文章编号:1004-0609(2014)06-1656-07
W(Ⅵ)-V(Ⅴ)-H 2O体系钨钒分离的热力学分析
张家靓,赵中伟
(中南大学 冶金与环境学院,长沙 410083)
摘 要:通过热力学计算绘制25 ℃时W(Ⅵ)-V(Ⅴ)-H2O系中存在的离子随pH、钨、钒浓度变化的热力学平衡图,并总结其变化规律。通过热力学分析,从理论上寻找到钨、钒在混合溶液中存在形式上的差异,并据此提出钨钒分离的技术路线。钒形成聚合离子的能力强于钨。在pH约为8.5~9.0的条件下,溶液中绝大部分的钒以聚合离子形式存在,而钨则几乎全部为单核离子,利用该差异通过合理的分离手段即可实现二者的有效分离;而在钨、钒浓度均较低时,体系会在pH较低的条件下形成VO2+离子,而此时钨的存在形式全部为阴离子,因而,采用阳离子萃取剂或阳离子树脂可望选择性地将VO2+从溶液中分离。
关键词:W(Ⅵ)-V(Ⅴ)-H 2O系;钨;钒;分离;热力学平衡;钨钒杂多酸根离子
中图分类号:TF801 文献标志码:A
Thermodynamic analysis for separation of tungsten and vanadium in W(Ⅵ)-V(Ⅴ)-H 2O system
ZHANG Jia-liang, ZHAO Zhong-wei
(School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: The thermodynamic equilibrium diagrams were drawn for the distribution of species at different pH and concentrations in the W(Ⅵ)-V(Ⅴ)-H 2O system at 25 ℃. The difference of existing forms between W and V in the mixed solution was found by the theoretical analysis, and accordingly the technical routes for separation of W and V were presented. The results show that the polymeric ability of VO43- is stronger than that of WO42-. At the pH range of 8.5-9.0, most of VO43- transform to polymeric anions, while almost all W ions still exist as the form of monomeric WO42-. This difference makes it possible that W and V can be separated efficiently by a proper method. In the solution with low concentration of W and V, VO2+ ions are formed under low pH conditions, while W ions exist as the form of anions. Consequently, VO2+ ions can be selectively separated by the cation extraction agent or exchange resin.
Key words: W(Ⅵ)-V(Ⅴ)-H 2O system; W; V; separation; thermodynamic equilibrium; tungstovanadate
