Trans. Nonferrous Met. Soc. China 26(2016) 2731-2737
Separation of W and Mo from their peroxoacids solutions by thermal decomposition
Wen-juan ZHANG1, Jiang-tao LI2, Zhong-wei ZHAO1, 2, Fei LI1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Key Laboratory of Hunan Province for Metallurgy and Material Processing of Rare Metals, Changsha 410083, China
Received 11 November 2015; accepted 5 May 2016
Abstract: A separation method for W and Mo from peroxoacids solution by thermal decomposition was studied. Thermal decomposition of peroxotungstic acid and peroxomolybdic acid was investigated respectively. The results confirmed that peroxomolybdic acid showed a preferable stability compared with peroxotungstic acid. This thermal stability difference was the basic principle of the separation of W and Mo. Experiments were performed to study the effects of temperature, stirring speed, free acid concentration and Mo concentration on the separation efficiency. The results indicated that peroxotungstic acid decomposed into tungstic acid (H2WO4) and precipitated selectively, while Mo was rejected in aqueous solution, realizing good separation of W and Mo. The separation factor of W and Mo reached 112 under the studied conditions, which indicated that this method has potential for use in separating W and Mo.
Key words: W; Mo; separation; thermal decomposition; peroxotungstic acid; peroxomolybdic acid
1 Introduction
Tungsten is usually accompanied by molybdenum in scheelite and these two metals are simultaneously extracted from minerals in metallurgical processes. Molybdenum is the strictly controlled impurity that impairs performance of tungsten products [1]. With progressive exhaustion of high-grade resources, most sources of tungsten located heretofore contain significant quantities of molybdenum [2]. In order to obtain qualified tungsten products, a process to separate Mo from W is necessary.
The separation of W and Mo from aqueous solution has been proven to be the most complicated problems in extractive metallurgy of tungsten. This is mainly due to the chemical and physical similarities of the two elements. Up to now, various methods for separation of W and Mo, such as solvent extraction, ion-exchange, adsorption, selective precipitation and emulsion liquid membrane, have been developed [3-7]. Any of the above methods is based on the differences in characteristics of W and Mo [8]. Previous work indicated that peroxide complexes of W and Mo exhibit different chemical properties. Based on the difference in these properties, several methods for the separation of W and Mo have been proposed, such as solvent extraction [9-11] and ion-exchange [12]. ZELIKMAN et al [11] achieved separation of the two metal peroxides with solvent extraction. This process utilized the preferential extraction of Mo into an organic phase with main part of W remaining in solution. The extraction rate of Mo reached 79% at one stage without W extracted from the pregnant solution which contained 19 g/L W and 1 g/L Mo. However, the use of the extractant, tributyl phosphate, has some drawbacks of high viscosity and poor phase disengagement.
An alternative method has been proposed, based on the poor stability of tungsten peroxide. W is initially separated from Mo by the selective decomposition of tungsten peroxide. JIANG et al [13] proposed homogeneous complexing precipitation, in which tungstic acid can be selectively precipitated from an aqueous acidic solution of tungsten and molybdenum containing hydrogen peroxide by SO2 reduction. However, the reducing agent is one kind of pungent, noxious gas to the human body and the environment. With the improvement of environment protection awareness, this method may not be suitable for separating W and Mo. By comparison, another decomposition method mentioned in the literature, thermal decomposition, was environment-friendly. But it was only used for analyzing tungsten in laboratory [14]. JIANG et al [13] even considered that the typical industrial sodium tungstate solution contained less than 0.03% Mo, and the thermal decomposition did not work well for it. But the details on the separation by thermal decomposition have not been investigated and reported.
Actually, the content of associated molybdenum in available resources of tungsten is higher and higher. For instance, the Mo/W mole ratio is greater than 1/8 in the scheelite middlings from Luanchuan Mo-W deposit, which occupies 10.4% of industrial reserves of tungsten in China [2]. For the resources with high Mo/W mole ratio, the content of Mo in sodium tungstate solution considerably is larger than 0.03% and it is not verified whether the method is feasible or not. In this work, we therefore investigated thermal decomposition as an alternative method for the separation of W and Mo from a solution with high Mo/W mole ratio. The thermal decomposition behavior of peroxotungstic acid and peroxomolybdic acid was investigated respectively. In addition, several affecting factors on the separation were also be studied.
