Equilibrium and kinetic data of adsorption and separation for zirconium and hafnium onto MIBK extraction resin
来源期刊:中国有色金属学报(英文版)2010年第8期
论文作者:徐志高 吴延科 张建东 张力 王力军
文章页码:1527 - 1533
Key words:equilibrium; kinetics; adsorption; methyl isobutyl ketone; extraction resin; zirconium; hafnium; separation
Abstract: The equilibrium and kinetics of methyl isobutyl ketone (MIBK) extraction resin for adsorption and separation of zirconium and hafnium were studied under the different conditions of acidity, initial total concentrations of zirconium and hafnium and temperature. The equilibrium data of both zirconium and hafnium are found to follow the Freundlich adsorption isotherm, and the Freundlich isotherm constants (KF) are 3.53 and 0.64 mg/g, respectively. The equilibrium data of zirconium also fit the Langmuir adsorption isotherm, and the saturation adsorption capacity (Qmax) and the Langmuir isotherm constant (KL) are 75.93 mg/g and -0.012 7 L/g, respectively. The obtained kinetic data of both zirconium and hafnium are found to fit the HO pseudo-second-order kinetic model, and the rate constants of pseudo-second-order equation (k2) are -0.019 and 0.41 g/(mg·min), respectively. Column tests show that the MIBK extraction resin could be used as efficient adsorbent material for separating hafnium from zirconium.

XU Zhi-gao (徐志高), WU Yan-ke (吴延科), ZHANG Jian-dong (张建东),
ZHANG Li (张 力), WANG Li-jun (王力军)
Division of Mineral Resources, Metallurgy and Materials,
General Research Institute for Nonferrous Metals, Beijing 100088, China
Received 22 December 2009; accepted 28 May 2010
Abstract: The equilibrium and kinetics of methyl isobutyl ketone (MIBK) extraction resin for adsorption and separation of zirconium and hafnium were studied under the different conditions of acidity, initial total concentrations of zirconium and hafnium and temperature. The equilibrium data of both zirconium and hafnium are found to follow the Freundlich adsorption isotherm, and the Freundlich isotherm constants (KF) are 3.53 and 0.64 mg/g, respectively. The equilibrium data of zirconium also fit the Langmuir adsorption isotherm, and the saturation adsorption capacity (Qmax) and the Langmuir isotherm constant (KL) are 75.93 mg/g and -0.012 7 L/g, respectively. The obtained kinetic data of both zirconium and hafnium are found to fit the HO pseudo-second-order kinetic model, and the rate constants of pseudo-second-order equation (k2) are -0.019 and 0.41 g/(mg·min), respectively. Column tests show that the MIBK extraction resin could be used as efficient adsorbent material for separating hafnium from zirconium.
Key words: equilibrium; kinetics; adsorption; methyl isobutyl ketone; extraction resin; zirconium; hafnium; separation
1 Introduction
The great difference between zirconium and hafnium for thermal neutron capture cross-section makes them to be cladding materials and controlling materials in nuclear reactors[1]. In recent years, methods for the separation of hafnium from zirconium metals, which are normally associated together in nature and separated difficultly from each other, have become very important due to increasing demands for the production of high grade hafnium and zirconium or compounds.
Methods of fractional crystallization, fractional precipitation, ion exchange, solvent extraction, molten salt distillation and selective reduction have been used to separate hafnium from zirconium. However, just the solvent extraction and molten salt distillation can be used in industry now. Traditional extractants, such as methyl isobutyl ketone (MIBK), N235 and TBP, are adopted in solvent extraction method. MIBK, which is used to extract hafnium from the zirconium-hafnium feed mixtures with less content of hafnium, has advantages of both great treatment capacity and high efficiency[2]. Nevertheless, MIBK results in solvent loss, atmospheric pollution and poor working environment due to the disadvantages of low flash point, high vapour pressure and solubility in the aqueous phase. Although there is no serious problem of environmental pollution for method with N235, the extract efficiency of zirconium is low. The TBP has a high extraction efficiency for zirconium from the solution containing zirconium and hafnium ions, but the equipments are seriously corroded. Furthermore, the emulsified phenomenon is found in continuous production. So, it has not been applied for scale production[3-4].