钨、钒同属于稀有高熔点金属,二者以其优良的物理化学性质,在国民经济和国防军工等诸多领域都发挥着日益重要的作用[1-2]。当前随着传统优质资源的不断消耗,从多金属的复杂矿物原料和二次资源中回收钨、钒所占的比例日益提高。在矿物资源方面,像在我国湖南和贵州等地产出的镍钼矿中就伴生有丰富的钨、钒等有价金属[3]。在二次资源中,包括一些废旧钢材、废合金以及废催化剂中钨和钒的含量均较高[4]。然而,为了保证材料的使用性能,钒、钨分别为钨、钒冶金产品中需严格控制的杂质元素。例如我国国家标准GB/T 10116—2007就规定0级仲钨酸铵产品中钒与三氧化钨的质量比不大于0.0010%[5]。因此,钨钒的深度分离成为上述资源利用过程中的关键技术问题。
由于钨、钒的物理化学性质较为相似,并且在湿法冶炼过程中,二者往往也具有相似的行为和走向,从而导致二者的分离极为困难[6-7]。国内外过往对于钨钒分离的研究并不多见[8-11],同时,由于目前也缺乏对该混合体系热力学性质的深入研究,致使为数不多的分离方法在技术路线以及工艺参数的选择上也往往存在一定的盲目性。在此背景下,有必要首先从热力学方面对钨钒混合体系进行研究,探明该溶液体系的物理化学性质,以期从理论上为钨钒的分离提供合理的技术路线。
钨、钒在水溶液中的存在形态十分复杂,除了存在各种单核离子以外,在溶液逐渐酸化的过程中,单核离子还会聚合成各种同多酸根离子,甚至还会相互反应形成多种钨钒杂多酸根离子。同时,上述离子还会随溶液浓度的不同而发生变化。正因为该体系的复杂性,在一定程度上造成了迄今为止鲜有文献对其热力学性质进行报道。有研究者考察了单一钨、钒水溶液体系的热力学行为,并绘制了体系中存在离子随pH、浓度变化的热力学平衡图[12-13]。但是上述研究均只是针对钨、钒单一体系进行研究,而忽略了二者之间的相互作用,即没有考虑生成钨钒杂多酸根离子所带来的影响。而在真实的钨钒混合溶液中,杂多根离子的形成是不可避免的,并且其对于钨钒分离有着极其不利的影响,因而不容忽视。
本文作者在对现有的热力学数据进行广泛的搜集和筛选基础上,全面考虑钨钒混合体系中存在的各种单核离子、同多酸根离子以及杂多酸根离子,并绘制了25 ℃时W(Ⅵ)-V(Ⅴ)-H 2O系中存在的各离子随pH、钨、钒浓度变化的热力学平衡图。通过热力学分析寻找到钨、钒在溶液中存在形式上的差异,并据此提出了钨钒分离可能的技术路线。
1 热力学数据及计算
1.1 热力学数据
W(Ⅵ)-V(Ⅴ)-H 2O系所涉及反应的方程式如下所示:
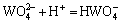 (1)
(1)
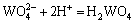 (2)
(2)
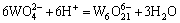 (3)
(3)
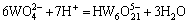 (4)
(4)
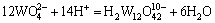 (5)
(5)
 (6)
(6)
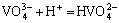 (7)
(7)
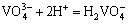 (8)
(8)
 (9)
(9)
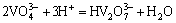 (10)
(10)
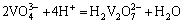 (11)
(11)
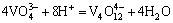 (12)
(12)
 (13)