2 Experimental
All feed solutions for experiments containing W and Mo were prepared by dissolving the exact quantities of Na2WO4·2H2O and Na2MoO4·2H2O at 20 °C. H2O2 was added in accordance with a certain proportion: 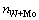 :
: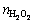 =1:1 and then a certain amount of HCl was mixed in the solution. All the reagents employed were analytical grade and the solutions were prepared with distilled water.
=1:1 and then a certain amount of HCl was mixed in the solution. All the reagents employed were analytical grade and the solutions were prepared with distilled water.
For thermal decomposition of single peroxoacid, 100 mL solution was put into a glass beaker and heated to desired temperature in a water bath. The concentration of W or Mo was analyzed using ICP-AES instrument during the decomposition. And the concentration of H2O2 was determined by titration with potassium permanganate.
Separation experiments were performed by placing 100 mL feed solution into a glass beaker and heating to desired temperature in the water bath under vigorously stirring. 0.5 mL liquor sample was periodically withdrawn from the reactor for determination of decomposition rate through analyzing the concentrations of W and Mo by ICP-AES instrument.
The distribution coefficient of W and Mo was calculated using the following equation:
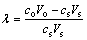 (1)
(1)
where co and cs are the initial and final concentrations of metals in solution, respectively, Vo and Vs are the initial and final volumes of solution, respectively.
The separation factor was defined as the ratio of the distribution coefficient of W to that of Mo, which was calculated as follows:
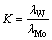 (2)
(2)
where λW and λMo are the distribution coefficients of W and Mo, repectively.
3 Results and discussion
3.1 Decomposition behavior of peroxotungstic acid and peroxomolybdic acid
A peroxomolybdic acid solution was prepared with H2O2/Mo mole ratio of 1:1 and decomposed at 60 °C. The H2O2 and Mo concentrations were analyzed during the decomposition. The results are illustrated in Table 1. It is noted that there is still not any precipitate formed in the solution when the H2O2 concentration reduces to 0.01 mol/L. This is probably because the Mo in acid solution is able to exist in the form of cationic molybdenum species such as 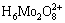 and
and 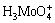 when the peroxide decomposes completely. The thermodynamic calculation also indicated that cationic molybdenum species become predominant along with the decrease of H2O2 concentration, as shown in Fig. 1, which is the distribution of molybdenum species with the variation in H2O2 concentration calculated from the data of Refs. [15-17].
when the peroxide decomposes completely. The thermodynamic calculation also indicated that cationic molybdenum species become predominant along with the decrease of H2O2 concentration, as shown in Fig. 1, which is the distribution of molybdenum species with the variation in H2O2 concentration calculated from the data of Refs. [15-17].
Table 1 H2O2 and Mo concentrations during peroxomolybdic acid decomposition (Free [H+]=1.0 mol/L, T=60 °C)
After decomposition of peroxide, a precipitate forms when the solution is further heated. This could be attributed to the decomposition of 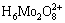 or
or 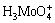 at high temperatures. To verify this, an experiment on the thermal stability of molybdic acid solution was carried out by mixing analytical pure Na2MoO4·2H2O and HCl solution, and then decomposing at 60 °C. The results presented in Fig. 2 show that the precipitation rate is higher with longer reaction time, which proves that the ion (
at high temperatures. To verify this, an experiment on the thermal stability of molybdic acid solution was carried out by mixing analytical pure Na2MoO4·2H2O and HCl solution, and then decomposing at 60 °C. The results presented in Fig. 2 show that the precipitation rate is higher with longer reaction time, which proves that the ion (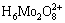 or
or 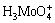 ) is not really stable and decomposes into solid H2MoO4 with heat. Additionally, it is observed that the decomposition curves of peroxomolybdic acid solution and molybdic acid solution are approximately parallel. This indicates that both solutions share the same decomposition behavior when the precipitate is formed. Based on the above analysis, it is concluded that the decomposition reaction of peroxomolybdic acid takes place in two steps. Peroxomolybdic acid decomposes into cationic molybdenum in the first step (Reactions 3 and 4).
) is not really stable and decomposes into solid H2MoO4 with heat. Additionally, it is observed that the decomposition curves of peroxomolybdic acid solution and molybdic acid solution are approximately parallel. This indicates that both solutions share the same decomposition behavior when the precipitate is formed. Based on the above analysis, it is concluded that the decomposition reaction of peroxomolybdic acid takes place in two steps. Peroxomolybdic acid decomposes into cationic molybdenum in the first step (Reactions 3 and 4).