The technique of solvent extraction resins where the conventional extractant is loaded onto macroporous polymer carriers combines the advantages of high selectivity to solvent extraction and high efficiency of ion exchange, and overcomes the disadvantages of serious pollution and difficulty of split phase in solvent extraction as well as high cost and difficulty of ion exchanger synthesizing. The performance of extraction resin can be estimated by solvent extraction data. In the last decade, it was well developed and had a firmly established place in the extraction, separation, and preconcentration of metals[5-8]. A few types of extraction resins were used in extraction and separation of zirconium and hafnium, except that ZHANG et al[9] introduced a method which was utilized to extract zirconium form the mixture of zirconium and hafnium by tributyl phosphate (TBP) extraction chromatography. MIBK extraction resin was thus prepared by impregnating onto macroporous adsorption resin to adsorb the hafnium from the feed solution of zirconium and hafnium (hafnium content of 1%-3%). The loss of MIBK that is highly volatile and has solubility of 19 g/L in water at 20 °C was reduced, because the MIBK extractant was fixed in micropore of macroporous adsorption resin and the MIBK extraction resin was packed in column.
The purpose of this study is to investigate the equilibrium and adsorption kinetics of MIBK extraction resin. The influence of acidity and initial metals concentration on the adsorption rate is studied. Two kinetic models of pseudo first-order and second-order equation are proposed to describe the adsorption process. The Langmuir and Freundlich equations are used to fit the equilibrium isotherms. The adsorption isotherm is measured in order to estimate the discrepancy between the experimental data and the theoretical equilibrium capacity predicted from the kinetic equations. Thermodynamic parameters are also evaluated through adsorption measurements. Besides, column test for the feasibility of separating hafnium from zirconium in the NH4SCN-HCl system is performed with the MIBK extraction resin at room temperature. These fundamental data will be valuable to further applications in separation of hafnium from zirconium.
2 Experimental
2.1 Chemicals
The methyl isobutyl ketone (MIBK) (purity: 99.9%) provided by Tianjin Jiashun Chemical Co., Ltd., China, was used without any further purification. HPD100 macroporous adsorption resin (granule diameter: 0.3-1.25 mm, average pore diameter: 8.5-9.0 nm, specific surface area: 650-700 m2/g) was provided by Cangzhou Bon Adsorber Technology Co., Ltd., Hebei Province, China. The MIBK extraction resin was self-made. Zirconium oxychloride (purity: 99.9%) was obtained from Zhejiang Shenghua Biok Biology Co., Ltd., China. Ammonium thiocyanate, hydrochloric acid, sulfuric acid, ammonium sulfate and ammonia are in analytical reagent grade. De-ionized water was used for the whole process. The feed mixtures, namely feed solution, were prepared by dissolving zirconium oxychloride and ammonium thiocyanate in appropriate amounts of de-ionized water, and the chemical composition of the feed mixtures is listed in Table 1.
Table 1 Chemical composition of feed mixtures (mol/L)

2.2 Preparation of MIBK extraction resin
MIBK extraction resin was prepared through immersion method. All polymers were extracted by ethanol for 8 h and then rinsed three times with de-ionized water before using macroporous adsorption resin. In 2 000 mL beaker, 600 g macroporous adsorption resin was swelled in 600 mL de-ionized water, then 224 mL MIBK was added. The mixture of resin, water and MIBK was stirred for 12 h to ensure that the micropore of macroporous adsorption resin was fully swelled with MIBK.
2.3 Experimental procedures
2.3.1 Bath test of zirconium and hafnium adsorption
Experiments were operated in a wide range of acidity, temperature, contact time as well as adsorption isotherms. The operation of separating hafnium from zirconium was usually carried out in batch vessels.