(13)
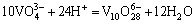 (14)
(14)
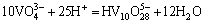 (15)
(15)
 (16)
(16)
 (17)
(17)
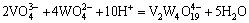 (18)
(18)
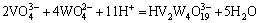 (19)
(19)
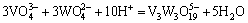 (20)
(20)
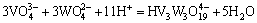 (21)
(21)
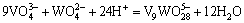 (22)
(22)
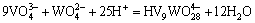 (23)
(23)
通过查阅文献,对上述反应的热力学数据进行了广泛的搜集和筛选,最终确定了各反应的平衡常数及计算式,如表1所列。由于高浓度的钨酸盐、钒酸盐溶液在pH=1.0和pH=2.0附近分别会形成H2WO4和V2O5沉淀,因此在相应浓度的溶液体系中,本文作者即对该体系在形成沉淀的pH以上的范围进行热力学分析。另外由于缺乏相关物质的活度系数,在计算过程中均以离子的浓度代替活度。
1.2 计算过程
设[W]T、[V]T分别为溶液中游离钨、游离钒的总浓度,“[ ]”为溶液中各游离组分的浓度,根据同时平衡和物质守恒原理,在W(Ⅵ)-V(Ⅴ)-H 2O体系中则可以得到如下方程:



 (24)
(24)
表1 涉及反应的平衡常数及计算式(25 ℃)
Table 1 Equilibrium constants and formulas of relevant reactions(25 ℃)
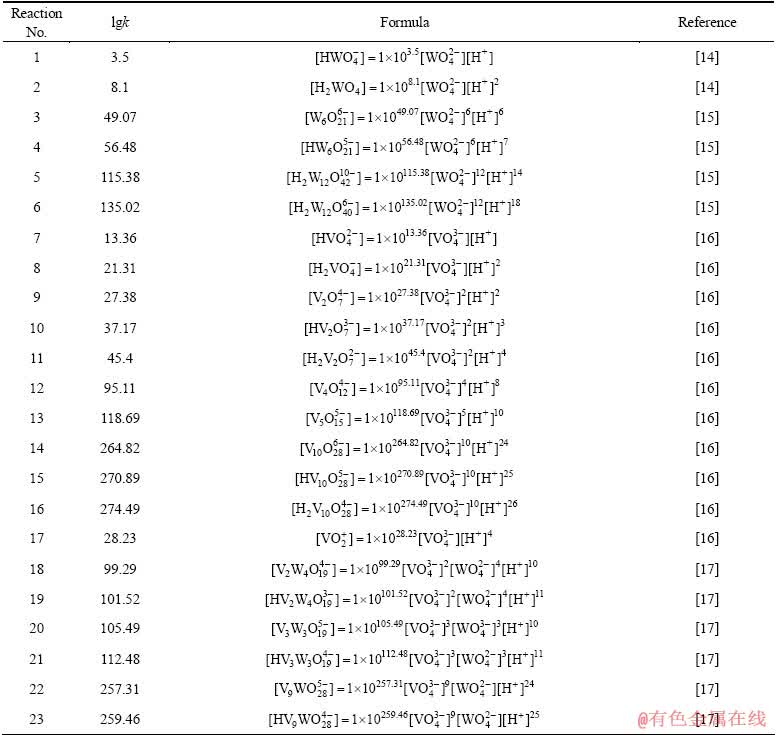
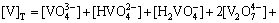
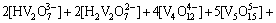
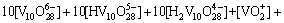

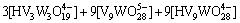 (25)
(25)
若分别给定体系中钨、钒的总浓度及溶液的pH值,通过联立式(1)~(25)求解,即可得到各离子的浓度。而各离子含钨、钒的摩尔浓度与总钨、总钒摩尔浓度的比值即为该离子占总钨、总钒的摩尔分数。可见,体系中存在的所有离子含钨与含钒的摩尔分数之和均为1。
2 结果与讨论
2.1 W(Ⅵ)-V(Ⅴ)-H 2O系热力学平衡分析
在W(Ⅵ)-V(Ⅴ)-H 2O系中,以pH值0.5为间隔,可计算出各个pH值下体系中存在的各离子含钨、钒的摩尔分数。图1所示为不同钨、钒浓度下各主要离子的摩尔分数与pH的关系图。
当溶液中[W]T=[V]T=0.05 mol/L时(见图1(a)),单钨酸根离子在pH约为8.5时开始聚合,聚合后的游离态物质主要为杂多酸根离子V2W4O194-。在pH为3.5~7.0的范围内,V2W4O194-含钨的摩尔分数在90%以上;当pH<3.5时,V2W4O194-则逐渐质子化转变为HV2W4O193-。相比于钨,钒在混合溶液酸化过程中的变化情况更为复杂。当溶液pH为10.0~12.0时,溶液中的钒除了以单核离子的HVO42-存在之外,还存在部分聚合的焦钒酸根离子V2O74-以及HV2O73- ;当pH继续下降时,上述离子则均逐渐转变为钒原子数更高的偏钒酸根离子V4O124-和V5O155-;当pH=8.5时,溶液中聚合的钒酸根离子的摩尔分数接近95%,这与此pH条件下仍以单核离子存在的钨存在明显的差异。继续降低pH,溶液中的钒则依次以V2W4O194-、V9WO285-以及上述两种离子的质子化形式存在。另外值得注意的是,当溶液酸度较低时,体系中会形成一定量的VO2+阳离子,当pH=1.0时,VO2+的摩尔分数达到50%以上。