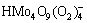 +5H++3H2O=
+5H++3H2O=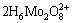 +2O2↑ (3)
+2O2↑ (3)
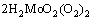 +2H+=
+2H+=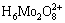 +2O2↑ (4)
+2O2↑ (4)
Thereafter, insoluble H2MoO4 is formed in the second step (Reactions 5 and 6).
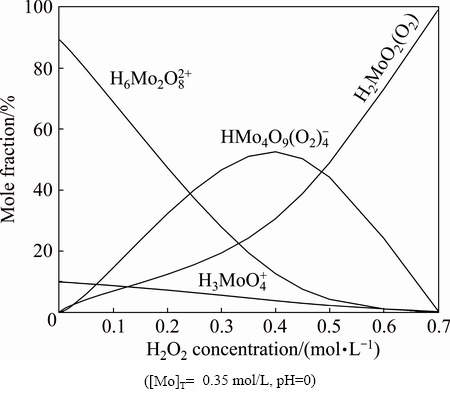
Fig. 1 Distribution diagram of molybdenum species
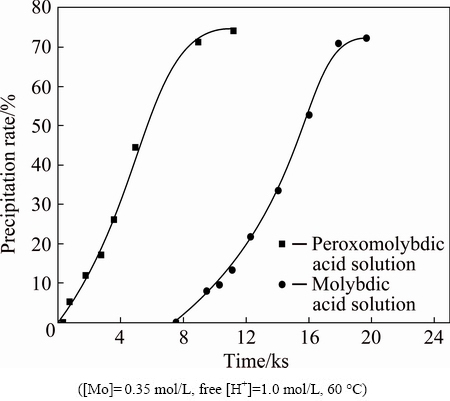
Fig. 2 Precipitation rate vs time for decomposition
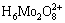 =2H2MoO4+2H+ (5)
=2H2MoO4+2H+ (5)
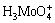 =H2MoO4+H+ (6)
=H2MoO4+H+ (6)
As a comparison, we also investigated the decomposition of peroxotungstic acid solution under the same conditions. The existing researches show that peroxotungstate chemistry is poorly understood [18,19], so, the form of peroxotungstate in this acidic solution is not determined through thermodynamic analysis. But we analyzed the H2O2 and W concentrations during the decomposition and discovered that the W/H2O2 mole ratio remained at around 2:1 throughout the precipitation of H2WO4 in solution, as shown in Table 2. It is assumed that peroxotungstic acid appears in the form of  in the decomposition course, which has been identified and the structure is likely to be that of
in the decomposition course, which has been identified and the structure is likely to be that of 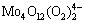 [18-20]. In conclusion, the decomposition reaction is simply expressed as
[18-20]. In conclusion, the decomposition reaction is simply expressed as
H4W4O12(O2)2+2H2O=4H2WO4+O2 (7)
As can be seen from Fig. 3, the decomposition of peroxotungstic acid is fast and approximately 95% of H2WO4 is precipitated within 31 min of reaction time, while no precipitate is formed after 125 min in peroxomolybdic acid solution. On the basis of the results obtained, it is evident that there exists a notable difference in thermal stability and decomposition mechanism between peroxotungstic acid and peroxomolybdic acid.
