。在3种不同浓度NaOH溶液中,银片表面的氧化物粒径大小比较一致,均匀地分布在银基体的表面,粒径约为150 nm。这也是在3种不同浓度NaOH溶液中银的恒电位阳极极化后的稳定电流值达到基本一致的原因。
3 结论
1) 银经过循环伏安扫描后,在正扫的阳极极化过程中形成了两个阳极氧化峰,分别对应于Ag2O和AgO的形成过程。反向扫描时,依次出现了AgO和Ag2O的阴极还原峰。
2) 银的动电位阳极极化曲线形成两个阳极氧化峰,第二个阳极氧化峰的电流密度大于第一个阳极氧化峰的电流密度,而且溶液中NaOH浓度对银的电化学腐蚀产生明显作用:在动电位阳极极化曲线的活化极化区,在相同电极电位条件下,随着NaOH浓度的增加,银片上的阳极电流密度逐渐增加,动电位极化后银片的质量增量也随之增加。
3) 银经过动电位阳极极化后,银片表面形成的腐蚀产物颗粒较大,并且比较均匀;而在较高浓度NaOH溶液中的腐蚀产物颗粒较小,形状不均匀,团聚现象较严重,存在更多的孔洞和微裂纹,为银的进一步电化学腐蚀提供了更多的通道,从而使电化学腐蚀更为严重。
4) 银在3种不同浓度NaOH溶液中进行恒电位阳极极化后,阳极电流密度很快达到一个相对稳定的值,并在银片表面形成粒径约150 nm、均匀分布的氧化物颗粒。NaOH浓度越高,银片表面的氧化物分布越致密。银片表面越粗糙,银的极化现象越明显。
REFERENCES
[1] EL-SHERBINYM, SALEM F. Tribological properties of PVD silver films[J]. ASLE Transactions, 1986, 29(2): 223-228.
[2] ROY R, SEN S K. The study of diffusion of copper in thin films of silver and Ag-Al alloys as a function of increasing aluminum concentration[J]. Thin Solid Films, 1993, 223: 189-195.
[3] ZHAO B, LIU Z R, LIUZ L, LIU G X, LI Z, WANG J X, DONG X T. Silver microspheres for application as hydrogen peroxide sensor[J]. Electrochemistry Communications, 2009, 11(8): 1707-1710.
[4] GUERRA M F, TISSOT I. The role of nuclear microprobes in the study of technology, provenance and corrosion of cultural heritage: the case of gold and silver items[J]. Nuclear Instruments and Methods in Physics Research B: Beam Interactions with Materials and Atoms, 2013, 306: 227-231.
[5] CHEN Z Y, LIANG D, MA G, FRANKEL G S, ALLEN H C, KELLY R G. Influence of UV irradiation and ozone on atmospheric corrosion of bare silver[J]. Corrosion Engineering, Science and Technology, 2010, 45: 169-180.
[6] ZHANG X, STEWART S, SHOESMITH D W, WREN J C. Interaction of aqueous iodine species with Ag2O/Ag surfaces[J]. Journal of the Electrochemical Society, 2007, 154: F70-F76.
[7] DROOG J M M, HUISMAN F. Electrochemical formation and reduction of silver oxides in alkaline media[J]. Journal of Power Sources, 1980, 115: 211-224.
[8] HUR T U, CHUNG W S. The mechanism of silver(Ⅰ) oxide to silver(Ⅱ) oxide formation on polycrystalline silver electrodes in 8 M KOH solution[J]. Journal of the Electrochemical Society, 2005, 152: A996-A1000.
[9]  . Electrochemical characteristics of binary silver alloys in alkaline solution[J]. Journal of Power Sources, 1999, 79: 1-8.
. Electrochemical characteristics of binary silver alloys in alkaline solution[J]. Journal of Power Sources, 1999, 79: 1-8.
[10] MUELLER H J, HIRTHE R W. Electrochemical characterization and immersion corrosion of a consolidated silver dental biomaterial[J]. Biomaterials, 2001, 22: 2635-2646.
[11] LEE K H, TAKAI O, LEE M H. Tribological and corrosive properties of silver thin films prepared bye-beam ion plating method[J]. Surface and Coatings Technology, 2003, 169/170: 695-698.
[12] 吴春莺, 易丹青, 周宏明, 王 斌, 李 荐. 稀土钇对银合金组织和性能的影响[J]. 贵金属, 2009, 30(2): 18-21.
WU Chun-ying, YI Dan-qing, ZHOU Hong-ming, WANG Bin, LI Jian. Effects of Y addition on the microstructure and performance of silver alloys[J]. Precious Metals, 2009, 30(2): 18-21.
[13] 张 蕾, 严川伟, 屈 庆, 孙 刚, 童靖宇, 李金洪, 曹楚南. 原子氧对金属银和有机防护涂层的侵蚀[J]. 材料研究学报, 2002, 16(3): 273-278.
ZHANG Lei, YAN Chuan-wei, QU Qing, SUN Gang, TONG Jing-yu, LI Jin-hong, CAO Chu-nan. Atomic oxygen effect on naked Ag and Ag with protective organic coating[J]. Chinese Journal of Materials Research, 2002, 16(3): 273-278.
[14] LANDER J J. Sealed zinc-silver oxide batteries[C]// Proceedings of Power Sources Division. US Army Signal Research & Development Laboratory, 1961, 15: 77.
[15] PAYER J H, BALL G, RICKETT B, KIM H S. Role of transport properties in corrosion product growth[J]. Materials Science and Engineering A, 1995, 198: 91-102.
[16] HUNG H, PAYER J. The effect of silver chloride formation on the kinetics of silver dissolution in chloride solution[J]. Electrochimica Acta, 2011, 56: 2781-2791.
[17] WAN Y, LI Y B, WANG Q, ZHANG K, WU Y H. The relationship of surface roughness and work function of pure silver by numerical modeling[J]. International Journal of Electrochemical Science, 2012, 7: 5204-5216.
[18] 周建龙, 李晓刚, 杜翠薇, 李云玲, 李 涛, 潘 莹. X80管线钢在NaHCO3溶液中的阳极电化学行为[J]. 金属学报, 2010, 46(2): 251-256.
ZHOU Jian-long, LI Xiao-gang, DU Cui-wei, LI Yun-ling, LI Tao, PAN Ying. Anodic electrochemical behavior of X80 pipeline steel in NaHCO3 solution[J]. Acta Metallurgica Sinica, 2010, 46(2): 251-256.
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51101106);国家自然科学基金重点项目(51131007);辽宁省高等学校优秀科技人才支持计划资助项目(LR2012019)
收稿日期:2013-04-28;修订日期:2013-08-19
通信作者:万 晔,副教授,博士;电话:024-24690313;E-mail: ywan@sjzu.edu.cn