Batch experiments were performed under kinetic and equilibrium conditions. A feed mixture (50 mL) was taken in a glass stopper bottle after adjusting its acidity to the optimum value. MIBK extraction resin of 0.6 g was added into the bottle and the mixture was shaken for different time at room temperature. The resin was then filtered, and the total concentrations of zirconium and hafnium in filter liquor were titrated by EDTA using xylenol orange as indicator. The acidity of filter liquor was titrated by standard sodium hydroxide using phenolphthalein as the indicator and sodium citrate as masking agent. The concentration of hafnium in filter liquor was determined by ICP-AES. The corresponding adsorption concentration was calculated by difference. The procedure of kinetic tests was identical to that of the equilibrium tests.
The equilibrium adsorption capacity (Qe, mg/g) and distribution coefficient (D) were calculated with the following formulas:
![]() (1)
(1)
![]() (2)
(2)
where c0 is the initial total metal concentration of zirconium or hafnium in solution, g/L; ce is the equilibrium concentration of zirconium or hafnium in solution, g/L; V is the total volume of solution, mL; and m is the mass of MIBK extraction resin, g.
2.3.2 Column test for adsorbing and separating metals
For continuous extraction, MIBK extraction resin was packed in a glass column (inner diameter: 35 mm). A total of 186 g resin was introduced (packing depth: 400 mm). The column was fed by upflow using a peristaltic pump. The flow rate was 600 mL/h. The column was preconditioned at the optimum acidity by flowing HSCN (acidity: 1.10 mol/L) at room temperature. The feed solution, containing both zirconium and hafnium, was passed downward through the column. The downstream effluent was collected at different fractions with each fraction measuring about 100 mL. The residual total metal concentrations of zirconium and hafnium in the aqueous phase were titrated by EDTA, and the concentration of hafnium was determined by ICP-AES. The resin was washed for zirconium with hydrochloric acid of 3.0 mol/L after reaching saturation adsorption for hafnium, then was eluted for hafnium with sulfuric acid of 2.5 mol/L. The resin was regenerated through washing several times with ammonia of 1.0 mol/L and de-ionized water[10].
3 Results and discussion
3.1 Influence of acidity on adsorption of zirconium and hafnium
The capacity of metals adsorption at different acidity values was determined by batch equilibration technique. A set of solutions (volume of 20 mL each) containing 1.24 mol/L of Zr4+ and Hf4+ were taken and the acidity values of the solutions in bottles were adjusted in the range of 0.5-4.5 mol/L with ammonia and hydrochloric acid. MIBK extraction resin of 0.6 g was individually added into each bottle. In order to reach the adsorption equilibrium, the bottle was then shaken for 20 min at room temperature. The influence of acidity on adsorption of MIBK extraction resin is shown in Fig.1.
Obviously, in Fig.1, the adsorption capacity of adsorbent is significantly affected by the initial acidity of the solution. The adsorption capacity is the highest when the acidity is 1.1 mol/L and decreases by either raising or lowering acidity under the experimental condition. Furthermore, the precipitation will be found in the feed solution when the acidity is below 0.5 mol/L, due to the hydroxylation of both zirconium and hafnium ions. The uptake of zirconium and hafnium ions is inhibited at the acidity of higher values, which can be attributed to the presence of H+ competing with zirconium and hafnium ions for the adsorption sites[11]. Therefore, all the following experiments were performed at the acidity of 1.1 mol/L in the feed solution.
3.2 Determination of adsorption rate constant
The adsorption kinetics was determined according to the following procedure: every 20 mL mixture solution
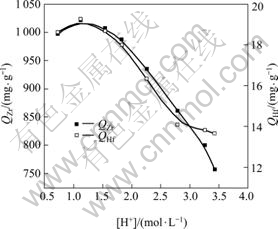
Fig.1 Effects of acidity on adsorption of zirconium and hafnium onto MIBK extraction resin
of feed solution with 1.0 mol/L of acidity was shaken with 0.6 g of MIBK extraction resin at 298.15 K. At predetermined intervals, the concentrations of both zirconium and hafnium in filter liquor were determined. After the concentrations of zirconium or hafnium in filter liquor kept constant, the adsorption rates of zirconium and hafnium onto MIBK extraction resin were obtained (Fig.2).