Table 2 H2O2 and W concentrations during peroxotungstic acid decomposition (free [H+]=1.0 mol/L, 60 °C)
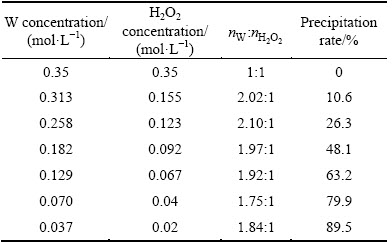
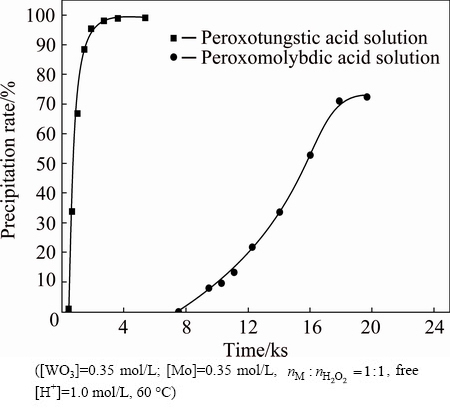
Fig. 3 Precipitation rate vs time for peroxoacids decomposition
3.2 Separation of W and Mo from solutions containing peroxotungstic acid and peroxo- molybdic acid
3.2.1 Effect of reaction time and temperature
The experimental results obtained by varying temperatures from 50 to 70 °C are shown in Fig. 4. In each case, there exists an introduction period. The possible reason for this phenomenon is the polymerization of soluble tungstic acid forming tungstic acid precipitate. The precipitation of peroxotungstic acid and peroxomolybdic acid is increased with the increase of temperature, which is consistent with the endothermic character of the decomposition reaction. At 55, 60 and 70 °C, the decomposition of peroxotungstic acid is completed quickly. By contrast, peroxomolybdic acid decomposes rather more slowly under the same conditions. This behavior indicates again that peroxomolybdic acid is thermodynamically more stable than peroxotungstic acid.
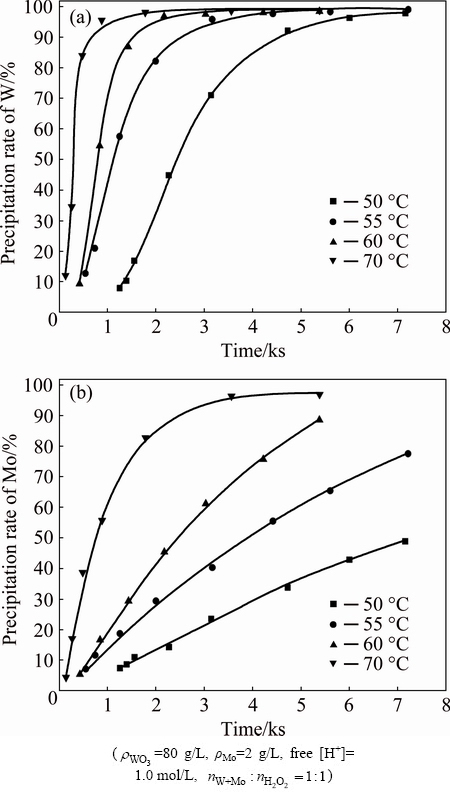
Fig. 4 Effect of reaction time and temperature on precipitation of W (a) and Mo (b)
As expected, the property can be used for the separation of W and Mo. Figure 5 shows that the separation factor increases and reaches a peak, and then declines along with the increase of the time. Temperature is determined to affect the separation factor that the higher the temperature is, the earlier the peak occurs. The reason for this phenomenon is a decrease of the difference in thermal stability between peroxotungstic acid and peroxomolybdic acid at elevated temperatures. Moreover, the co-precipitation of Mo is significantly increased with the increase of temperature as shown in Fig. 4.
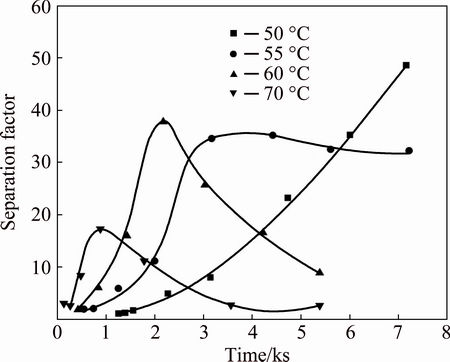
Fig. 5 Effect of reaction time and temperature on separation factor of W and Mo
3.2.2 Effect of Mo concentration
In order to investigate the effect of Mo concentration, experiments were conducted by varying Mo concentration in the range of 2-11.3 g/L. The results presented in Fig. 6 show an increase in precipitation rates of both W and Mo with increasing Mo concentration against time. This behavior may originate from the increase of Mo concentration, accelerating the co-precipitation of W and Mo. Besides, Mo concentration has an influence on separation factor which decreases with increasing Mo concentration. Figure 7 indicates that separation factor is still above 20 at ρMo=11.3 g/L. Consequently, it is predicted that this method could be applied for preliminary separation of Mo and W from solutions with high content of Mo.