当溶液中总钨与总钒浓度均升高至0.5 mol/L时(见图1(b)),WO42-开始聚合形成杂多酸根离子V2W4O194-的pH升高至9.0,而在pH为2.5~8.0的范围内,V2W4O194-均为钨的主要存在形式。对于溶液中的钒来说,当pH为10.5~12.0时,钒仍主要以HVO42-和焦钒酸根离子V2O74-、HV2O73- 存在,只是由于钒浓度的升高使得此时钒原子数更高的焦钒酸根离子在体系中占据优势。浓度的升高使上述离子转变为偏钒酸根离子的起始pH上升至10.5。当pH=9.0时(体系中钨为WO42-),溶液中钒的同多酸根离子的摩尔分数达到90%以上。pH继续降低,钒的同多酸根离子则逐渐转变为各种杂多酸根离子。
当体系中总钨浓度高于总钒浓度时(见图1(c)),WO42-在pH约为9.0时开始聚合,形成聚合离子除了在前述两种浓度条件下均存在的杂多酸根离子V2W4O194-之外,还包括3种同多酸根离子W6O216-、HW6O215-、H2W12O4210-。当pH继续降低,上述离子则逐步转变为偏钨酸根离子H2W12O406-和杂多酸根 离子HV2W4O193- 。在此浓度条件下,溶液中的钒在pH=10.0时,从HVO42-和焦钒酸根离子转变为偏钒酸根离子,但由于浓度上处于弱势,偏钒酸根离子占据优势的pH被压缩至8.5~9.5的范围内。但当pH=9.0时,体系中聚合的钒酸根离子的摩尔分数仍高达75%以上。当pH低于8.0时,溶液中的钒几乎全部以杂多酸根离子V2W4O194- 和HV2W4O193-存在。
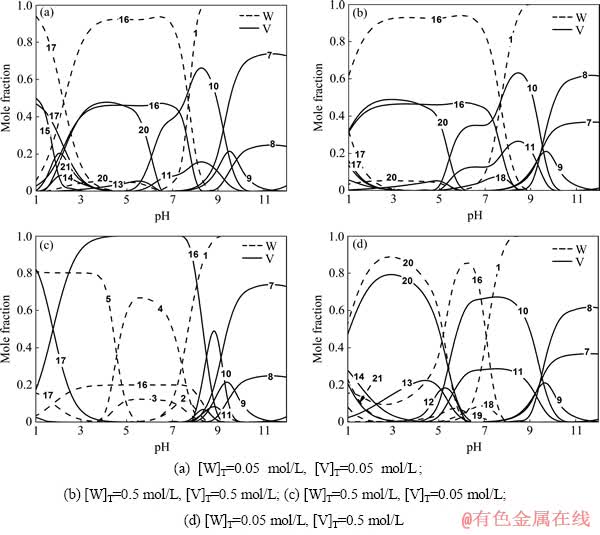
图1 不同钨钒浓度下W(Ⅵ)-V(Ⅴ)-H 2O系主要存在离子含钨、钒的摩尔分数与pH的关系(25 ℃)
Fig. 1 Mole fraction of W and V in main ions as a function of pH at different concentrations of W[T] and V[T] in W(Ⅵ)-V(Ⅴ)-H 2O system (25 ℃) (1—WO42-; 2—W6O216-; 3—HW6O215-; 4—H2W12O4210-; 5—H2W12O406-; 6—VO43-; 7—HVO42-; 8—V2O74-; 9—HV2O73-; 10—V4O124-; 11—V5O105-; 12—V10O286-; 13—HV10O285-; 14—H2V10O284-; 15—VO2+; 16—V2W4O194-; 17—HV2W4O193-; 18—V3W3O195-; 19—HV3W3O194-; 20—V9WO285-; 21—HV9WO284-)
而当体系中总钨浓度低于总钒浓度时(见图1(d)),钨在pH=8.5开始聚合后除了形成V2W4O194-之外,还会形成少量的V3W3O195-和HV3W3O194-。之后,随着pH的降低,上述杂多酸根离子则逐渐转变为钒钨原子比更高的V9WO285-和HV9WO284-。对于钒来说,HVO42-和焦钒酸根离子在pH=10.5时开始转变为偏钒酸根离子,并在7.0~9.5的pH区间内,偏钒酸根离子在体系中都占据绝对的优势。当pH=8.5时,体系中钒的同多酸根离子的摩尔分数接近100%。当pH由7.0继续下降时,体系中则逐渐形成大量的V9WO285-以及一定量的十聚同多酸根离子(V10O286-、HV10O285-、HV10O284-)。