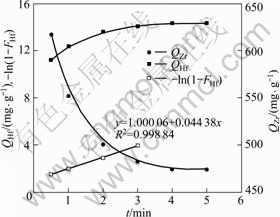
Fig.2 Curves of adsorption rate of zirconium and hafnium onto MIBK extraction resin
The adsorption rate of both zirconium and hafnium onto MIBK extraction resin are completely fast, and the equilibrium time is about 3.5 min. The equilibrium adsorption capacity of zirconium and hafnium (Qm, Zr and Qm, Hf) are 473.801 mg/g and 14.333 mg/g, respectively. Furthermore, zirconium is firstly adsorbed on the resin, and then zirconium adsorbed is exchanged from the resin by hafnium. This means that the adsorption ability for hafnium onto MIBK extraction resin is stronger than for zirconium. Because the concentration of zirconium is much higher than that of hafnium, the MIBK extraction is enclosed by zirconium while less contacted by the hafnium, and the zirconium is adsorbed onto resin prior to the hafnium before the hafnium is contacted by the MIBK extraction resin through diffusion. The predominating step of the adsorption process may be intraparticle diffusion for zirconium while film diffusion for hafnium.
The adsorption capacity of zirconium decreases with increasing the adsorption time. From the adsorption capacity of zirconium calculated by Eq.(1), the concentration of zirconium in influent increases with the adsorption time increasing after the empty volume. This also means that the adsorption ability for hafnium onto MIBK extraction resin is stronger than for zirconium.
In general, the adsorption process may be described as a series of steps: mass transfer from fluid phase to the particle surface across the boundary layer, diffusion within the porous particle and adsorption onto the surface[12]. To determine which one (film diffusion or intraparticle diffusion) is the predominating step of the adsorption process and also to find the rate parameters for film diffusion or intraparticle diffusion, sorption kinetic data were further processed.
According to Boyd method[13], the adsorption rate constant K could be calculated by
![]() (3)
(3)
where F (F=Qt/Q∞) is the fractional attainment of the equilibrium, Qt and Q∞ are the adsorption amounts at certain time and at reaching adsorption equilibrium, respectively, and K is the adsorption rate constant.
The experimental results accorded with Eq.(3) and a straight line was obtained by plotting -ln(1-F) against t (Fig.2). The adsorption rate constant is 4.438×10-2 s-1, which can be found from the slope of the straight line. The correlation coefficient (R2 = 0.998 84) was obtained via linear fitting. According to Boyd from the linear relationship of -ln(1-F) vs t, it can be deduced that the film diffusion is the predominating step of the adsorption process for hafnium.
3.3 Adsorption isotherms
Adsorption isotherms were carried out with different initial total concentrations of zirconium and hafnium (ce, Zr+Hf) in the range of 30-120 g/L-1 at the acidity of 1.1 mol/L-1 (as shown in Fig.3).
The adsorption capacity of MIBK extraction resin for zirconium and hafnium (QZr and QHf) increases with the increase of ce, Zr+Hf. When ce, Zr+Hf is 120 g/L, not QZr but QHf can reach the equilibrium, and the equilibrium adsorption capacity of QHf is 13.33 mg/g.
An adsorption isotherm describes the relationship between the amount of adsorbate adsorbed on the adsorbent and the concentration of dissolved adsorbate in
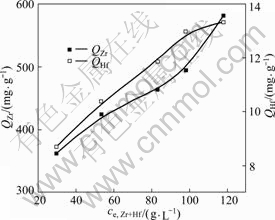
Fig.3 Adsorption isotherms of zirconium and hafnium by MIBK extraction resin
the liquor at equilibrium. The analysis of the adsorption isotherm data by fitting them to different isotherm models is an important step to find the suitable model. The adsorption capacity of zirconium and hafnium onto MIBK extraction resin can be used for design of adsorption systems. The adsorption isotherm studies were carried out on two isotherm models, the Langmuir isotherm and the Freundlich isotherm.