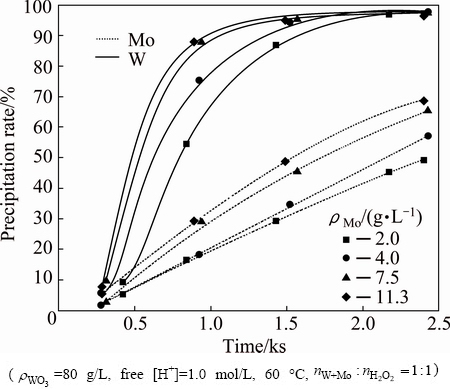
Fig. 6 Effect of Mo concentration on precipitation rates of W and Mo
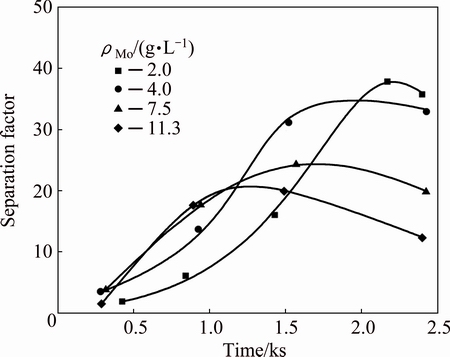
Fig. 7 Effect of Mo concentration on separation factor of W and Mo
3.2.3 Effect of free acid concentration
The effects of free acid concentration are presented in Figs. 8 and 9. It can be seen that there is an increase in the precipitation rates of W and Mo with increase in free acid concentration. The decomposition of W is fast and 95.8% of W is precipitated at the initial of 5 min with free acid concentration of 2.5 mol/L. About 22.3% of Mo is precipitated under the same condition. However, peroxotungstic acid and peroxomolybdic acid are more stable when initial acid concentration is 0.6 mol/L. As shown in Fig. 8, the precipitation rates of W and Mo reach 31.2% and 9.8%, respectively, when the decomposition time is 20 min. However, the acid concentration affects not only the stability of peroxotungstic acid and peroxomolybdic acid, but also the solubility of decomposition products (H2WO4 and H2MoO4) and in consequence affecting the separation factor indirectly. As reported in Ref. [1], the solubility of H2WO4 in HCl solution is far less than that of H2MoO4 [1]. The H2MoO4 solubility increases with an increasing amount of HCl solution. The maximum separation factor of 112 is obtained at free acid concentration of 2.5 mol/L. Thus, higher HCl concentration may favor for the separation of W and Mo.
3.2.4 Effect of stirring speed
Figure 10 shows the effect of stirring speed on the decomposition of peroxotungstic acid and peroxomolybdic acid. The precipitation of W is not affected and the precipitation of Mo is slightly dependent on the stirring speed. The slight effect of stirring may be attributed to the mixing of oxygen bubbles produced by peroxide decomposition. In addition, stirring can accelerate the decomposition of hydrogen peroxide.
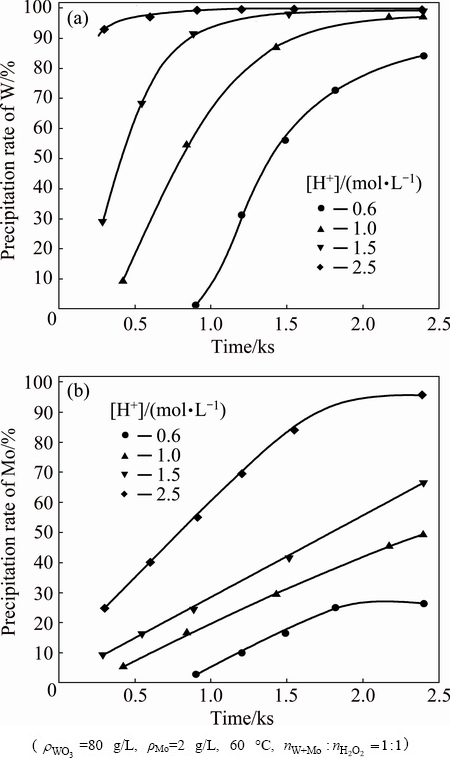
Fig. 8 Effect of free acid concentration on precipitation rates of W (a) and Mo (b)
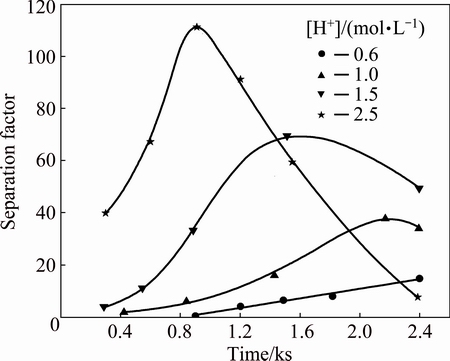
Fig. 9 Effect of free acid concentration on separation factor
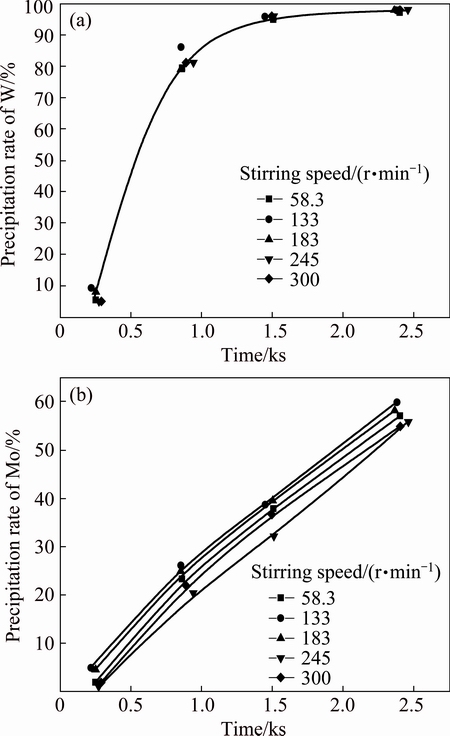
Fig. 10 Effect of stirring speed on decomposition
4 Conclusions
1) The thermal decomposition of peroxomolybdic acid takes place in two steps. Firstly, peroxomolybdic acid transforms into cationic molybdenum as peroxide decomposes completely. Secondly, the insoluble H2MoO4 forms when the solution is further heated. By comparison, peroxotungstic acid decomposes into insoluble H2WO4 directly.