2.2 钨钒分离路线的提出
相似元素分离的关键在于找到元素之间性质上的差异,之后再借助于合理的分离手段即可实现二者的有效分离。如之前所分析的,在W(Ⅵ)-V(Ⅴ)-H 2O体系中存在离子的类型可分为单核离子(此处将VO2+也包括在内)、同多酸根离子以及杂多酸根离子。将W(Ⅵ)-V(Ⅴ)-H 2O体系中这3种类型的离子分别求和,得到不同钨钒浓度下各类型离子含钨、钒的摩尔分数随pH变化的曲线图,如图2所示。从图2中可以看到,单核钨离子聚合的起始pH主要与溶液中的钨浓度有关,当[W]T=0.5 mol/L,聚合的起始pH为9.0,而当[W]T=0.05 mol/L时,起始pH则为8.5。聚合之后除了高钨低钒体系外,其余溶液体系中钨均以杂多酸根离子形式存在,而在W[T]=0.5 mol/L,V[T]=0.05 mol/L的高钨低钒溶液中,除了生成含钨摩尔分数为20%左右的杂多酸根离子之外。另外,80%的含钨物质则为钨的同多酸根离子,并且这种摩尔分数的数值在pH<6.5的范围内几乎保持不变,这主要是因为此时溶液中的钒几乎全部以钨、钒摩尔比为2的杂多酸根离子存在。
对于溶液中的钒来说,在pH=12.0时,部分的钒即以聚合的同多酸根离子存在(主要为二聚的焦钒酸根离子),且钒浓度越高,聚合离子的摩尔分数越大。随后在酸化的过程中,单核钒离子进一步转变为同多酸根离子。总的来说,当[W]T=0.5 mol/L时,钒的同多酸根离子的摩尔分数在pH=9.0时达到最大;而当[W]T=0.05 mol/L时,则在pH=8.5时达到最大,这恰巧也是在相应溶液中钨开始形成聚合离子的pH值。在该pH条件下,溶液中绝大部分的钒以聚合离子形式存在,而钨则几乎全部以单核离子存在,可见此时钒、钨在存在形式上存在着差异,同时这也表明钒形成聚合离子的能力强于钨的。
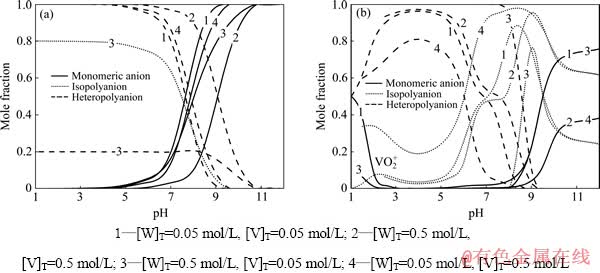
图2 不同钨钒浓度下W(Ⅵ)-V(Ⅴ)-H 2O系中单核离子、同多酸根离子、杂多酸离子含钨、钒的摩尔分数随pH的变化(25 ℃)
Fig. 2 Mole fractions of W(a) and V(b) in monomeric anions, isopolyanions, heteropolyanions as function of pH at different concentrations of [W]T and [V]T in W(Ⅵ)-V(Ⅴ)-H2O system (25 ℃)
当继续降低pH时,溶液中钒的同多酸根离子的摩尔分数逐渐降低,杂多酸根离子的摩尔分数则逐渐升高。在pH为2.5~6.5的范围内,溶液中有大量的钨钒杂多酸根离子形成,这对于钨钒的分离显然是极其不利的。因此,在不破坏体系中杂多酸的前提下,该pH区间内是无法进行钨钒的有效分离的。而在溶液中钨钒浓度均较低的情况下,体系会在酸度较低时形成一定量的VO2+离子,而此时体系中钨的存在形式则全部为阴离子,这也是钨、钒在水溶液中存在形式上的另外一个差异。
基于以上对W(Ⅵ)-V(Ⅴ)-H 2O系的热力学分析,找到钨、钒在该体系中存在形式上的两个差异,利用这些差异,提出了以下两种可能的钨钒分离的技术路线:
1) 利用钨钒形成聚合离子能力的差异
如之前所分析的,钒形成聚合离子的能力强于钨,当pH约为8.5~9.0时,溶液中绝大部分的钒以聚合离子形式存在,而钨则几乎全部以单核离子形式存在。聚合离子在沉淀结晶性能以及被某些吸附剂所吸附的吸附特性等方面与单核离子有着较大的差异,进而可通过沉淀、萃取、吸附等方式对钨钒进行分离。以高价离子(Fe3+、Mn4+、Sn4+等)水合氧化物的吸附举例来说,相对于未聚合的WO42-,聚合钒酸根离子(主要为偏钒酸根离子V4O124-和V5O155-)电价更高,而高价离子的水合氧化物实质上是一种弱碱性的阴离子交换剂,因而其与聚合钒酸根离子应具有更强的亲和力。同时聚合钒酸根离子的钒原子数与电价之比相对更大,这意味着每交换单位电价所吸附的钒量也是要大于钨的。基于上述原理,预期采用高价离子的水合氧化物在pH为8.5~9.0的条件下可选择性地吸附混合溶液中的钒,进而实现钨钒的有效分离。