3.3.1 Langmuir isotherm
Langmuir isotherm model[14] is represented by
![]() (4)
(4)
where Qmax is the amount of adsorbate at complete monolayer coverage, mg/g, which gives the maximum adsorption capacity of adsorbent, and KL is the Langmuir isotherm constant which relates to the energy of adsorption, L/g.
The Langmuir adsorption isotherms of zirconium and hafnium by MIBK extraction resin are shown in Fig.4. A straight line was obtained by plotting of ce/qe
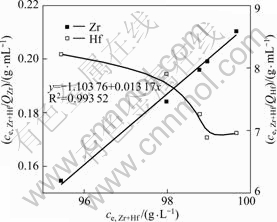
Fig.4 Langmuir adsorption isotherms of zirconium and hafnium by MIBK extraction resin
against ce together with experimental data of zirconium (R2>0.99), while a curve was obtained by plotting the data of hafnium. These indicate that the adsorption of hafnium onto MIBK extraction resin is not fitted by means of the Langmuir isotherm but zirconium. The saturation adsorption capacity (Qmax) of zirconium is estimated from the linear slope (1/Qmax) as 75.93 mg/g. The Langmuir isotherm constant (KL) of -0.012 7 L/g is estimated from the intercept (![]() ). The smaller the KL is, the weaker the adsorption capacity between the adsorbate and adsorbent is. That is to say, the adsorption capacity of hafnium is stronger than that of zirconium.
). The smaller the KL is, the weaker the adsorption capacity between the adsorbate and adsorbent is. That is to say, the adsorption capacity of hafnium is stronger than that of zirconium.
In order to find out the feasibility of the isotherm, the essential characteristics of the Langmuir isotherm could be expressed in terms of a dimensionless constant separation factor (RL)[15]:
![]() (5)
(5)
where c0 is the initial total concentration of both zirconium and hafnium, mol/L; while KL is the Langmuir isotherm constant. The RL values between 0 and 1 indicate favorable adsorption, the RL values more than 1 indicate unfavorable adsorption, the RL values equal to 1 indicate linear adsorption, and the RL values equal to 0 indicate irreversible adsorption[16].
The RL calculated by Eq.(5) is approximately equal to 1, which indicates that the adsorption of zirconium onto MIBK extraction resin is linear adsorption.
3.3.2 Freundlich isotherm
Freundlich isotherm model[17] is represented by
![]() (6)
(6)
where KF is the Freundlich isotherm constant, mg/g; n is the adsorption intensity.
The plots of lg Qe vs lg ce of both zirconium and hafnium are linear, which indicates the applicability of Freundlich isotherm (Fig.5). The slopes (1/n) of lines of zirconium and hafnium are 0.29 and 0.31, respectively. The adsorption process is favorable when the value of 1/n lies between 0.1 and 1. The KF of zirconium and hafnium, 3.53 mg/g and 0.64 mg/g, are calculated from the intercept of these lines, respectively.
3.4 Adsorption kinetic models
The most commonly used pseudo-first-order and pseudo-second-order models were employed to explain the solid/liquid adsorption. A simple Lagergren pseudo-first-order kinetic model and HO pseudo- second-order kinetic model[18] are given as
![]() (7)
(7)
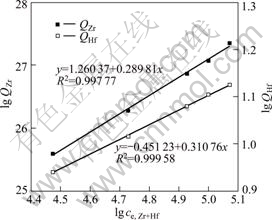
Fig.5 Plot of lg Q vs lg ce for adsorption of zirconium and hafnium from solution
![]() (8)
(8)
where Qt and Qe are the adsorption amounts at certain time and at equilibrium time, respectively, mg/g; k1 is the pseudo-first-order rate constant, min-1; and k2 is the rate constant of pseudo-second-order equation, g/(mg·min).
