KINETICS OF ALUMINUM EXTRACTION WITH DI-2-ETHYLHEXYL PHOSPHORIC ACID
来源期刊:中国有色金属学报(英文版)1992年第4期
论文作者:Ma Yun Zhu Tun
文章页码:14 - 20
Key words:aluminum ; solvent extraction; kinetics ; constant interfacial area cell; di-2-ethylhexyl phosphoric acid.
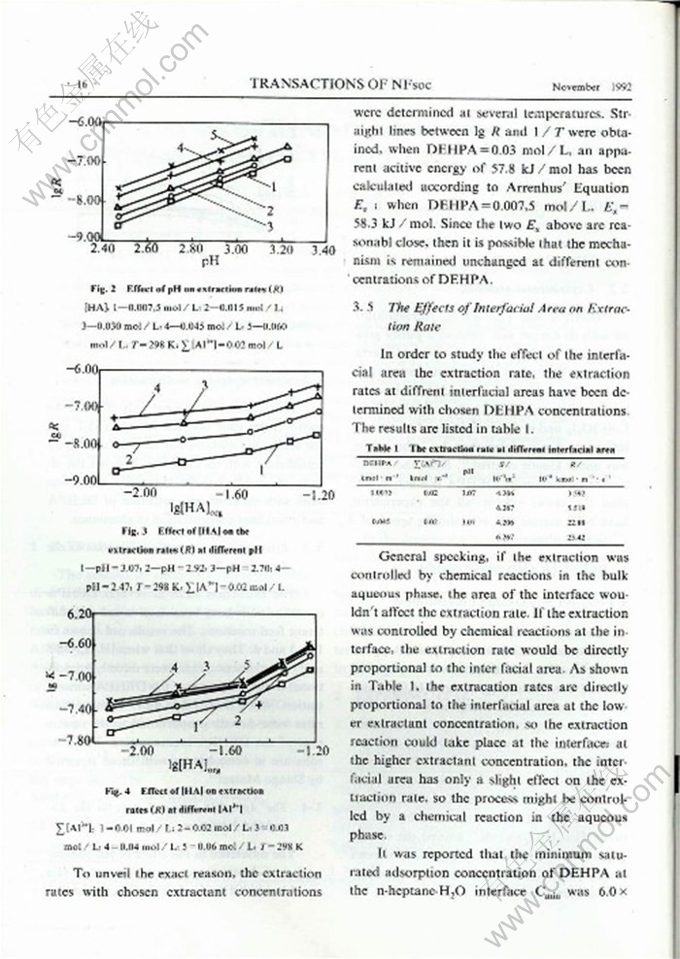
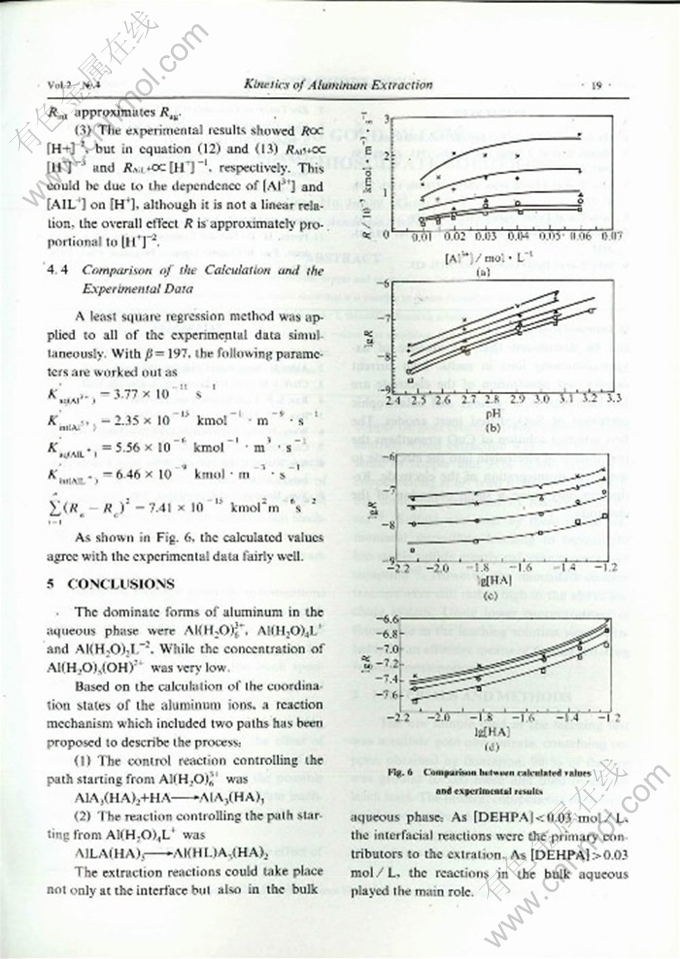
Abstract: The kinetics of solvent,extraction of aluminum with di-2-ethylhexyl phosphoric acid (DEHPA) in n-heptane have been studied in a constant interfacial area cell. A HCl-KHC8H404 (potassium biphthalate> KHL) buffer solution was used to maintain a constant pH during extraction. The effects of the concentration of aluminum, pH,the concentration of the extractant, the interfacial area and the temperature on the extraction rate were investigated. A method has been invented to determine amont of the extracted aluminum in the organic phase with 8-hydroxyquinoline. Aased on calculation of the coordination states of the aluminum ions and their contribution to the reaction rate, a raaction mechanism which includes two main reaction paths, has been proposed to describe the process. One path starts from Al(H2O)63+,and the other starts from Al(H2O)4L+. The reaction could take place both in the aqueous phase and at the interface. The main reaction region could be changed as the conditions of extraction were changed. When [HA] < 0.03 mol / L the process was controlled by the interfacial reaclion, and when [HA]>0.03 mol / L it was shifted to a homogeneous aqueous solution reaction.