2) 利用钒在溶液中可形成阳离子的差异
热力学分析表明:当钨、钒浓度均较低时,溶液会在pH较低的条件下形成一定量的VO2+阳离子,而此时含钨离子仍全部为阴离子。基于这一差异,采用阳离子萃取剂或阳离子树脂可望选择性地将VO2+从溶液中分离。但同时从图2 中也可以看到,此pH条件下体系中仍有一部分的钒是以杂多酸根阴离子形式存在,因此势必要采取一定的措施破坏杂多酸根离子。此时,可适当向溶液中加入一定量的H2O2、EDTA、酒石酸等配合试剂,促进杂多酸根离子的解聚,从而改善钨钒的分离效果。
除了从理论上提出了上述两种钨钒分离的技术路线之外,热力学分析还可进一步对上述方法的分离条件进行优化。由于钨、钒在溶液中的存在形式对pH的变化较为敏感,因而适宜进行分离的pH值的范围也较为狭窄,并且该pH范围还会随着钨、钒浓度的不同而发生改变。这就需要在分离不同的料液时,对溶液的pH进行相应的调整,以获得最优的分离效果。传统上只能通过试验得到适宜的分离条件,这在实际生产中显然是不可能实现的。此时,针对该体系的热力学分析可以全部或部分的代替试验探索,来对分离条件进行优化,从而为钨钒分离的实现提供理论指导。
3 结论
1) 全面考虑了钨钒混合体系中存在的各种单核离子、同多酸根离子以及杂多酸根离子,通过热力学计算,绘制了25 ℃时W(Ⅵ)-V(Ⅴ)-H 2O系中存在离子随pH、钨钒浓度变化的热力学平衡图,并总结了其变化规律。
2) 钒形成聚合离子的能力强于钨的,在pH约为8.5~9.0的条件下,溶液中绝大部分的钒以聚合离子的形式存在,而钨则几乎全部以单核离子形式存在。利用该差异,采用高价离子水合氧化物吸附等方法预期可实现钨钒的有效分离。
3) 在溶液中钨、钒浓度均较低的情况下,体系会在pH较低时形成VO2+离子,而此时钨的存在形式则全部为阴离子。基于该差异采用阳离子萃取剂或阳离子树脂可望选择性地将VO2+从混合溶液中分离。
4) 通过W(Ⅵ)-V(Ⅴ)-H 2O体系的热力学分析,提出钨钒分离的技术路线,同时还可便利地对分离不同浓度料液的pH条件进行优化,从而为钨钒分离的实现提供理论指导。
REFERENCES
[1] 赵中伟. 钨冶炼的理论与应用[M]. 北京: 清华大学出版社, 2013: 1-4.
ZHAO Zhong-wei. Tungsten metallurgy: Fundamentals and applications[M]. Beijing: Tsinghua University Press, 2013: 1-4.
[2] GUPTA C K, KRISHNAMURTHY N. Extractive metallurgy of vanadium[M]. Amsterdam: Elsevier Science Publishers, 1992: 2-7.
[3] 贾帅广, 陈星宇, 刘旭恒, 李月星. 镍钼矿研究现状及发展趋势[J]. 中国钨业, 2013, 27(6): 8-12.
JIA Shuai-guang, CHEN Xing-yu, LIU Xu-heng, LI Yue-xing. Research status and development trends of Ni-Mo ore[J]. China Tungsten Industry, 2013, 27(6): 8-12.