The experimental adsorption data of zirconium and hafnium onto the extraction resin are not fitted with the Lagergren pseudo-first-order kinetic model but agree with the HO pseudo-second-order kinetic model (R2>0.99). The plots of t/Qt vs t for both zirconium and hafnium ions adsorption are shown in Fig.6. The equilibrium adsorption capacity (Qe) is evaluated from the slope (1/Qe) of linear plots obtained and found to be 462.96 mg/g and 14.88 mg/g for zirconium and hafnium, respectively. The calculated Qe values of zirconium and hafnium fairly agree with the experimental values (Qm, Zr=473.801 mg/g, Qm, Hf=14.333 mg/g). The rate constant of pseudo-second-order equation (k2) of -0.019 and 0.41 g/(mg·min) for zirconium and hafnium, respectively, are
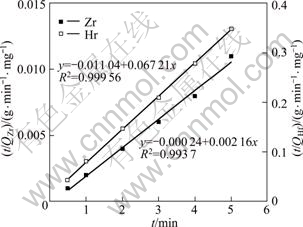
Fig.6 Kinetic curves of adsorption of zirconium and hafnium onto MIBK extraction resin
evaluated from the intercept (![]() ), respectively. While linear plots could not be obtained by the plot of lg(Qe-Qt) vs t for zirconium and hafnium. These results suggest that the pseudo-second-order adsorption mechanism is predominant and the rate of each ion is controlled by the chemisorption process.
), respectively. While linear plots could not be obtained by the plot of lg(Qe-Qt) vs t for zirconium and hafnium. These results suggest that the pseudo-second-order adsorption mechanism is predominant and the rate of each ion is controlled by the chemisorption process.
3.5 Thermodynamic studies
The influence of temperature on distribution ratios of zirconium and hafnium is shown in Fig.7. The distribution ratios of both zirconium and hafnium (DZr and DHf) decrease with the increase of temperature in the range of 10-60 °C. The results clearly show that the adsorption process of both zirconium and hafnium by the MIBK extraction resin is exothermic and imposes a negative influence with temperature. The influence of temperature on adsorption process of zirconium by the MIBK extraction resin is stronger than that of hafnium.
According to Van’t Hoff equation[19]:
![]() (9)
(9)
where D is the distribution ratio, T is the absolute temperature, R is the ideal gas constant, and ?H is the enthalpy change, kJ/mol.
Two straight lines are obtained by plotting lg D for zirconium and hafnium vs T-1, respectively, as shown in Fig.7.
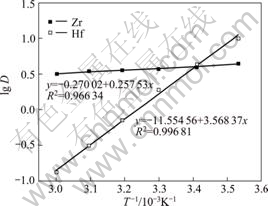
Fig.7 Influence of temperature on distribution ratios of zirconium and hafnium
The slope of straight line for hafnium is greater than for zirconium. The enthalpy changes of adsorption for zirconium and hafnium onto the MIBK extraction resin, -4.93 kJ/mol and -68.32 kJ/mol, are calculated from the slopes of the Van’t Hoff plots, respectively. A negative value of ?H indicates the exothermic nature of adsorption of zirconium and hafnium onto the MIBK extraction resin. Furthermore, the value of ?H for hafnium is less than for zirconium, which indicates that the adsorption capacity for hafnium onto the resin is stronger than for zirconium. This is in agreement with the heretofore discussed results in adsorption rate constant.
An adsorption free energy equation is obtained by combining adsorption free energy formula with Freundlich isotherm equation as following equation[20]:
?G=-nRT (10)
where ?G is the adsorption free energy, and n is the adsorption intensity of Freundlich isotherm equation.
The values of ?G for zirconium and hafnium are calculated by Eq.(10) and found to be -8.5 kJ/mol and -8.0 kJ/mol at 298.15 K, respectively. The negative value of ?G at 298.15 K indicates the feasibility of the adsorbent and spontaneity of the adsorption process.
According to Gibbs-Helmholtz equation:
?S=(?H-?G)/T (11)
where ?S is the entropy change.
The values of ?S for zirconium and hafnium (?SZr and ?SHf) are calculated by Eq.(11) and found to be 11.97 J/(mol·K) and -202.31 J/(mol·K) at 298.15 K, respectively. The positive value suggests increased randomness at the solid/solution interface during the adsorption of zirconium ion onto the MIBK extraction resin. While the negative value suggests the adsorption of hafnium is bounded in two-dimensional space and the confusion degree of the system reduces. These also indicate that the adsorption capacity for hafnium onto the resin is stronger than for zirconium.