2) In mixture solution of peroxotungstic acid and peroxomolybdic acid, peroxotungstic acid shows a lower thermal stability than peroxomolybdic acid. The difference can be utilized for the removal of W from an aqueous acidic solution of W and Mo containing hydrogen peroxide. In the process, peroxotungstic acid is decomposed into tungstic acid, while Mo is rejected in solution.
3) The precipitation rate and the separation factor are slightly dependent on the stirring speed, but are considerably dependent on the temperature and acid concentration. And the higher the temperature or acid concentration is, the faster the peroxoacids decompose. The separation factor reaches 112 at reaction temperature of 60 °C and free acid concentration of 2.5 mol/L.
References
[1] LI Hong-gui, YANG Jian-gao, LI kun. Tungsten metallurgy [M]. Changsha: Central South University Press, 2010: 36-38. (in Chinese)
[2]  Ying, LI Hong-gui. Utilization of tungsten concentrates of high molybdenum rationally [J]. China Tungsten Industry, 2005, 20(5): 15-16. (in Chinese)
Ying, LI Hong-gui. Utilization of tungsten concentrates of high molybdenum rationally [J]. China Tungsten Industry, 2005, 20(5): 15-16. (in Chinese)
[3] ZHAO Zhong-wei, CAO Cai-fang, CHEN Xing-yu. Separation of macro amounts of tungsten and molybdenum by precipitation with ferrous salt [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(12): 2758-2763.
[4] ZHAO Zhong-wei, ZHANG Jia-liang, CHEN Xing-yu, HE Li-hua, LI Jiang-tao. Separation of tungsten and molybdenum using macroporous resin: Equilibrium adsorption for single and binary systems [J]. Hydrometallurgy, 2013, 140: 120-127.
[5] LU Xiao-ying, HUO Guang-sheng, LIAO Chun-hua. Separation of macro amounts of tungsten and molybdenum by ion exchange with D309 resin [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(9): 3008-3013.
[6] LI Hong-gui, ZHAO Zhong-wei, HUO Guang-sheng. Deep separation of resemble elements [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(1): 234-240. (in Chinese)
[7] LI Shao-xiu, WAN Xiang-de, ZHANG Xiu-juan. Separation of tungsten and molybdenum by emulsion liquid membrane weak basic separation system [J]. Membrane Science and Technology, 1996, 16(3): 33-38. (in Chinese)
[8] LIU Xu-heng, SUN Fang, ZHAO Zhong-wei. The latest development of separation of molybdenum from tungsten [J]. Rare Metals and Cemented Carbides, 2007, 35(4): 42-45. (in Chinese)
[9] OZENSOY E, BURKIN A R. Separation of tungsten and molybdenum by solvent extraction: US patent, 4275039[P]. 1981-06-23.