[4] 廖世明, 柏谈论. 国外钒冶金[M]. 北京: 冶金工业出版社, 1985: 4-19.
LIAO Shi-ming, BO Tan-lun. Extractive metallurgy of vanadium[M]. Beijing: Metallurgical Industry Press, 1985: 4-19.
[5] GB/T 10116—2007. 仲钨酸铵[S]. 北京: 中国标准出版社, 2007.
GB/T 10116—2007. Ammoniumparatungstate[S]. Beijing: China Standards Press, 2007.
[6] 李洪桂, 羊建高, 李 昆. 钨冶金学[M]. 长沙: 中南大学出版社, 2010: 211-228.
LI Hong-gui, YANG Jian-gao, LI Kun. Tungsten metallurgy[M]. Changsha: Central South University Press, 2010: 211-228.
[7] 杨守志. 钒冶金[M]. 北京: 冶金工业出版社, 2010: 65-66.
YANG Shou-zhi. Vanadium metallurgy[M]. Beijing: Metallurgical Industry Press, 2010: 65-66.
[8] 宋 阜, 朱宾权. 离子交换法分离富集钨酸钠溶液中的钒[J]. 稀有金属与硬质合金, 2006, 34(3): 5-7.
SONG Fu, ZHU Bin-quan. Extraction of vanadium from sodium tungstate by ion exchange[J]. Rare Metals and Cemented Carbides, 2006, 34(3): 5-7.
[9] 罗 琳, 藤田丰久, 治进藤. 从废弃的钨金属制品中回收钨和钒[J]. 矿冶, 2002, 11(1): 50-54.
LUO Lin, TOYOHISA F, OSAMU S. Recovery of tungsten and vanadium from tungsten scrap[J]. Mining & Metallurgy, 2002, 11(1): 50-54.
[10] LUO L, MIYAZAKI T, SHIBAYAMA A, YEN W, FUJITA T. A novel process for recovery of tungsten and vanadium from a leach solution of tungsten alloy scrap[J]. Minerals Engineering, 2003, 16(7): 665-670.
[11] LUO L, LIU K, SHIBAYAMA A, YEN W, FUJITA T, SHINDO O, KATAI A. Recovery of tungsten and vanadium from tungsten alloy scrap[J]. Hydrometallurgy, 2004, 72(1): 1-8.
[12] CRUYWAGEN J J. Protonation, oligomerization and condensation reactions of vanadate(Ⅴ), molybdate(Ⅵ) and tungstate(Ⅵ)[J]. Advances in Inorganic Chemistry, 2000, 49: 127-182.
[13] NEKOVAR P, SCHROTTEROVA D. Extraction of V(Ⅴ), Mo(Ⅵ) and W(Ⅵ) polynuclear species by primene JMT[J]. Chemical Engineering Journal, 2000, 79: 229-233.
[14] BAES C F, MESMER R E. The hydrolysis of cations[M]. New York: Wiley, 1976: 259.
[15] CRUYWAGEN J J, MERWE F J. Tungsten(Ⅵ) equilibria: a potentiometric and calorimetric investigation[J]. Journal of the Chemical Society, Dalton Transactions, 1987(7): 1701-1705.
[16] ELVINGSON K, FRITZSCHE M, REHDER D, PETTERSON L. Speciation in vanadium bioinorganic systems. 1. A potentiometric and V-51 NMR-study of aqueous equilibria in the H+-vanadate(Ⅴ)-L-alpha-alanyl-L-histidine system[J]. Acta Chemica Scandinavica, 1994, 48(11): 878-885.
[17] ANDERSSON I, HASTINGS J J, HOWARTH O W. Aqueous tungstovanadate equilibria[J]. Journal of the Chemical Society, Dalton Transactions, 1996(13): 2705-2711.
(编辑 李艳红)
基金项目:国家自然科学基金重点项目(51334008)
收稿日期:2014-01-05;修订日期:2014-05-10
通信作者:赵中伟,教授,博士;电话:0731-88830476;E-mail: zhaozw@csu.edu.cn