3.6 Column test
In order to evaluate the amount of metal in the column, the column experiment was carried out using the MIBK extraction resin in a fixed-bed column with the feed solution (Table 1). The column was preconditioned by flowing HSCN solution (acidity: 1.10 mol/L) through the fixed-bed for the zirconium and hafnium adsorption, especially, for hafnium adsorption. In the column test, the effluent solution at every 100 mL was collected and was analyzed by EDTA for both zirconium and hafnium and by ICP for hafnium. The efficiency of adsorbing zirconium and hafnium is presented by breakthrough curves showing concentration ratios ce/c0 as a function of throughput volumes, where ce is the instantaneous concentration of zirconium or hafnium in effluents and c0 is the initial total metal concentration of influent.
Fig.8 shows the adsorption curves for zirconium and hafnium. In this test, the nominal resident time of the influent solution in the resin column was 40 min. It is shown that the zirconium broke through the column almost immediately (at 400 mL) after feeding and the column took approximately 800 mL before being completely exhausted with zirconium. On the other hand, the hafnium was adsorbed for a substantial period of time, not achieving breakthrough until 2 500 mL, and reached exhaustion after 2 800 mL of throughput volume.
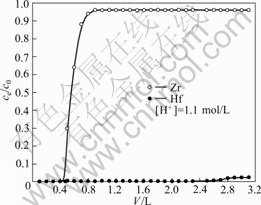
Fig.8 Column adsorption curves for zirconium and hafnium
The resin was washed with hydrochloride of 2.5 mol/L, then was eluted for hafnium with sulfuric acid of 3.5 mol/L. The separation of hafnium from zirconium was fairly completed, and the separation coefficient (β) was 25-30. In each of the effluent fractions which were collected prior to 1 900 mL, the hafnium concentration was below 0.015%, whereas zirconium was retained in the solution, and therefore, high purity zirconium could be recovered subsequently to this separation. After six regeneration tests of the resin were carried out, the ability of the resin for adsorption and separation zirconium and hafnium was unchanged. The results show that the resin has a good ability to be regenerated and reused.
4 Conclusions
1) The adsorption capacity of the MIBK extraction resin for zirconium and hafnium acidity is strongly influenced by the acidity of feed solution. The acidity of 1.1 mol/L was optimal in the adsorption process.
2) The capacity of hafnium onto the MIBK extraction resin is more than that of zirconium. The HO pseudo-second-order kinetic model is employed to fit the experimental adsorption data of zirconium and hafnium onto the extraction resin.
3) The Langmuir equation is only suitable to the adsorption of zirconium, while the Freundlich equation is more suitable to the adsorption of both zirconium and hafnium. The relation of adsorption of zirconium onto MIBK extraction resin is linear.
4) Column test reveals that MIBK extraction resin is effective in separating hafnium from zirconium.
References
[1] XIONG Bing-kun, WEN Wang-guang, YANG Xin-min, LI Hui-yuan, LUO Fang-cheng, ZHANG Wei, GUO Jing-mao. Zirconium and hafnium metallurgy [M]. Beijing: Metallurgical Industry Press, 2006, 95-108. (in Chinese)
[2] SOMMERS J A, PERRINE J G. Method for separation hafnium from zirconium. US200301438A1[P]. 2003-7-31.
[3] ZHANG Li. The development and the status of zirconium and hafnium separation process [J]. Rare Metals Letters, 2004, 23(5): 26-29. (in Chinese)
[4] ZHANG Li. Review and comparison for zirconium hafnium separation by wet extraction [J]. Rare Metals Letters, 2007, 26(1): 115-118. (in Chinese)
[5] LIU J S, CHEN H, CHEN X Y, GUO Z L, HU Y C, LIU C P, SUN Y Z. Extraction and separation of In(Ⅲ), Ga(III) and Zn(II) from sulfate solution using extraction resin [J]. Hydrometallurgy, 2006, 82: 137-143.