[10] GUAN Wen-juan, ZHANG Gui-qing, CAO Cong-jie. Precursor solution prepared by evaporation deamination complex method for solvent separation of Mo and W by H2O2-complexation [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 1139-1146.
[11] ZELIKMAN A N, VOLDMAN G M, RUMYANTSEV V K, ZIBEROV G N, KAGERMANIAN V S. Process for separation of tungsten and molybdenum by extraction: US patent, 3969478[P]. 1976-07-13.
[12] YUAN Bin, DENG Shun-qin. Removeal of molybdenum from tungsten solution by ion exchange [J]. Hydrometallurgy of China, 2003, 22(2): 69-78.
[13] JIANG An-ren, OU Yang-shi, PANG Zhen. Separation of molybdenum from tungsten by complexed homogeneous precipitation with H2O2 [J]. Rare Metals, 1988, 12(4): 245-248.
[14] MURAU P. Dissolution of tungsten by hydrogen peroxide [J]. Analytical Chemistry, 1961, 33(8): 1125-1126.
[15] PETTERSSON L, ANDERSSON I, TAUBE F, TOTH I, HASHIMOTO M, HOWARTH O W. 17O NMR study of aqueous peroxoisopolymolybdate equilibria at lower peroxide/Mo ratios [J]. Dalton Transactions, 2003(1): 146-152.
[16] TAUBE F, HASHIMOTO M, ANDERSSON I, PETTERSSON L. Characterization of aqueous peroxomolybdate catalysts applicable to pulp bleaching [J]. Dalton Transactions, 2002(6): 1002-1008.
[17] OLAZABAL M, ORIVE M, FERNANDEZ L, MADARIAGA J. Selective extraction of vanadium (V) from solutions containing molybdenum (VI) by ammonium salts dissolved in toluene [J]. Solvent Extraction and Ion Exchange, 1992, 10(4): 623-635.
[18] HOWARTH O W, PETTERSSON L, ANDERSSON I. Aqueous peroxoisopolyoxometalates, polyoxometalate chemistry from topology via self-assembly to applications [M]. Heidelberg: Springer, 2001: 145-159.
[19] HOWARTH O W. Oxygen-17 NMR study of aqueous peroxotungstates [J]. Dalton Transactions, 2004(3): 476-481.
[20] JIANG An-ren, PANG Zhen, GU Yi-dong. Preparation of tungstic acid at room temperature and low acidity [J]. Chemical Research in Chinese Universities, 1987, 8(5): 403-405. (in Chinese).
热分解法从钨钼过氧酸溶液中分离钨和钼
张文娟1,李江涛2,赵中伟1,2,李 飞1
1. 中南大学 冶金与环境学院,长沙 410083;
2. 稀有金属冶金与材料制备湖南省重点实验室,长沙 410083
摘 要:研究了一种从含钨钼过氧酸溶液中分离钨和钼的方法。对过氧钨酸和过氧钼酸的热分解过程分别进行研究,证实了过氧钼酸比过氧钨酸具有更高的稳定性,并利用这一差异来分离钨和钼。研究反应温度、搅拌速度、游离酸浓度和溶液钼浓度对钨、钼分离效果的影响。结果表明:在钨、钼过氧酸溶液的热分解过程中,过氧钨酸分解为钨酸沉淀,而钼仍存在于溶液中,从而实现钨和钼的分离。在所研究的实验条件下,钨和钼的分离系数可达到112,该结果表明热分解法在钨、钼分离过程中具有良好的应用前景。
关键词:W;Mo;分离;热分解;过氧钨酸;过氧钼酸
(Edited by Wei-ping CHEN)
Foundation item: Project (51334008) supported by the National Natural Science Foundation of China; Project (2010FJ1011) supported by the Key Program of Science and Technology of Hunan Province, China
Corresponding author: Zhong-wei ZHAO; Tel: +86-731-88830476; E-mail: zhaozw@csu.edu.cn
DOI: 10.1016/S1003-6326(16)64402-3