[6] MU F T, JIA Q, TIAN Y M, SHANG Q K. Extraction of cobalt (Ⅱ) and nickel (Ⅱ) by a solvent impregnated resin containing bis(2, 4, 4-trimethylpentyl) monothiophosphinic acid [J]. Adsorption, 2008, 14: 31-36.
[7] EL-SOFANY E A. Removal of lanthanum and gadolinium from nitrate medium using Aliquat-336 impregnated onto Amberlite XAD-4 [J]. Journal of Hazardous Materials, 2008, 153: 948-954.
[8] LIU Jun-shen, YUAN Yang-xu. Adsorption property of P507 levextrel resin for indium (Ⅲ) from hydrochloric acid system [J]. Rare Metals and Cemented Carbides, 2008, 36(4): 1-4. (in Chinese)
[9] ZHANG Li, WANG Li-jun, LANG Shu-ling, CHEN Song, LUO Yuan-hui, HUANG Yong-zhang. Method for separating zirconium hafnium by tributyl phosphate extraction chromatography method. China Patent, CN101209858 [P]. 2008-7-2. (in Chinese)
[10] XIONG C H, YAO C P, WANG Y J. Sorption behaviour and mechanism of ytterbium(Ⅲ) on imino-diacetic acid resin [J]. Hydrometallurgy, 2006, 82(3/4): 190-194.
[11] XIONG C H, YAO C P. Study on the adsorption of cadmium(Ⅱ) from aqueous solution by D152 resin [J]. Journal of Hazardous Materials, 2009, 166: 815-820.
[12] PAN B C, XIONG Y, SU Q, LI A M, CHEN J L, ZHANG Q X. Role of amination of a polymeric adsorbent on phenol adsorption from aqueous solution [J]. Chemosphere, 2003, 51: 953-962.
[13] SRIVASTAVAA V C, MALLB I D, MISHRA I M. Adsorption thermodynamics and isosteric heat of adsorption of toxic metal ions onto bagasse fly ash (BFA) and rice husk ash (RHA) [J]. Chemical Engineering Journal, 2007, 132(1/3): 267-268.
[14] GODEA F, PEHLIVAN E. Removal of chromium(Ⅲ) from aqueous solutions using Lewatit S 100: The effect of pH, time, metal concentration and temperature [J]. Journal of Hazardous Materials, 2006, 136(2): 330-337.
[15] WEBER T W, CHAKRAVORTI R K. Pore and solid diffusion models for fixed bed adsorbers [J]. American Institute of Chemical Engineers, 1974, 20: 228-238.
[16] MALIK P K. Dye removal form wastewater using activated carbon developed from sawdust: Adsorption equilibrium and kinetics [J]. Journal of Hazardous Materials, 2004, 113: 81-88.
[17] BILGILI M S. Adsorption of 4-chlorophenol from aqueous solutions by xad-4 resin: Isotherm, kinetic, and thermodynamic analysis [J]. Journal of Hazardous Materials, 2006, 137(1): 157-164.
[18] HO Y S. Second order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods [J]. Water Research, 2006, 40: 119-125.
[19] JIA Q, WANG Z H, LI D Q, NIU C J. Adsorption of heavy rare earth (Ⅲ) with extraction resin containing bis(2,4,4-trimethylpentyl) mono-thiophosphinic acid [J]. Journal of Alloys and Compounds, 2004, 374: 434-437.
[20] LIU Jun-shen, LI Gui-hua, CHEN Hou, LIU Chun-ping, MA Song-mei. Adsorption properties and thermodynamics of platinum (IV) with trialkylamines extraction resin [J]. Chinese Journal of Rare Metals, 2005, 29(4): 509-512. (in Chinese)
Corresponding author: WANG Li-jun, Tel: +86-10-82241308; Fax: +86-10-62355399; E-mail: gold@grinm.com
DOI: 10.1016/S1003-6326(09)60333-2

